Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Expression and Identification of p30 Protein
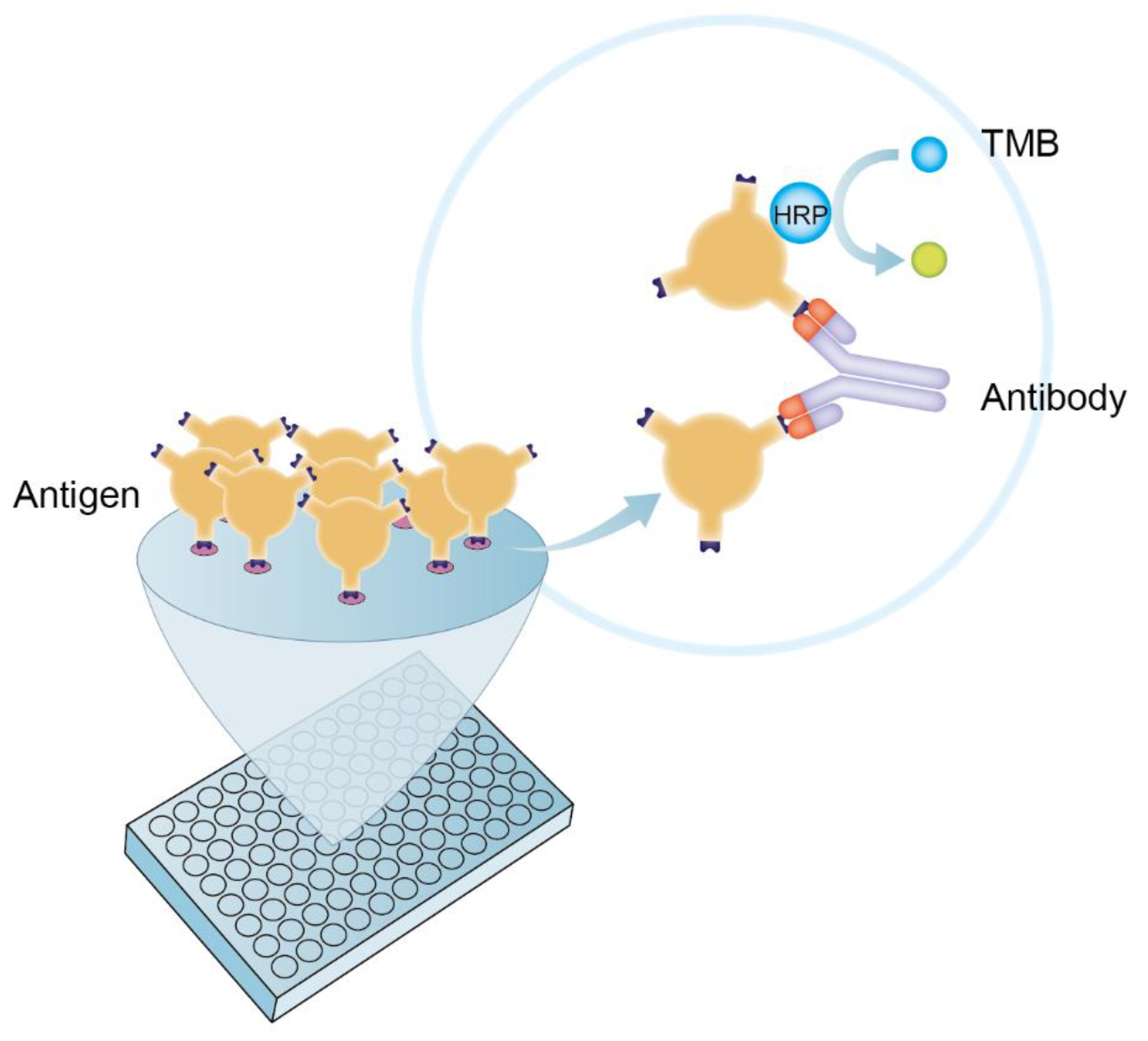
2.3. The Establishment of DAgS-ELISA Based on p30 Protein
2.4. Determination of the Cut-Off Value
2.5. Assessment of the Diagnostic Sensitivity and Specificity
2.6. Reproducibility of DagS-ELISA
2.7. Comparison of DagS-ELISA with Commercial Kits
2.8. Statistical Analysis
3. Results
3.1. Expression and Purification of p30 Protein
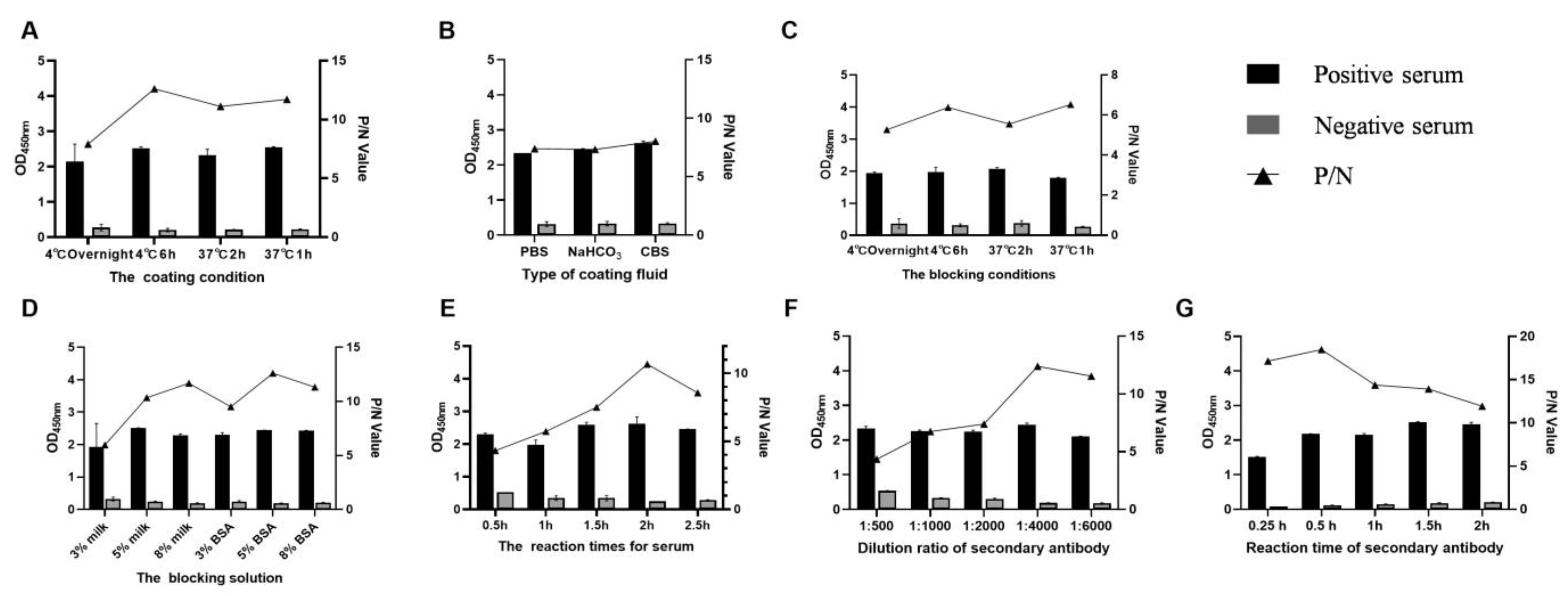
3.2. Standardization of the DAgS-ELISA Procedure
3.3. Cut-Off Value of the DAgS-ELISA
3.4. Diagnostic Sensitivity and Specificity of DAgS-ELISA
3.5. Repeatability of the DAgS-ELISA
3.6. Clinical Serum Sample Detection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernándezpinero, J.; Markowskadaniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How To Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Y.; Wang, Y.; Li, S.; Zhao, Z.; Bi, Y.; Sun, J.; Peng, R.; Song, H.; Zhu, D.; et al. Cryo-EM Structure of the African Swine Fever Virus. Cell Host Microbe 2019, 26, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 15, 9–21. [Google Scholar] [CrossRef]
- Gao, G.F. From “A”IV to “Z”IKV: Attacks from Emerging and Re-emerging Pathogens. Cell 2018, 172, 1157–1159. [Google Scholar] [CrossRef]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A proteomic atlas of the African swine fever virus particle. J. Virol. 2018, 92, 23. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Quintas, A.; Nogal, M.; Castelló, A.; Revilla, Y. African swine fever virus controls the host transcription and cellular machinery of protein synthesis. Virus Res. 2013, 173, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, B.; Escribano, J.; Alonso, C. African swine fever virus protein p30 interaction with heterogeneous nuclear ribonucleoprotein K (hnRNP-K) during infection. FEBS Lett. 2008, 582, 3275–3280. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, P.; Takamatsu, H.; Werling, D.; Dixon, L.; Chapman, D. Correlation of cell surface marker expression with African swine fever virus infection. Vet. Microbiol. 2014, 168, 413–419. [Google Scholar] [CrossRef]
- Alcaraz, C.; De Diego, M.; Pastor, M.J.; Escribano, J.M.J. Comparison of a radioimmunoprecipitation assay to immunoblotting and ELISA for detection of antibody to African swine fever virus. J. Vet. Diagn. Investig. 1990, 2, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, e12. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Maghsoudlou, P. Enzyme-linked immunosorbent assay (ELISA): The basics. Br. J. Hosp. Med. 2016, 77, C98–C101. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Mao, P.; Hou, J.; Hong, S.; Zhu, L.; Hu, Y.; Bai, Y. Establishment of a double-antigen sandwich ELISA for detecting total antibodies to human immunodeficiency virus type 1/2. Chin. J. Exp. Clin. Virol. 2002, 16, 288–291. [Google Scholar]
- He, J.; Xiu, B.; Wang, G.; Chen, K.; Feng, X.; Song, X.; Zhu, C.; Yang, X.; Bai, G.; Ling, S.; et al. Construction, expression, purification and biotin labeling of a single recombinant multi-epitope antigen for double-antigen sandwich ELISA to detect hepatitis C virus antibody. Protein Pept. Lett. 2011, 18, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Xinju, G. Clinical study on double antigen sandwich method of hepatitis C virus antibody. Chin. J. Mod. Drug Appl. 2016, 10, 2. [Google Scholar]
- Bao, J.; Wang, Q.; Lin, P.; Liu, C.; Li, L.; Wu, X.; Chi, T.; Xu, T.; Ge, S.; Liu, Y.; et al. Genome comparison of African swine fever virus China/2018/AnhuiXCGQ strain and related European p72 Genotype II strains. Transbound. Emerg. Dis. 2019, 66, 1167–1176. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Qiu, H. African swine fever: An unprecedented disaster and challenge to China. Infect. Dis. Poverty 2018, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahma, K.A.; Ghazy, A.A.; Ata, E.B. Better Understanding of Important Aspects Associated with Vaccines Development for Controlling Viral Diseases in Animals. Int. J. Dairy Sci. 2020, 15, 114–122. [Google Scholar] [CrossRef]
- Teklue, T.; Sun, Y.; Muhammad, A.; Luo, Y.; Qiu, H.J. Current status and evolving approaches to African swine fever vaccine development. Transbound. Emerg. Dis. 2020, 67, 529–542. [Google Scholar] [CrossRef]
- Cubillos, C.; Gómez-Sebastian, S.; Moreno, N.; Nuñez, M.; Blanco, E. African swine fever virus serodiagnosis: A general review with a focus on the analyses of African serum samples. Vet Res. 2013, 173, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ata, E.; Li, Z.; Shi, C.; Yang, G.; Yang, W.; Wang, C. African swine fever virus: A raised global upsurge and a continuous threaten to pig husbandry. Microb. Pathog. 2022, 167, 105561. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Zhu, M.; Yuan, Q.; Deng, Z.; Feng, S.; Liu, D.; Yuan, X. Development of an Indirect ELISA to Detect African Swine Fever Virus pp62 Protein-Specific Antibodies. Front. Vet. Sci. 2021, 8, 798559. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, X.; Chen, X.; Li, D.; Xu, Q.; Yao, L.; Sun, Q.; Ghonaim, A.; Ku, X.; Fan, S. Establishment of a Blocking ELISA Detection Method for Against African Swine Fever Virus p30 Antibody. Front. Vet. Sci. 2021, 8, 781373. [Google Scholar] [CrossRef]
- Qiang, S.; Yanqin, L.; Baoming, H. Comparative study on detection of hepatitis C virus antibody by dual antigen sandwich method and indirect method. Shaanxi Med. J. 2019, 48, 4. [Google Scholar]
- Xia, N.; Wang, G.; Gong, W. Serological Test is an Efficient Supplement of RNA Detection for Confirmation of SARS-CoV-2 Infection. Preprints 2020, 3, 184. [Google Scholar]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Murgia, M.V.; Mogler, M.; Certoma, A.; Green, D.; Monaghan, P.; Williams, D.T.; Rowland, R.R.R.; Gaudreault, N.N. Evaluation of an African swine fever (ASF) vaccine strategy incorporating priming with an alphavirus-expressed antigen followed by boosting with attenuated ASF virus. Arch. Virol. 2019, 164, 359–370. [Google Scholar] [CrossRef]
- Giménez-Lirola, L.; Mur, L.; Rivera, B.; Mogler, M. Detection of African Swine Fever Virus Antibodies in Serum and Oral Fluid Specimens Using a Recombinant Protein 30 (p30) Dual Matrix Indirect ELISA. PLoS ONE 2016, 11, e0161230. [Google Scholar] [CrossRef]




| Dilution of Sera | Antigen at Different Concentrations (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 6 | 8 | ||
| 1:5 | P | 2.635 | 2.7043 | 2.593 | 1.751 | 1.264 | 1.433 |
| N | 0.333 | 0.2111 | 0.222 | 0.238 | 0.256 | 0.267 | |
| P/N | 7.917 | 12.811 | 11.693 | 7.349 | 4.938 | 5.357 | |
| 1:10 | P | 2.430 | 2.560 | 2.437 | 0.904 | 0.528 | 0.479 |
| N | 0.252 | 0.192 | 0.218 | 0.224 | 0.237 | 0.252 | |
| P/N | 9.638 | 13.316 | 11.192 | 4.027 | 2.225 | 1.903 | |
| 1:20 | P | 2.235 | 2.247 | 1.836 | 1.836 | 0.264 | 0.406 |
| N | 0.261 | 0.194 | 0.187 | 0.187 | 0.262 | 0.284 | |
| P/N | 8.579 | 11.572 | 9.831 | 1.760 | 1.007 | 1.427 | |
| 1:40 | P | 1.656 | 1.616 | 0.813 | 0.309 | 0.256 | 0.255 |
| N | 0.3046 | 0.160 | 0.172 | 0.201 | 0.225 | 0.239 | |
| P/N | 5.435 | 10.131 | 4.736 | 1.540 | 1.137 | 1.067 | |
| 1:80 | P | 0.971 | 0.739 | 0.446 | 0.322 | 0.267 | 0.296 |
| N | 0.314 | 0.167 | 0.179 | 0.223 | 0.198 | 0.259 | |
| P/N | 3.089 | 4.414 | 2.500 | 1.448 | 1.348 | 1.143 | |
| Sample No. | Intra-assay CV (%) | Inter-assay CV (%) | |||
|---|---|---|---|---|---|
| X ± SD | CV% | X ± SD | CV% | ||
| Positive samples | 1 | 1.687 ± 0.049 | 2.88 | 1.680 ± 0.063 | 3.74 |
| 2 | 1.645 ± 0.021 | 1.28 | 1.534 ± 0.049 | 3.22 | |
| 3 | 1.667 ± 0.052 | 3.15 | 1.668 ± 0.105 | 6.32 | |
| 4 | 1.682 ± 0.057 | 3.37 | 1.753 ± 0.115 | 6.54 | |
| 5 | 1.893 ± 0.033 | 1.76 | 2.028 ± 0.193 | 9.50 | |
| Negative samples | 6 | 0.099 ± 0.002 | 1.62 | 0.086 ± 0.002 | 2.70 |
| 7 | 0.087 ± 0.003 | 3.73 | 0.070 ± 0.005 | 7.38 | |
| 8 | 0.090 ± 0.002 | 2.74 | 0.086 ± 0.008 | 9.51 | |
| 9 | 0.079 ± 0.003 | 3.63 | 0.077 ± 0.002 | 2.38 | |
| 10 | 0.085 ± 0.003 | 3.85 | 0.084 ± 0.004 | 5.10 | |
| No. of Clinical Samples | DAgS-ELISA | Commercial Kits | ||
|---|---|---|---|---|
| No. of Positive | Positive Rate (%) | No. of Positive | Positive Rate (%) | |
| 120 | 26 | 21.7% | 21 | 17.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Song, J.; Sun, J.; Du, Y.; Qin, X.; Xia, L.; Wu, Y.; Zhang, G. Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus. Viruses 2022, 14, 2170. https://doi.org/10.3390/v14102170
Wang M, Song J, Sun J, Du Y, Qin X, Xia L, Wu Y, Zhang G. Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus. Viruses. 2022; 14(10):2170. https://doi.org/10.3390/v14102170
Chicago/Turabian StyleWang, Mengxiang, Jinxing Song, Junru Sun, Yongkun Du, Xiaodong Qin, Lu Xia, Yanan Wu, and Gaiping Zhang. 2022. "Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus" Viruses 14, no. 10: 2170. https://doi.org/10.3390/v14102170
APA StyleWang, M., Song, J., Sun, J., Du, Y., Qin, X., Xia, L., Wu, Y., & Zhang, G. (2022). Development of an Effective Double Antigen Sandwich ELISA Based on p30 Protein to Detect Antibodies against African Swine Fever Virus. Viruses, 14(10), 2170. https://doi.org/10.3390/v14102170






