SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Antibody Measurements
2.3. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
3.2. Seropositivity by Luminex
3.3. Factors Affecting Antibody Responses
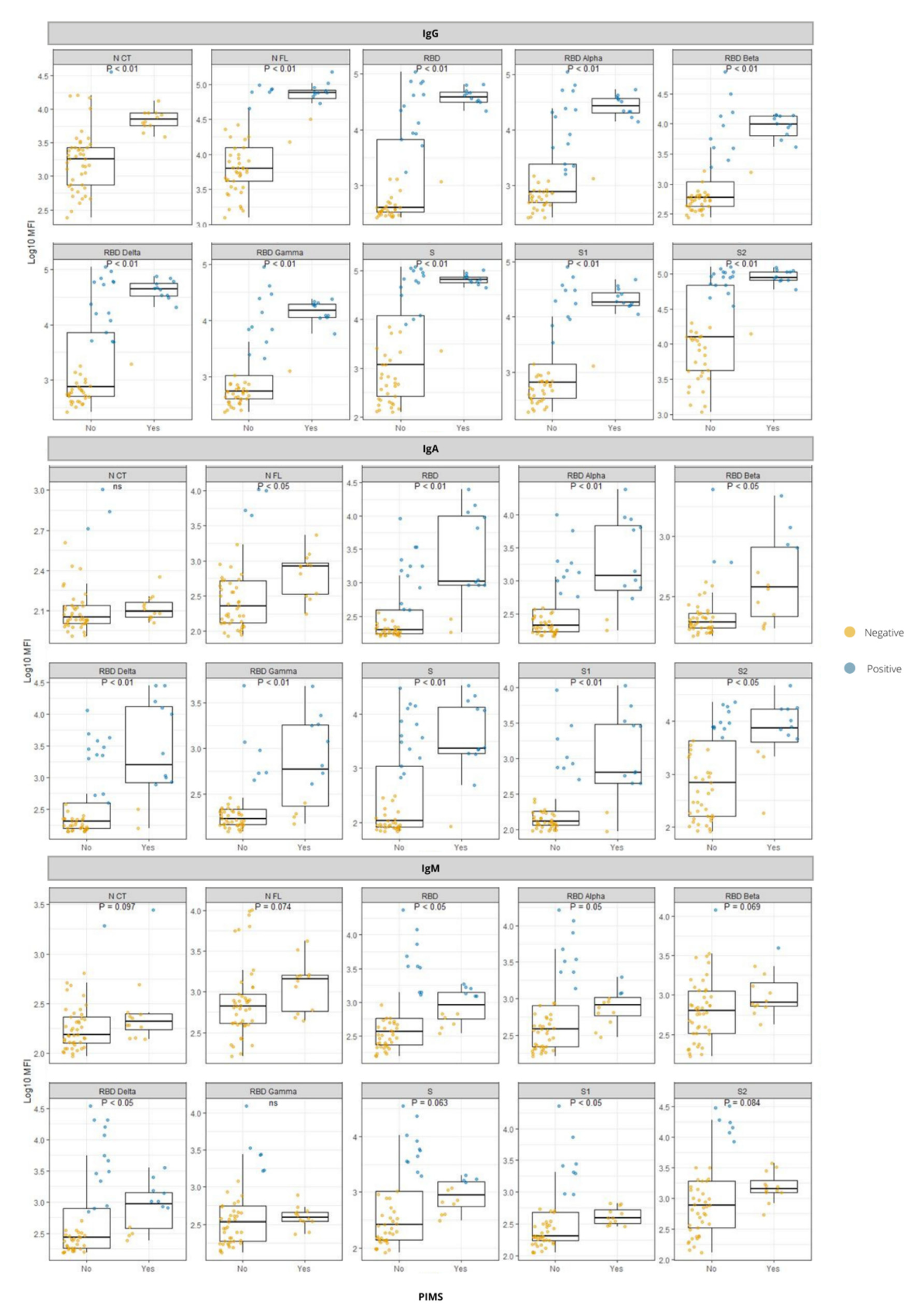
3.4. Antibody Responses in Relation to PIMS-TS
3.5. Comparison between ELISA and Luminex Serology
3.6. Antibody Responses to RBD from VoCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The Emergence of COVID-19 as a Global Pandemic: Understanding the Epidemiology, Immune Response and Potential Therapeutic Targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Lee, E.; Oh, J.E. Humoral Immunity against SARS-CoV-2 and the Impact on COVID-19 Pathogenesis. Mol. Cells 2021, 44, 392–400. [Google Scholar] [CrossRef]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic Changes in Anti-SARS-CoV-2 Antibodies during SARS-CoV-2 Infection and Recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef] [PubMed]
- Altawalah, H. Antibody Responses to Natural SARS-CoV-2 Infection or after COVID-19 Vaccination. Vaccines 2021, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Vidal, M.; Santano, R.; Jiménez, A.; Chi, J.; Barrios, D.; Ruiz-Olalla, G.; Rodrigo Melero, N.; Carolis, C.; Parras, D.; et al. Highly Sensitive and Specific Multiplex Antibody Assays To Quantify Immunoglobulins M, A, and G against SARS-CoV-2 Antigens. J. Clin. Microbiol. 2021, 59, e01731-20. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 12 May 2022).
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune Response to SARS-CoV-2 Variants of Concern in Vaccinated Individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased Transmissibility and Global Spread of SARSCoV- 2 Variants of Concern as at June 2021. Eurosurveillance 2021, 26, 2100509. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The Biological and Clinical Significance of Emerging SARS-CoV-2 Variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among Front-Line Health-Care Workers and the General Community: A Prospective Cohort Study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 Antibodies and Associated Factors in Healthcare Workers: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.A.; Hassan Khamis, A.; Ho, S.B. COVID-19 and Healthcare Workers: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Mehta, N.S.; Mytton, O.T.; Mullins, E.W.S.; Fowler, T.A.; Falconer, C.L.; Murphy, O.B.; Langenberg, C.; Jayatunga, W.J.P.; Eddy, D.H.; Nguyen-Van-Tam, J.S. SARS-CoV-2 (COVID-19): What Do We Know about Children? A Systematic Review. Clin. Infect. Dis. 2020, 71, 2469–2479. [Google Scholar] [CrossRef]
- Dobaño, C.; Alonso, S.; Vidal, M.; Jiménez, A.; Rubio, R.; Santano, R.; Barrios, D.; Pons Tomas, G.; Melé Casas, M.; Hernández García, M.; et al. Multiplex Antibody Analysis of IgM, IgA and IgG to SARS-CoV-2 in Saliva and Serum From Infected Children and Their Close Contacts. Front. Immunol. 2022, 13, 751705. [Google Scholar] [CrossRef]
- Dobaño, C.; Alonso, S.; Fernández de Sevilla, M.; Vidal, M.; Jiménez, A.; Pons Tomas, G.; Jairoce, C.; Melé Casas, M.; Rubio, R.; Hernández García, M.; et al. Antibody Conversion Rates to SARS-CoV-2 in Saliva from Children Attending Summer Schools in Barcelona, Spain. BMC Med. 2021, 19, 309. [Google Scholar] [CrossRef]
- Harwood, R.; Allin, B.; Jones, C.E.; Whittaker, E.; Ramnarayan, P.; Ramanan, A.V.; Kaleem, M.; Tulloh, R.; Peters, M.J.; Almond, S.; et al. A National Consensus Management Pathway for Paediatric Inflammatory Multisystem Syndrome Temporally Associated with COVID-19 (PIMS-TS): Results of a National Delphi Process. Lancet Child Adolesc. Health 2021, 5, 133–141. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of Thrombosis in Children and Adolescents Hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef]
- Gottlieb, M.; Bridwell, R.; Ravera, J.; Long, B. Multisystem Inflammatory Syndrome in Children with COVID-19. Am. J. Emerg. Med. 2021, 49, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Porritt, R.A.; Binek, A.; Paschold, L.; Rivas, M.N.; McArdle, A.; Yonker, L.M.; Alter, G.; Chandnani, H.K.; Lopez, M.; Fasano, A.; et al. The Autoimmune Signature of Hyperinflammatory Multisystem Inflammatory Syndrome in Children. J. Clin. Investig. 2021, 131, e151520. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Actualización de La Situación Epidemiológica de Las Variantes de SARS-CoV-2 En España. Centro de Coordinación de Alertas y Emergencias Sanitarias. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20211213.pdf (accessed on 22 May 2022).
- Ortega, N.; Ribes, M.; Vidal, M.; Rubio, R.; Aguilar, R.; Williams, S.; Barrios, D.; Alonso, S.; Hernández-Luis, P.; Mitchell, R.A.; et al. Seven-Month Kinetics of SARS-CoV-2 Antibodies and Role of Pre-Existing Antibodies to Human Coronaviruses. Nat. Commun. 2021, 12, 4740. [Google Scholar] [CrossRef] [PubMed]
- Moncunill, G.; Mayor, A.; Santano, R.; Jiménez, A.; Vidal, M.; Tortajada, M.; Sanz, S.; Méndez, S.; Llupià, A.; Aguilar, R.; et al. SARS-CoV-2 Seroprevalence and Antibody Kinetics among Health Care Workers in a Spanish Hospital after 3 Months of Follow-Up. J. Infect. Dis. 2021, 223, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)-A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Varona, J.F.; Madurga, R.; Peñalver, F.; Abarca, E.; Almirall, C.; Cruz, M.; Ramos, E.; Castellano Vázquez, J.M. Seroprevalence of SARS-CoV-2 Antibodies in over 6000 Healthcare Workers in Spain. Int. J. Epidemiol. 2021, 50, 400–409. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A Nationwide, Population-Based Seroepidemiological Study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Vogelzang, E.H.; Loeff, F.C.; Derksen, N.I.L.; Kruithof, S.; Ooijevaar-de Heer, P.; van Mierlo, G.; Linty, F.; Mok, J.Y.; van Esch, W.; de Bruin, S.; et al. Development of a SARS-CoV-2 Total Antibody Assay and the Dynamics of Antibody Response over Time in Hospitalized and Nonhospitalized Patients with COVID-19. J. Immunol. 2020, 205, 3491–3499. [Google Scholar] [CrossRef]
- Dufloo, J.; Grzelak, L.; Staropoli, I.; Madec, Y.; Tondeur, L.; Anna, F.; Pelleau, S.; Wiedemann, A.; Planchais, C.; Buchrieser, J.; et al. Asymptomatic and Symptomatic SARS-CoV-2 Infections Elicit Polyfunctional Antibodies. Cell Rep. Med. 2021, 2, 100275. [Google Scholar] [CrossRef]
- Maine, G.N.; Lao, K.M.; Krishnan, S.M.; Afolayan-Oloye, O.; Fatemi, S.; Kumar, S.; VanHorn, L.; Hurand, A.; Sykes, E.; Sun, Q. Longitudinal Characterization of the IgM and IgG Humoral Response in Symptomatic COVID-19 Patients Using the Abbott Architect. J. Clin. Virol. 2020, 133, 104663. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody Seroconversion in Asymptomatic and Symptomatic Patients Infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Transl. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A Comparison Study of SARS-CoV-2 IgG Antibody between Male and Female COVID-19 Patients: A Possible Reason Underlying Different Outcome between Sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef]
- Arnold, C.G.; Libby, A.; Vest, A.; Hopkinson, A.; Monte, A.A. Immune Mechanisms Associated with Sex-Based Differences in Severe COVID-19 Clinical Outcomes. Biol. Sex Differ. 2022, 13, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Rostad, C.A.; Chahroudi, A.; Mantus, G.; Lapp, S.A.; Teherani, M.; Macoy, L.; Tarquinio, K.M.; Basu, R.K.; Kao, C.; Linam, W.M.; et al. Quantitative SARS-CoV-2 Serology in Children With Multisystem Inflammatory Syndrome (MIS-C). Pediatrics 2020, 146, e2020018242. [Google Scholar] [CrossRef] [PubMed]
- Lapp, S.A.; Abrams, J.; Lu, A.T.; Hussaini, L.; Kao, C.M.; Hunstad, D.A.; Rosenberg, R.B.; Zafferani, M.J.; Ede, K.C.; Ballan, W.; et al. Serologic and Cytokine Signatures in Children with Multisystem Inflammatory Syndrome and Coronavirus Disease 2019. Open Forum Infect. Dis. 2022, 9, ofac070. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Henderson, L.A.; Yeung, R.S.M. MIS-C: Early Lessons from Immune Profiling. Nat. Rev. Rheumatol. 2021, 17, 75–76. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (Mis-c) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Perez-Gomez, R. The Development of SARS-CoV-2 Variants: The Gene Makes the Disease. J. Dev. Biol. 2021, 9, 58. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St. Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. SARS-CoV-2 Variants of Concern. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 25 May 2022).
- Deshpande, A.; Harris, B.D.; Martinez-Sobrido, L.; Kobie, J.J.; Walter, M.R. Epitope Classification and RBD Binding Properties of Neutralizing Antibodies Against SARS-CoV-2 Variants of Concern. Front. Immunol. 2021, 12, 691715. [Google Scholar] [CrossRef] [PubMed]
- Mengist, H.M.; Kombe Kombe, A.J.; Mekonnen, D.; Abebaw, A.; Getachew, M.; Jin, T. Mutations of SARS-CoV-2 Spike Protein: Implications on Immune Evasion and Vaccine-Induced Immunity. Semin. Immunol. 2021, 55, 101533. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shayestehpour, M.; Mirzaei, H. The Impact of Spike Mutated Variants of SARS-CoV2 [Alpha, Beta, Gamma, Delta, and Lambda] on the Efficacy of Subunit Recombinant Vaccines. Braz. J. Infect. Dis. 2021, 25, 101606. [Google Scholar] [CrossRef]
- Boehm, E.; Kronig, I.; Neher, R.A.; Eckerle, I.; Vetter, P.; Kaiser, L. Novel SARS-CoV-2 Variants: The Pandemics within the Pandemic. Clin. Microbiol. Infect. 2021, 27, 1109–1117. [Google Scholar] [CrossRef]
- Tatsi, E.B.; Filippatos, F.; Michos, A. SARS-CoV-2 Variants and Effectiveness of Vaccines: A Review of Current Evidence. Epidemiol. Infect. 2021, 149, 536–544. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, C.; Hong, W.; Zhang, K.; Wei, X. The Challenges of COVID-19 Delta Variant: Prevention and Vaccine Development. MedComm 2021, 2, 846–854. [Google Scholar] [CrossRef]
- Santano, R.; Barrios, D.; Crispi, F.; Crovetto, F.; Vidal, M.; Chi, J.; Izquierdo, L.; Gratacós, E.; Moncunill, G.; Dobaño, C. Agreement between Commercially Available ELISA and In-House Luminex SARS-CoV-2 Antibody Immunoassays. Sci. Rep. 2021, 11, 18984. [Google Scholar] [CrossRef]


| Children | Adults | ||
|---|---|---|---|
| Timepoint | 0 (baseline) | 0 | 1 |
| N | 57 | 190 | 172 |
| Median age, years (IQR) | 10 (3.25–12.75) | 44 (37–54) | NA |
| Sex (M/F) | 1.04 | 1.06 | 0.81 |
| Symptoms *, n (%) | 41 (72) | 35 † (29) | NA |
| Median days from symptoms onset to serological study (IQR) | 9 (6–11) | NS | NA |
| Positive SARS-CoV-2 RT-PCR, n (%) | 23 (43) § | 1 (1) ‡ | NA |
| Hospitalized, n (%) | 33 (58) | NA | NA |
| PIMS, n (%) | 12 (21) | NA | NA |
| Time periods of recruitment ǂ | |||
| 1. n (%) | 18 (32) | 190 (100) | 172 (100) |
| 2. n (%) | 24 (42) | ||
| 3. n (%) | 15 (26) | ||
| Children (n = 57) | Adults Timepoint 0 (n = 190) | Adults Timepoint 1 (n = 172) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Undeter-mined | Positive + Undeter. | Positive | Undeter-mined | Positive + Undeter. | Positive | Undeter-mined | Positive + Undeter. | |||||||
| n | % | n | % | % | n | % | n | % | % | n | % | n | % | % | |
| Overall | |||||||||||||||
| 31 | 54.39 | 7 | 12.28 | 66.67 | 37 | 19.37 | 28 | 14.66 | 34.03 | 32 | 18.60 | 20 | 11.63 | 30.23 | |
| By isotype | |||||||||||||||
| IgG | 28 | 49.12 | 3 | 5.26 | 54.39 | 21 | 10.99 | 11 | 5.76 | 16.75 | 18 | 9.94 | 5 | 2.76 | 12.71 |
| IgA | 26 | 45.61 | 5 | 8.77 | 54.39 | 11 | 5.76 | 15 | 7.85 | 13.61 | 8 | 4.42 | 12 | 6.63 | 11.05 |
| IgM | 20 | 35.09 | 4 | 7.02 | 42.11 | 14 | 7.33 | 14 | 7.33 | 14.66 | 7 | 3.87 | 12 | 6.63 | 10.5 |
| By isotype-antigen combination | |||||||||||||||
| IgG N CT | 0 | 0 | 1 | 1.75 | 1.75 | 0 | 0 | 2 | 1.05 | 1.05 | 0 | 0 | 1 | 0.55 | 0.55 |
| IgG N FL | 3 | 5.26 | 13 | 22.81 | 28.07 | 2 | 1.05 | 8 | 4.19 | 5.24 | 2 | 1.1 | 4 | 2.21 | 3.31 |
| IgG RBD Wuhan | 24 | 42.11 | 1 | 1.75 | 43.86 | 9 | 4.71 | 4 | 2.09 | 6.81 | 8 | 4.42 | 1 | 0.55 | 4.97 |
| IgG S | 22 | 38.6 | 3 | 5.26 | 43.86 | 4 | 2.09 | 4 | 2.09 | 4.19 | 3 | 1.66 | 4 | 2.21 | 3.87 |
| IgG S1 | 21 | 36.84 | 1 | 1.75 | 38.6 | 6 | 3.14 | 3 | 1.57 | 4.71 | 5 | 2.76 | 3 | 1.66 | 4.42 |
| IgG S2 | 19 | 33.33 | 8 | 14.04 | 47.37 | 2 | 1.05 | 7 | 3.66 | 4.71 | 4 | 2.21 | 1 | 0.55 | 2.76 |
| IgA N CT | 2 | 3.51 | 1 | 1.75 | 5.26 | 3 | 1.57 | 2 | 1.05 | 2.62 | 0 | 0 | 3 | 1.66 | 1.66 |
| IgA N FL | 2 | 3.51 | 2 | 3.51 | 7.02 | 2 | 1.05 | 4 | 2.09 | 3.14 | 0 | 0 | 2 | 1.1 | 1.1 |
| IgA RBD Wuhan | 19 | 33.33 | 3 | 5.26 | 38.6 | 2 | 1.05 | 1 | 0.52 | 1.57 | 2 | 1.1 | 1 | 0.55 | 1.66 |
| IgA S | 24 | 42.11 | 1 | 1.75 | 43.86 | 4 | 2.09 | 6 | 3.14 | 5.24 | 4 | 2.21 | 3 | 1.66 | 3.87 |
| IgA S1 | 15 | 26.32 | 3 | 5.26 | 31.58 | 3 | 1.57 | 0 | 0 | 1.57 | 0 | 0 | 3 | 1.66 | 1.66 |
| IgA S2 | 7 | 12.28 | 13 | 22.81 | 35.09 | 1 | 0.52 | 6 | 3.14 | 3.66 | 0 | 0 | 5 | 2.76 | 2.76 |
| IgM N CT | 0 | 0 | 2 | 3.51 | 3.51 | 0 | 0 | 4 | 2.09 | 2.09 | 0 | 0 | 2 | 1.1 | 1.1 |
| IgM N FL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.52 | 0.52 | 0 | 0 | 0 | 0 | 0 |
| IgM RBD Wuhan | 7 | 12.28 | 9 | 15.79 | 28.07 | 2 | 1.05 | 7 | 3.66 | 4.71 | 0 | 0 | 6 | 3.31 | 3.31 |
| IgM S | 9 | 15.79 | 6 | 10.53 | 26.32 | 1 | 0.52 | 6 | 3.14 | 3.66 | 0 | 0 | 4 | 2.21 | 2.21 |
| IgM S1 | 6 | 10.53 | 2 | 3.51 | 14.04 | 0 | 0 | 3 | 1.57 | 1.57 | 0 | 0 | 1 | 0.55 | 0.55 |
| IgM S2 | 2 | 3.51 | 5 | 8.77 | 12.28 | 2 | 3.51 | 5 | 8.77 | 12.28 | 0 | 0 | 2 | 1.1 | 1.1 |
| Children (n = 57) | Adults Timepoint 0 (n = 190) | Adults Timepoint 1 (n = 172) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Undeter-mined | Positive + Undeter. | p value * | Positive | Undeter-mined | Positive + Undeter. | p value * | Positive | Undeter-mined | Positive + Undeter | p value * | |||||||
| n | % | n | % | % | n | % | n | % | % | n | % | n | % | % | ||||
| IgG RBD | 0.89 | 0.35 | 0.35 | |||||||||||||||
| IgG RBD Wuhan | 24 | 42.11 | 1 | 1.75 | 43.86 | 9 | 4.71 | 4 | 2.09 | 6.81 | 8 | 4.42 | 1 | 0.55 | 4.97 | |||
| IgG RBD Alpha | 22 | 38.6 | 4 | 7.02 | 45.61 | 8 | 4.19 | 8 | 4.19 | 8.38 | 8 | 4.42 | 4 | 2.21 | 6.63 | |||
| IgG RBD Beta | 19 | 33.33 | 2 | 3.51 | 36.84 | 3 | 1.57 | 4 | 2.09 | 3.66 | 4 | 2.21 | 1 | 0.55 | 2.76 | |||
| IgG RBD Gamma | 21 | 36.84 | 1 | 1.75 | 38.6 | 4 | 2.09 | 5 | 2.62 | 4.71 | 3 | 1.66 | 4 | 2.21 | 3.87 | |||
| IgG RBD Delta | 23 | 40.35 | 3 | 5.26 | 45.61 | 6 | 3.14 | 6 | 3.14 | 6.28 | 4 | 2.21 | 6 | 3.31 | 5.52 | |||
| IgA RBD | 0.0001 | NA | NA | |||||||||||||||
| IgA RBD Wuhan | 19 | 33.33 | 3 | 5.26 | 38.6 | 2 | 1.05 | 1 | 0.52 | 1.57 | 2 | 1.1 | 1 | 0.55 | 1.66 | |||
| IgA RBD Alpha | 19 | 33.33 | 1 | 1.75 | 35.09 | 3 | 1.57 | 2 | 1.05 | 2.62 | 2 | 1.1 | 1 | 0.55 | 1.66 | |||
| IgA RBD Beta | 3 | 5.26 | 4 | 7.02 | 12.28 | 0 | 0 | 3 | 1.57 | 1.57 | 0 | 0 | 4 | 2.21 | 2.21 | |||
| IgA RBD Gamma | 9 | 15.79 | 5 | 8.77 | 24.56 | 1 | 0.52 | 1 | 0.52 | 1.05 | 0 | 0 | 2 | 1.1 | 1.1 | |||
| IgA RBD Delta | 21 | 36.84 | 1 | 1.75 | 38.6 | 2 | 1.05 | 2 | 1.05 | 2.09 | 2 | 1.1 | 3 | 1.66 | 2.76 | |||
| IgM RBD | 0.0003 | NA | NA | |||||||||||||||
| IgM RBD Wuhan | 7 | 12.28 | 9 | 15.79 | 28.07 | 2 | 1.05 | 7 | 3.66 | 4.71 | 0 | 0 | 6 | 3.31 | 3.31 | |||
| IgM RBD Alpha | 8 | 14.04 | 4 | 7.02 | 21.05 | 3 | 1.57 | 4 | 2.09 | 3.66 | 2 | 1.1 | 1 | 0.55 | 1.66 | |||
| IgM RBD Beta | 1 | 1.75 | 1 | 1.75 | 3.51 | 0 | 0 | 2 | 1.05 | 1.05 | 0 | 0 | 0 | 0 | 0 | |||
| IgM RBD Gamma | 1 | 1.75 | 5 | 8.77 | 10.53 | 1 | 0.52 | 1 | 0.52 | 1.05 | 1 | 0.55 | 0 | 0 | 0.55 | |||
| IgM RBD Delta | 14 | 24.56 | 7 | 12.28 | 36.84 | 3 | 1.57 | 10 | 5.24 | 6.81 | 1 | 0.55 | 6 | 3.31 | 3.87 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prados de la Torre, E.; Obando, I.; Vidal, M.; de Felipe, B.; Aguilar, R.; Izquierdo, L.; Carolis, C.; Olbrich, P.; Capilla-Miranda, A.; Serra, P.; et al. SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays. Viruses 2022, 14, 2039. https://doi.org/10.3390/v14092039
Prados de la Torre E, Obando I, Vidal M, de Felipe B, Aguilar R, Izquierdo L, Carolis C, Olbrich P, Capilla-Miranda A, Serra P, et al. SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays. Viruses. 2022; 14(9):2039. https://doi.org/10.3390/v14092039
Chicago/Turabian StylePrados de la Torre, Esther, Ignacio Obando, Marta Vidal, Beatriz de Felipe, Ruth Aguilar, Luis Izquierdo, Carlo Carolis, Peter Olbrich, Ana Capilla-Miranda, Pau Serra, and et al. 2022. "SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays" Viruses 14, no. 9: 2039. https://doi.org/10.3390/v14092039
APA StylePrados de la Torre, E., Obando, I., Vidal, M., de Felipe, B., Aguilar, R., Izquierdo, L., Carolis, C., Olbrich, P., Capilla-Miranda, A., Serra, P., Santamaria, P., Blanco-Lobo, P., Moncunill, G., Rodríguez-Ortega, M. J., & Dobaño, C. (2022). SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays. Viruses, 14(9), 2039. https://doi.org/10.3390/v14092039







