Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Proteins, Cells, and Plasmids
2.2. Production of mAbs against ASFV CD2v Protein
2.3. Western Blotting for CD2v-ex Protein
2.4. Hemadsorption (HAD) and Indirect Immunofluorescence Analysis (IFA)
2.5. Blocking/Competitive ELISA Analysis of ASFV Positive Serum
2.6. Identification of Linear B Cell Epitopes
2.7. B Cell Epitope Conservation
2.8. Statistical Analysis
3. Results
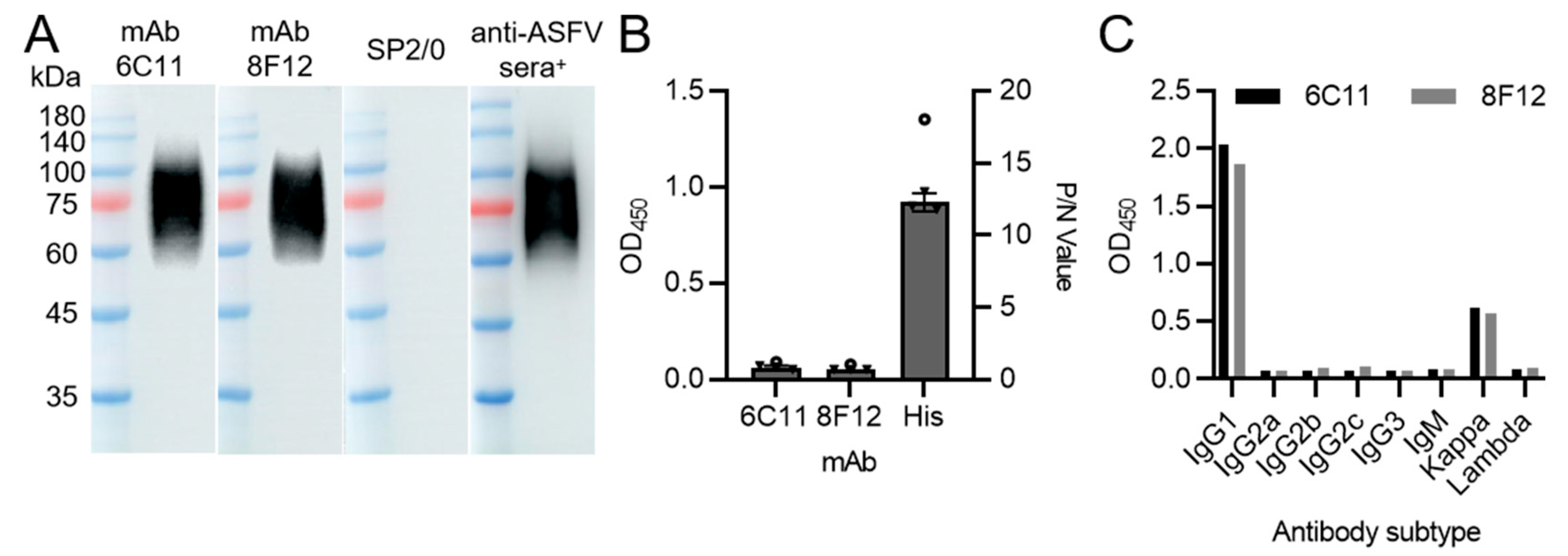
3.1. Production and Characterization of CD2v Protein-Specific Monoclonal Antibodies
3.2. Localization of CD2v Protein in Transfected Cells
3.3. Reactivity of ASFV-Positive Serum against Anti-CD2v Monoclonal Antibodies
3.4. Precise Localization of the mAb Epitopes
3.5. Sequence Alignment Analysis of Epitope Conservation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Zsak, L.; Delhon, G.; Diel, D.G.; Shobogorov, N.M.; Morgunov, Y.P.; Morgunov, S.Y.; Kutish, G.F.; et al. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J. Gen. Virol. 2016, 97, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.A.; Darby, A.C.; Da Silva, M.; Upton, C.; Radford, A.D.; Dixon, L.K. Genomic analysis of highly virulent Georgia 2007/1 isolate of African swine fever virus. Emerg. Infect. Dis. 2011, 17, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Malogolovkin, A.; Sereda, A. African Swine Fever Virus Hemadsorption Inhibition Assay. Methods Mol. Biol. 2022, 2503, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A Proteomic Atlas of the African Swine Fever Virus Particle. J. Virol. 2018, 92, e01293-18. [Google Scholar] [CrossRef]
- Brown, V.R.; Bevins, S.N. A Review of African Swine Fever and the Potential for Introduction into the United States and the Possibility of Subsequent Establishment in Feral Swine and Native Ticks. Front. Vet. Sci. 2018, 5, 11. [Google Scholar] [CrossRef]
- Jori, F.; Bastos, A.D. Role of wild suids in the epidemiology of African swine fever. Ecohealth 2009, 6, 296–310. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Salas, M.L.; Andrés, G. African swine fever virus morphogenesis. Virus. Res. 2013, 173, 29–41. [Google Scholar] [CrossRef]
- Andrés, G. African Swine Fever Virus Gets Undressed: New Insights on the Entry Pathway. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever (ASF): Five years around Europe. Vet. Microbiol. 2013, 165, 45–50. [Google Scholar] [CrossRef]
- Gallardo, M.C.; Reoyo, A.T.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African swine fever: A global view of the current challenge. Porcine Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef]
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.A.J. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef]
- Galindo, I.; Cuesta-Geijo, M.A.; Hlavova, K.; Muñoz-Moreno, R.; Barrado-Gil, L.; Dominguez, J.; Alonso, C. African swine fever virus infects macrophages, the natural host cells, via clathrin- and cholesterol-dependent endocytosis. Virus Res. 2015, 200, 45–55. [Google Scholar] [CrossRef]
- Hernaez, B.; Alonso, C. Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J. Virol. 2010, 84, 2100–2109. [Google Scholar] [CrossRef]
- Dixon, L.K.; Islam, M.; Nash, R.; Reis, A.L. African swine fever virus evasion of host defences. Virus Res. 2019, 266, 25–33. [Google Scholar] [CrossRef]
- Reis, A.L.; Netherton, C.; Dixon, L.K. Unraveling the Armor of a Killer: Evasion of Host Defenses by African Swine Fever Virus. J. Virol. 2017, 91, e02338-16. [Google Scholar] [CrossRef]
- Breese, S.S., Jr.; Pan, I.C. Electron microscopic observation of African swine fever virus development in Vero cells. J. Gen. Virol. 1978, 40, 499–502. [Google Scholar] [CrossRef]
- Goatley, L.C.; Dixon, L.K. Processing and localization of the african swine fever virus CD2v transmembrane protein. J. Virol. 2011, 85, 3294–3305. [Google Scholar] [CrossRef]
- Borca, M.V.; Kutish, G.F.; Afonso, C.L.; Irusta, P.; Carrillo, C.; Brun, A.; Sussman, M.; Rock, D.L. An African swine fever virus gene with similarity to the T-lymphocyte surface antigen CD2 mediates hemadsorption. Virology 1994, 199, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Abrams, C.C.; Bowick, G.; Goatley, L.C.; Kay-Jackson, P.C.; Chapman, D.; Liverani, E.; Nix, R.; Silk, R.; Zhang, F. African swine fever virus proteins involved in evading host defence systems. Vet. Immunol. Immunopathol. 2004, 100, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Duarte, M.M.; Boinas, F.; Hutchings, G.; Dixon, L.K. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology 2009, 393, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, S.; Delhon, G.A.; Khatiwada, S.; Rock, D.L. African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells. Viruses 2021, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Núñez, D.; Castillo-Rosa, E.; Vigara-Astillero, G.; García-Belmonte, R.; Gallardo, C.; Revilla, Y. Identification and Isolation of Two Different Subpopulations Within African Swine Fever Virus Arm/07 Stock. Vaccines 2020, 8, 625. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, e01058-17. [Google Scholar] [CrossRef]
- Teklue, T.; Wang, T.; Luo, Y.; Hu, R.; Sun, Y.; Qiu, H.J. Generation and Evaluation of an African Swine Fever Virus Mutant with Deletion of the CD2v and UK Genes. Vaccines 2020, 8, 763. [Google Scholar] [CrossRef]
- Lopez, E.; van Heerden, J.; Bosch-Camós, L.; Accensi, F.; Navas, M.J.; López-Monteagudo, P.; Argilaguet, J.; Gallardo, C.; Pina-Pedrero, S.; Salas, M.L.; et al. Live Attenuated African Swine Fever Viruses as Ideal Tools to Dissect the Mechanisms Involved in Cross-Protection. Viruses 2020, 12, 1474. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, J.; Liang, W.; Chen, W.; Li, Z.; Chen, Q.; Cai, S. The recombinant pseudorabies virus expressing African swine fever virus CD2v protein is safe and effective in mice. Virol. J. 2020, 17, 180. [Google Scholar] [CrossRef]
- Lopera-Madrid, J.; Osorio, J.E.; He, Y.; Xiang, Z.; Adams, L.G.; Laughlin, R.C.; Mwangi, W.; Subramanya, S.; Neilan, J.; Brake, D.; et al. Safety and immunogenicity of mammalian cell derived and Modified Vaccinia Ankara vectored African swine fever subunit antigens in swine. Vet. Immunol. Immunopathol. 2017, 185, 20–33. [Google Scholar] [CrossRef]
- Sunwoo, S.Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.G.; Gaudreault, N.N.; Trujillo, J.D.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, D.; Li, L.; Wan, B.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Song, J.; Zhu, Z.; et al. Development of an indirect ELISA for the identification of African swine fever virus wild-type strains and CD2v-deleted strains. Front. Vet. Sci. 2022, 9, 1006895. [Google Scholar] [CrossRef]
- Wang, M.; Song, J.; Sun, J.; Du, Y.; Qin, X.; Xia, L.; Wu, Y.; Zhang, G. Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus. Viruses 2022, 14, 2107. [Google Scholar] [CrossRef]
- Li, G.; Wang, A.; Chen, Y.; Sun, Y.; Du, Y.; Wang, X.; Ding, P.; Jia, R.; Wang, Y.; Zhang, G. Development of a Colloidal Gold-Based Immunochromatographic Strip for Rapid Detection of Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein. Front. Immunol. 2021, 12, 635677. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Y.; Ge, J.; Ma, Q.; Yan, W.; Zhang, Y.; Zhao, L.; Chen, H. Identification and characterization of a novel B-cell epitope on Aleutian Mink Disease virus capsid protein VP2 using a monoclonal antibody. Virus Res. 2018, 248, 74–79. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.; Cao, Y.; Wang, N.; Sun, P.; Shi, Q.; Ji, X.; Bao, H.; Li, D.; Chen, Y.; et al. Development of a Blocking ELISA Using a Monoclonal Antibody to a Dominant Epitope in Non-Structural Protein 3A of Foot-and-Mouth Disease Virus, as a Matching Test for a Negative-Marker Vaccine. PLoS ONE 2017, 12, e0170560. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Zhang, G.; Liu, H.; Ding, P.; Jia, R.; Zhou, J.; Chen, Y.; Qi, Y.; Du, J.; Liang, C.; et al. Screening and identification of B cell epitope of the nucleocapsid protein in SARS-CoV-2 using the monoclonal antibodies. Appl. Microbiol. Biotechnol. 2022, 106, 1151–1164. [Google Scholar] [CrossRef]
- Ge, S.; Li, J.; Fan, X.; Liu, F.; Li, L.; Wang, Q.; Ren, W.; Bao, J.; Liu, C.; Wang, H.; et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018, 24, 2131–2133. [Google Scholar] [CrossRef]
- Busch, F.; Haumont, C.; Penrith, M.L.; Laddomada, A.; Dietze, K.; Globig, A.; Guberti, V.; Zani, L.; Depner, K. Evidence-Based African Swine Fever Policies: Do We Address Virus and Host Adequately? Front. Vet. Sci. 2021, 8, 637487. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Carrillo, C.; Zsak, L.; Laegreid, W.W.; Kutish, G.F.; Neilan, J.G.; Burrage, T.G.; Rock, D.L. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 1998, 72, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; O’Donnell, V.; Holinka, L.G.; Risatti, G.R.; Ramirez-Medina, E.; Vuono, E.A.; Shi, J.; Pruitt, S.; Rai, A.; Silva, E.; et al. Deletion of CD2-like gene from the genome of African swine fever virus strain Georgia does not attenuate virulence in swine. Sci. Rep. 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Lirola, L.G.; Mur, L.; Rivera, B.; Mogler, M.; Sun, Y.; Lizano, S.; Goodell, C.; Harris, D.L.; Rowland, R.R.; Gallardo, C.; et al. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef] [PubMed]
- Tesfagaber, W.; Wang, L.; Tsegay, G.; Hagoss, Y.T.; Zhang, Z.; Zhang, J.; Huangfu, H.; Xi, F.; Li, F.; Sun, E.; et al. Characterization of Anti-p54 Monoclonal Antibodies and Their Potential Use for African Swine Fever Virus Diagnosis. Pathogens 2021, 10, 178. [Google Scholar] [CrossRef]
- Yuan, F.; Petrovan, V.; Gimenez-Lirola, L.G.; Zimmerman, J.J.; Rowland, R.R.R.; Fang, Y. Development of a Blocking Enzyme-Linked Immunosorbent Assay for Detection of Antibodies against African Swine Fever Virus. Pathogens 2021, 10, 760. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Kuriga, A.; Niemyjski, R.; Tarasiuk, G.; Pomorska-Mól, M. African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine-Current Data. Viruses 2021, 13, 1212. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Revilla, Y.; Pérez-Núñez, D.; Richt, J.A. African Swine Fever Virus Biology and Vaccine Approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar] [CrossRef]
- Lopez, E.; Bosch-Camós, L.; Ramirez-Medina, E.; Vuono, E.; Navas, M.J.; Muñoz, M.; Accensi, F.; Zhang, J.; Alonso, U.; Argilaguet, J.; et al. Deletion Mutants of the Attenuated Recombinant ASF Virus, BA71ΔCD2, Show Decreased Vaccine Efficacy. Viruses 2021, 13, 1678. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef]
- Wang, T.; Luo, R.; Sun, Y.; Qiu, H.J. Current efforts towards safe and effective live attenuated vaccines against African swine fever: Challenges and prospects. Infect. Dis. Poverty 2021, 10, 137. [Google Scholar] [CrossRef]
- Barasona, J.A.; Cadenas-Fernández, E.; Kosowska, A.; Barroso-Arévalo, S.; Rivera, B.; Sánchez, R.; Porras, N.; Gallardo, C.; Sánchez-Vizcaíno, J.M. Safety of African Swine Fever Vaccine Candidate Lv17/WB/Rie1 in Wild Boar: Overdose and Repeated Doses. Front. Immunol. 2021, 12, 761753. [Google Scholar] [CrossRef]
- Leitão, A.; Cartaxeiro, C.; Coelho, R.; Cruz, B.; Parkhouse, R.M.E.; Portugal, F.C.; Vigário, J.D.; Martins, C.L.V. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol. 2001, 82, 513–523. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Gladue, D.P.; O’Donnell, V.; Ramirez-Medina, E.; Rai, A.; Pruitt, S.; Vuono, E.A.; Silva, E.; Velazquez-Salinas, L.; Borca, M.V. Deletion of CD2-Like (CD2v) and C-Type Lectin-Like (EP153R) Genes from African Swine Fever Virus Georgia-∆9GL Abrogates Its Effectiveness as an Experimental Vaccine. Viruses 2020, 12, 1185. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, C.; Shi, W.; Huang, C.; Zeng, S.; Sun, J.; Wu, J.; Hua, Q. Development of a triplex real-time PCR assay for detection and differentiation of gene-deleted and wild-type African swine fever virus. J. Virol. Methods 2020, 280, 113875. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Jiang, D.; Li, L.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Liu, B.; Ji, P.; Zhang, G. Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses 2023, 15, 131. https://doi.org/10.3390/v15010131
Jiang W, Jiang D, Li L, Wang J, Wang P, Shi X, Zhao Q, Liu B, Ji P, Zhang G. Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses. 2023; 15(1):131. https://doi.org/10.3390/v15010131
Chicago/Turabian StyleJiang, Wenting, Dawei Jiang, Lu Li, Jiabin Wang, Panpan Wang, Xuejian Shi, Qi Zhao, Boyuan Liu, Pengchao Ji, and Gaiping Zhang. 2023. "Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies" Viruses 15, no. 1: 131. https://doi.org/10.3390/v15010131
APA StyleJiang, W., Jiang, D., Li, L., Wang, J., Wang, P., Shi, X., Zhao, Q., Liu, B., Ji, P., & Zhang, G. (2023). Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses, 15(1), 131. https://doi.org/10.3390/v15010131





