Epidemiological and Genomic Characterisation of Middelburg and Sindbis Alphaviruses Identified in Horses with Febrile and Neurological Infections, South Africa (2014–2018)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Specimens
2.3. Viral RNA Extraction, Detection, and Sanger Sequencing
2.4. RNA Replication for MIDV and SINV Positive Follow-Up Specimens
2.5. Virus Isolation and Sequence-Independent Single-Primer Amplification (SISPA) with Rapid Amplification of cDNA Ends (RACE) for Full Genome Sequencing
2.6. Illumina Full Genome Sequencing
2.7. Phylogenetic Analysis
2.8. Statistical Analysis
2.9. Recombination Analysis
3. Results
3.1. MIDV and SINV Detection and Clinical Disease Description
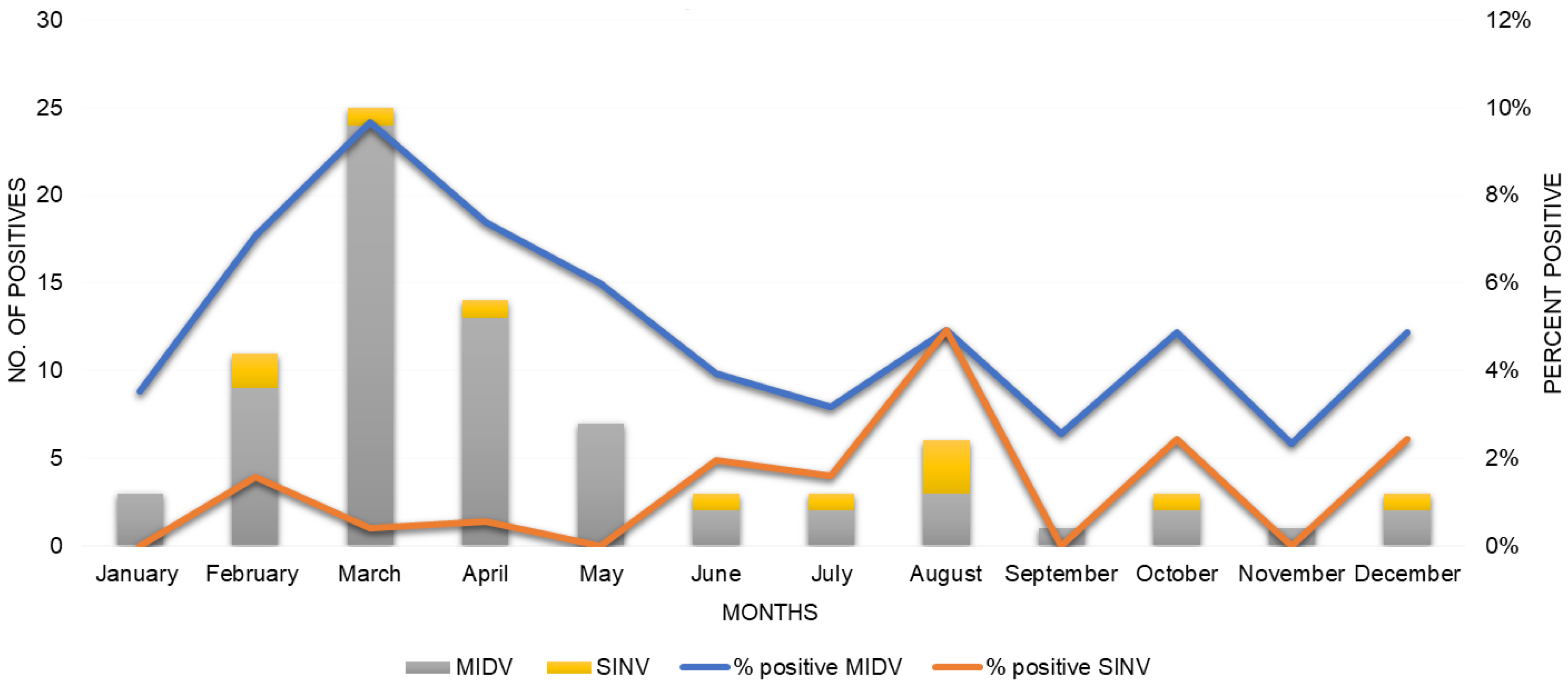
3.2. Seasonality of Alphaviruses in South Africa
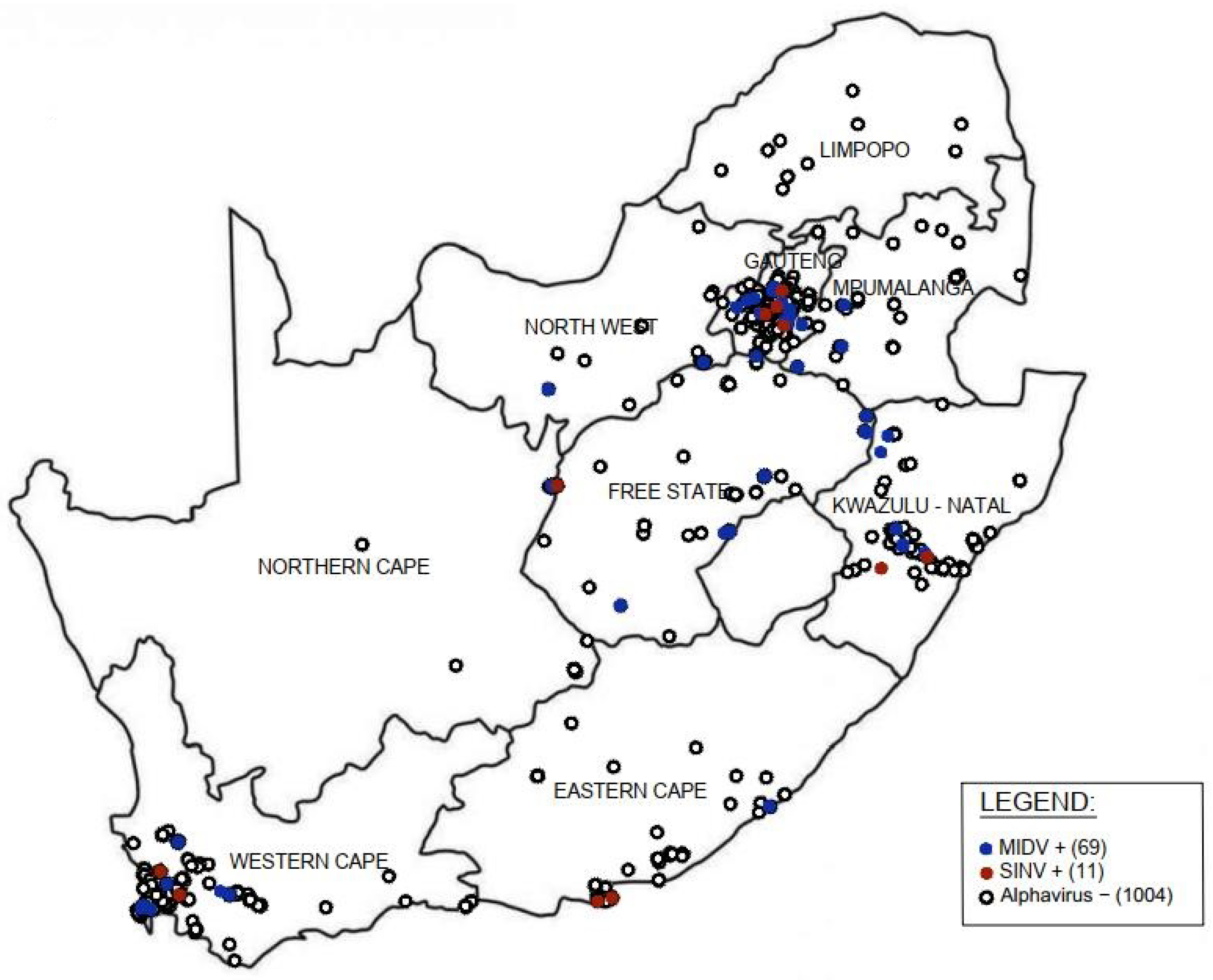
3.3. Geographic Distribution
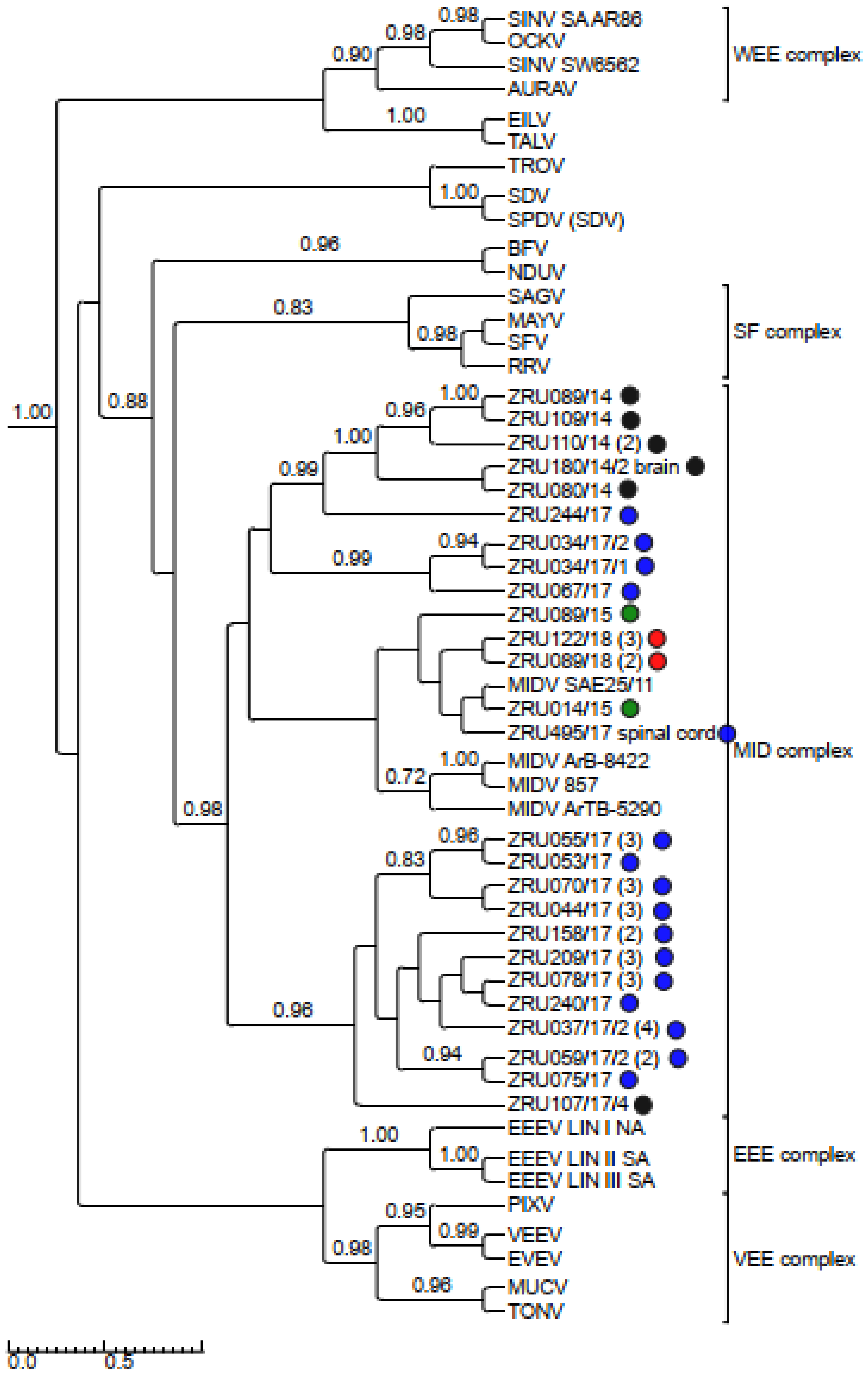
3.4. Detection and Phylogenetic Analysis of Partial Sequences
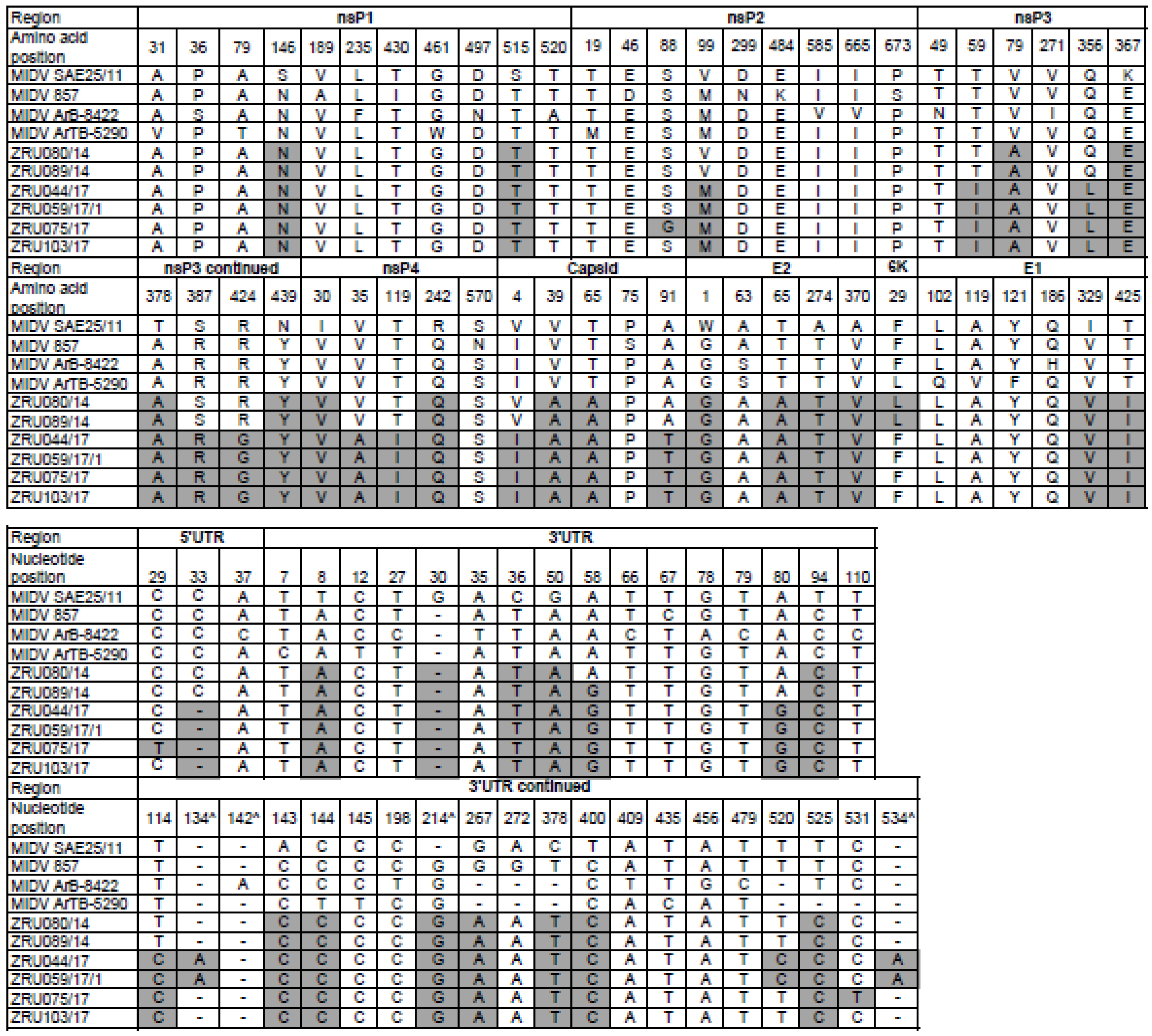
3.5. Virus Isolations for Full Genome Sequence Analysis
3.6. Recombination Event Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrera, J.-P.; Forrester, N.; Wang, E.; Vittor, A.Y.; Haddow, A.D.; López-Vergès, S.; Abadía, I.; Castaño, E.; Sosa, N.; Báez, C.; et al. Eastern equine encephalitis in Latin America. N. Engl. J. Med. 2013, 369, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An emerging viral threat? Emerg. Microbes. Infect. 2018, 7, 163. [Google Scholar] [CrossRef]
- Lundstrom, J.O.; Turell, M.J.; Niklasson, B. Viremia in three orders of brids (Anseriforms, Galliformes and Passeriformes) inoculated with Ockelbo virus. J. Wildl. Dis. 1993, 29, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen-epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Hurlbut, H.S.; Work, T.H.; Kingston, J.R.; Frothingham, T.E. Sindbis Virus: A Newly Recognized Arthropod-Transmitted Virus1. Am. J. Trop. Med. Hyg. 1955, 4, 844–862. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, B.M.; Jupp, P.G.; Dos Santos, I.; Meenehan, G.M. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. South Afr. J. Sci. 1976, 72, 295–300. [Google Scholar]
- Storm, N.; Weyer, J.; Markotter, W.; Kemp, A.; Leman, P.A.; Dermaux-Msimang, V.; Nel, L.H.; Paweska, J.T. Human cases of Sindbis fever in South Africa, 2006-2010. Epidemiol. Infect. 2014, 142, 234–238. [Google Scholar] [CrossRef]
- Division of Public Health Surveillance and Response; Centre for Emerging ZaPD, NICD-NHLS. Cluster of Sindbis virus infections in Gauteng Province: An update. In Division of Public Health Surveillance and Response; Centre for Emerging ZaPD, NICD-NHLS, Ed.; NICD Division of Public Health Surveillance and Response: Johannesburg, South Africa, 2017; p. 1. [Google Scholar]
- Kokernot, R.H.; De Meillon, B.; Paterson, H.E.; Heymann, C.S.; Smithburn, K.C. Middelburg virus; a hitherto unknown agent isolated from Aedes mosquitoes during an epizootic in sheep in the eastern Cape Province. S. Afr. J. Med. Sci. 1957, 22, 145–153. [Google Scholar]
- McIntosh, B.M. The Epidemiology of Arthropod-Borne Viruses in Southern Africa Pretoria (SA). Doctoral Dissertation, University of Pretoria, Hatfield, South Africa, 1980. [Google Scholar]
- Human, S.; Steyl, J.; Williams, J.; Last, R.; van Niekerk, S.; Venter, M. Sindbis and Middelburg Viruses as a Cause of Disease in Animals in South Africa: The Molecular Epidemiology. In Proceedings of the 9th Annual Congress of the Southern African Society for Veterinary Epidemiology and Preventive Medicine, Farm Inn, Pretoria, South Africa, 18–20 August 2010. [Google Scholar]
- Attoui, H.; Sailleau, C.; Mohd Jaafar, F.; Belhouchet, M.; Biagini, P.; Cantaloube, J.F.; de Micco, P.; Mertens, P.; Zientara, S. Complete nucleotide sequence of Middelburg virus, isolated from the spleen of a horse with severe clinical disease in Zimbabwe. J. Gen. Virol. 2007, 88 Pt 11, 3078–3088. [Google Scholar] [CrossRef]
- van Niekerk, S.; Human, S.; Williams, J.; van Wilpe, E.; Pretorius, M.; Swanepoel, R.; Venter, M. Sindbis and Middelburg Old World Alphaviruses Associated with Neurologic Disease in Horses, South Africa. Emerg. Infect. Dis. 2015, 21, 2225–2229. [Google Scholar] [CrossRef]
- Steyn, J.; Fourie, I.; Steyl, J.; Williams, J.; Stivaktas, V.; Botha, E.; van Niekerk, S.; Reininghaus, B.; Venter, M. Zoonotic Alphaviruses in Fatal and Neurologic Infections in Wildlife and Nonequine Domestic Animals, South Africa. Emerg. Infect. Dis. 2020, 26, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Seco, M.P.; Rosario, D.; Quiroz, E.; Guzmán, G.; Tenorio, A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J. Virol. Methods 2001, 95, 153–161. [Google Scholar] [CrossRef]
- Zaayman, D.; Human, S.; Venter, M. A highly sensitive method for the detection and genotyping of West Nile virus by real-time PCR. J. Virol. Methods 2009, 157, 155–160. [Google Scholar] [CrossRef]
- van Eeden, C.; Harders, F.; Kortekaas, J.; Bossers, A.; Venter, M. Genomic and phylogenetic characterization of Shuni virus. Arch Virol. 2014, 159, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Storm, N.; Weyer, J.; Markotter, W.; Leman, P.A.; Kemp, A.; Nel, L.H.; Paweska, J.T. Phylogeny of Sindbis virus isolates from South Africa. South. Afr. J. Infect. Dis. 2013, 28, 207–214. [Google Scholar] [CrossRef]
- Jansen van Vuren, P.; Wiley, M.; Palacios, G.; Storm, N.; McCulloch, S.; Markotter, W.; Birkhead, M.; Kemp, A.; Paweska, J.T. Isolation of a Novel Fusogenic Orthoreovirus from Eucampsipoda africana Bat Flies in South Africa. Viruses 2016, 8, 65. [Google Scholar] [CrossRef]
- Forrester, N.L.; Palacios, G.; Tesh, R.B.; Savji, N.; Guzman, H.; Sherman, M.; Weaver, S.C.; Lipkin, W.I. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J. Virol. 2012, 86, 2729–2738. [Google Scholar] [CrossRef]
- Nasar, F.; Palacios, G.; Gorchakov, R.V.; Guzman, H.; Da Rosa, A.P.; Savji, N.; Popov, V.L.; Sherman, M.B.; Lipkin, W.I.; Tesh, R.B.; et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc. Natl. Acad. Sci. USA 2012, 109, 14622–14627. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. (Eds.) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info™, a Database and Statistics Program for Public Health Professionals. CDC: Atlanta, GA, USA, 2011. Available online: https://www.cdc.gov/epiinfo (accessed on 20 November 2019).
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rybicki, E. RDP: Detection of recombination amongst aligned sequences. Bioinformatics 2000, 16, 562–563. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Sawyer, S.; Fauquet, C.M. Possible emergence of new geminiviruses by frequent recombination. Virology 1999, 265, 218–225. [Google Scholar] [CrossRef]
- Posada, D. Evaluation of methods for detecting recombination from DNA sequences: Empirical data. Mol. Biol. Evol. 2002, 19, 708–717. [Google Scholar] [CrossRef]
- Gibbs, M.J.; Armstrong, J.S.; Gibbs, A.J. Sister-scanning: A Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 2000, 16, 573–582. [Google Scholar] [CrossRef]
- Martin, D.P.; Posada, D.; Crandall, K.A.; Williamson, C. A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res. Hum. Retrovir. 2005, 21, 98–102. [Google Scholar] [CrossRef]
- Smith, J.M. Analyzing the mosaic structure of genes. J. Mol. Evol. 1992, 34, 126–129. [Google Scholar] [CrossRef]
- Lam, H.M.; Ratmann, O.; Boni, M.F. Improved Algorithmic Complexity for the 3SEQ Recombination Detection Algorithm. Mol. Biol. Evol. 2018, 35, 247–251. [Google Scholar] [CrossRef]
- Tricou, V.; Berthet, N.; Descorps-Declere, S.; Nakoune, E.; Kazanji, M. Complete genome sequences of two middelburg viruses isolated from arthropods in the central african republic. Genome Announc. 2014, 2, e01078-14. [Google Scholar] [CrossRef] [PubMed]
- Venter, M. Assessing the zoonotic potential of arboviruses of African origin. Curr. Opin. Virol. 2018, 28, 74–84. [Google Scholar] [CrossRef]
- Fourie, I.; Williams, J.; Ismail, A.; Jansen van Vuren, P.; Stoltz, A.; Venter, M. Detection and genome characterization of Middelburg virus strains isolated from CSF and whole blood samples of humans with neurological manifestations in South Africa. PLoS Negl. Trop. Dis. 2022, 16, e0010020. [Google Scholar] [CrossRef]
- Sane, J.; Kurkela, S.; Levanov, L.; Nikkari, S.; Vaheri, A.; Vapalahti, O. Development and evaluation of a real-time RT-PCR assay for Sindbis virus detection. J. Virol. Methods 2012, 179, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.C.; Shivas, M.A.; May, F.J.; Pyke, A.T.; Onn, M.B.; Lodo, K.; Hall-Mendelin, S.; McMahon, J.L.; Montgomery, B.L.; Darbro, J.M.; et al. Epidemiologic, Entomologic, and Virologic Factors of the 2014-15 Ross River Virus Outbreak, Queensland, Australia. Emerg. Infect. Dis. 2019, 25, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Weaver, S.C. Eastern Equine Encephalomyelitis Virus: Epidemiology and Evolution of Mosquito Transmission; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Advances in Virus Research; Academic Press: Cambridge, MA, USA, 1989; Volume 37, pp. 277–328. [Google Scholar]
- Jupp, P.G.; Blackburn, N.K.; Thompson, D.L.; Meenehan, G.M. Sindbis and West Nile virus infections in the Witwatersrand-Pretoria region. S. Afr. Med. J. 1986, 70, 218–220. [Google Scholar]
- Jupp, P.G.T.D.L.; Cornel, A.J. Isolations of Middelburg virus from Aedes (Ochlerotatus) juppi McIntosh (Diptera: Culicidae) suggestive of a reservoir vector. J. Entomol. Soc. South. Afr. 1987, 50, 393–397. [Google Scholar]
- Hopkins, B. Vegetation Map of Africa. The Vegetation of Africa: A Descriptive Memoir to Accompany the Unesco/AETFAT/UNSO Vegetation map of Africa. J. Ecol. 1987, 75, 1214–1216. [Google Scholar] [CrossRef]
- South African Weather Service Annual Report 2016/2017 [Press Release]; South African Weather Service: Centurion, South Africa, 2017.
- Lim, E.X.Y.; Lee, W.S.; Madzokere, E.T.; Herrero, L.J. Mosquitoes as Suitable Vectors for Alphaviruses. Viruses 2018, 10, 84. [Google Scholar] [CrossRef]
- Jose, J.; Snyder, J.E.; Kuhn, R.J. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 2009, 4, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-borne arboviruses of African origin: Review of key viruses and vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Gorchakov, R.; Frolova, E.; Frolov, I. Inhibition of transcription and translation in Sindbis virus-infected cells. J. Virol. 2005, 79, 9397–9409. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Sun, C.; Metthew Lam, L.K.; Gardner, C.L.; Ryman, K.D.; Klimstra, W.B. Host translation shutoff mediated by non-structural protein 2 is a critical factor in the antiviral state resistance of Venezuelan equine encephalitis virus. Virology 2016, 496, 147–165. [Google Scholar] [CrossRef]
- Mathiot, C.C.; Grimaud, G.; Garry, P.; Bouquety, J.C.; Mada, A.; Daguisy, A.M.; Georges, A.J. An outbreak of human Semliki Forest virus infections in Central African Republic. Am. J. Trop. Med. Hyg. 1990, 42, 386–393. [Google Scholar] [CrossRef]
- Willems, W.; Kaluza, G.; Boschek, C.; Bauer, H.; Hager, H.; Schutz, H.; Feistner, H. Semliki forest virus: Cause of a fatal case of human encephalitis. Science 1979, 203, 1127–1129. [Google Scholar] [CrossRef]
- Milner, A.R.; Marshall, I.D. Pathogenesis of in utero infections with abortogenic and non-abortogenic alphaviruses in mice. J. Virol. 1984, 50, 66–72. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Johnson, T.; Braack, L.; Guarido, M.; Venter, M.; Gouveia Almeida, A.P. Mosquito community composition and abundance at contrasting sites in northern South Africa, 2014–2017. J. Vector Ecol. 2020, 45, 104–117. [Google Scholar] [CrossRef]




| PCR | Primer Name (Orientation/Channel Dye) | Sequence 5′–3′ | Position (MIDV/SINV) | Region and Amplicon Size | Reference |
|---|---|---|---|---|---|
| Alpha first round | Alpha1+ (sense) | GAYGCITAYYTIGAYATGGTIGAIGG | 5888–6368/ 6162–6642 | nsP4 480 bp | Sánchez-Seco et al., 2001 [15] |
| Alpha1− (antisense) | KYTCYTCIGTRTGYTTIGTICCIGG | ||||
| Alpha nested | Alpha2+ (sense) | GIAAYTGYAAYGTIACICARATG | 6066–6264/ 6340–6538 | nsP4 198 bp | |
| Alpha2− (antisense) | GCRAAIARIGCIGCIGCYYTIGGICC | ||||
| MIDV probe | GCTTTAAGAAGTACGCATGCAACA | 6132–6155 | nsP4 N/A | van Niekerk et al., 2015 [13] | |
| SINV probe | ATGACGAGTATTGGGAGGAGTTTG | 6427–6450 | |||
| Nested specific MIDV | MNF (sense | GCAGCCTTTTGTCCGTCYAA | 5936–6283 | nsP4 347 bp | Steyn et al., 2020 [14] |
| MNR (antisense) | GGCTTCAAGTCRTAGGTTT | ||||
| Nested specific SINV | SNF (sense) | GCAACCTTYTGCCCCGCYAA | 6209–6556 | nsP4 347 bp | |
| SNR (antisense) | GGGACCAAATTATRCGTCT | ||||
| MIDV E first round | MIDV EF (sense) | TTGTCAACGGAGAGAGCAC | 10,231–11,048 | E1 817 bp | This study |
| MIDV ER (antisense) | CTATGGGCGGAGCTACTGTG | ||||
| MIDV E nested | MIDV EN 9F (sense) | ACCGGGTAGATTTGGGGACT | 10,379–10,930 | E1 551 bp | This study |
| MIDV 10,911 EN (antisense) | CACTTTGCTGTGCAAGTGGT | van Niekerk et al., 2015 [13] |
| MIDV and SINV Positive Fatal Cases in Horses January 2014–December 2018 | |||||||
|---|---|---|---|---|---|---|---|
| Year | 2014 | 2015 | 2016 | 2017 | 2018 | Total | |
| Virus | No. samples received | 195 | 197 | 129 | 427 | 136 | 1084 |
| MIDV | % Positive | 4.61% (9/195) | 5.07% (10/197) | 077% (1/129) | 9.84% (42/427) | 6.48% (7/108) | 6.36% (69/1084) |
| % Fatal | 22.22% (2/9) | 10.00% (1/10) | 0.00% (0/1) | 7.14% (3/42) | 0.00% (0/7) | 8.69% (6/69) | |
| % Fatal co-infections | 100% (2 WNV/2) | 0.00% (0/1) | N/A | 66.66% (2 WNV/3) | N/A | 66.66% (4/6) | |
| SINV | % Positive | 1.02% (2/195) | 4.06% (8/197) | 0.00% (0/0) (0/129) | 0.00% (0/427) | 0.92% (1/108) | 1.01% (11/1084) |
| % Fatal | 100% (2/2) | 0.00% (0/8) | 0.00% (0/0) | 0.00% (0/0) | 0.00% (0/1) | 18.18% (2/11) | |
| % Fatal co-infections | 50.00% (1 AHSV/EEV/2) | 0.00% (0/0) | 0.00% (0/0) | 0.00% (0/0) | 0.00% (0/0) | 50.00% (1/2) | |
| Middelburg | Sign | MIDV Positive n = 69 (%) | MIDV Negative n = 1015 (%) | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Died or euthanized | 6 (8.70) | 235 (23.15) | 0.004 | 0.31 (0.13–0.73) | |
| Neurological signs | Any neurological signs | 51 (73.91) | 667 (65.71) | 0.188 | 1.48 (0.85–2.59) |
| Ataxia | 34 (49.23) | 388 (38.23) | 0.074 | 1.56 (0.96–2.55) | |
| Paresis | 11 (15.94) | 122 (12.02) | 0.342 | 1.38 (0.70–2.71) | |
| Hind leg paralysis | 6 (8.70) | 47 (4.63) | 0.142 | 1.96 (0.80–4.76) | |
| Recumbency | 6 (8.70) | 159 (15.67) | 0.163 | 0.51 (0.21–1.20) | |
| Paralysis | 3 (4.35) | 97 (9.56) | 0.195 | 0.43 (0.13–1.39) | |
| Tremors/fasciculations | 3 (4.35) | 28 (2.76) | 0.441 | 1.6 (0.47–5.40) | |
| Seizures | 1 (1.45) | 49 (4.83) | 0.365 | 0.28 (0.03–2.13) | |
| Stiffness | 6 (8.70) | 7 (0.69) | 0.0001 | 13.71 (4.47–42.02) | |
| Fever | Fever | 52 (75.36) | 529 (52.12) | 0.00025 | 2.81 (1.60–4.92) |
| Fever only | 13 (18.84) | 168 (16.55) | 1.000 | 1.015 (0.543–1.895) | |
| Fever and neurological signs | 38 (55.07) | 188 (18.52) | 2 × 10−10 | 5.25 (3.18–8.66) | |
| Fever, neurological and respiratory signs | 1 (1.45) | 3 (0.29) | 0.235 | 4.85 (0.49–47.38) | |
| Other | Icterus | 20 (28.99) | 118 (11.63) | 0.00021 | 3.1 (1.78–5.40) |
| Anorexia/inappetence | 20 (28.99) | 189 (18.62) | 0.039 | 1.78 (1.03–3.07) | |
| Pallor | 10 (14.49) | 69 (5.91) | 0.010 | 2.69 (1.31–5.53) | |
| Sindbis | Sign | SINV Positive n = 11 (%) | SINV Negative n = 1073 (%) | p-Value | Odds Ratio (95% CI) |
| Died or euthanized | 2 (18.18) | 239 (22.27) | 1 | 0.77 (0.16–3.61) | |
| Neurological signs | Any neurological signs | 6 (54.54) | 713 (66.45) | 0.522 | 0.42 (0.12–1.38) |
| Ataxia | 3 (27.27) | 419 (39.05) | 0.543 | 0.58 (0.15–2.21) | |
| Recumbency | 2 (18.18) | 163 (15.19) | 0.678 | 1.24 (0.26–5.79) | |
| Circling/paddling | 1 (9.09) | 17 (1.58) | 0.168 | 6.21 (0.75–51.27) | |
| Facial nerve paralysis | 1 (9.09) | 3 (0.28) | 0.040 | 35.67 (3.41–372.98) | |
| Seizures | 1 (9.19) | 49 (4.57) | 0.406 | 2.08 (0.26–16.65) | |
| Tongue paralysis | 1 (9.09) | 7 (0.65) | 0.078 | 15.22 (1.71–135.51) | |
| Tremors/fasciculations | 1 (9.09) | 30 (2.80) | 0.274 | 3.47 (0.43–28.03) | |
| Fever | Fever | 8 (72.73) | 573 (53.40) | 0.237 | 2.33 (0.61–8.81) |
| Fever only | 3 (27.27) | 179 (16.68) | 0.406 | 1.88 (0.49–7.17) | |
| Fever and neurological signs | 4 (36.36) | 220 (20.50) | 0.260 | 2.13 (0.61–7.36) | |
| Fever, neurological and respiratory signs | 0 (0.00) | 4 (0.37) | 1.000 | Undefined(undefined) | |
| Other | Anorexia/inappetence | 4 (36.36) | 205 (19.11) | 0.237 | 2.41 (0.71–8.34) |
| Flaccid tail | 1 (9.09) | 7 (0.65) | 0.078 | 15.22 (1.71–135.51) | |
| Icterus | 1 (9.09) | 137 (12.77) | 1 | 0.68 (0.08–5.37) | |
| Nasal discharge | 2 (18.18) | 19 (1.78) | 0.021 | 12.26 (2.48–60.64) |
| Sample Group. | Horse 1 | Horse 2 | Horse 3 | Horse 4 |
|---|---|---|---|---|
| Sample ID’s | ZRU074/17 ZRU141/17/6 | ZRU060/17 ZRU141/17/1 | ZRU034/18 ZRU031/18 ZRU038/18 | ZRU194/17/3 ZRU209/17 |
| Date sick | 2017/03/07 | 2017/03/03 | 2018/02/03 | 2017/04/03 |
| Date first sampled | 2017/03/07 | 2017/03/03 | 2018/02/05 | 2017/04/03 |
| Days from onset for first sample (PCR positive) | 0 days | 0 days | 2 days | 0 days |
| Initial signs | Fever & Icterus | Ataxia, Fever & Icterus | Fever | Anorexia/inappetence, Fever, Icterus, Pallor |
| Virus result on first submission (test method) | MIDV/EEV (PCR) | MIDV (PCR) | MIDV (PCR) | MIDV (PCR) |
| Date follow-up sampled | 2017/03/24 | 2017/03/24 | 2018/02/07 2018/02/17 | 2017/04/10 |
| Days from onset for follow-up sample (PCR positive) | 17 days | 21 days | 4 and 14 days | 7 days |
| Signs at follow-up | Healthy | Healthy | Fever, Healthy | Icterus |
| Virus result for follow-up specimen (test method) | MIDV/EEV (PCR) | MIDV (PCR) | MIDV (PCR) | MIDV/WNV (MIDV PCR/WNV IgM ELISA) |
| Location | Benoni | Benoni | Bapsfontein | Onderstepoort |
| ZRU Number | Year | Signs | Location (Province, City) | Co-Infection | Clinical Outcome |
|---|---|---|---|---|---|
| ZRU080/14 | 2014 | Stiffness | North West, Vryburg | None detected | Alive |
| ZRU089/14 | 2014 | Ataxia, icterus, paresis, fever | Free State, Ladybrand | AHSV | Alive |
| ZRU044/17 | 2017 | Ataxia, icterus, fever | Gauteng, Pretoria | None detected | Alive |
| ZRU059/17/1 | 2017 | Ataxia, icterus, fever | Gauteng, Pretoria | None detected | Alive |
| ZRU075/17 | 2017 | Ataxia, fever | Gauteng, Benoni | None detected | Alive |
| ZRU103/17 | 2017 | Fever | Gauteng, Benoni | None detected | Alive |
| Possible Event | Parental Sequence | RDP4 Recombination Detection Model and Corresponding p-Values | |||||
|---|---|---|---|---|---|---|---|
| SP nt position | Major | Minor | RDP | MaxChi | Chimaera | SciScan | 3Seq |
| 2582–3093 (509 nt) | SFV | Unknown | 1.833 × 10−2 | 3.921 × 10−4 | 8.034 × 10−4 | 5.921 × 10−11 | 1.242 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fourie, I.; Snyman, J.; Williams, J.; Ismail, A.; Jansen van Vuren, P.; Venter, M. Epidemiological and Genomic Characterisation of Middelburg and Sindbis Alphaviruses Identified in Horses with Febrile and Neurological Infections, South Africa (2014–2018). Viruses 2022, 14, 2013. https://doi.org/10.3390/v14092013
Fourie I, Snyman J, Williams J, Ismail A, Jansen van Vuren P, Venter M. Epidemiological and Genomic Characterisation of Middelburg and Sindbis Alphaviruses Identified in Horses with Febrile and Neurological Infections, South Africa (2014–2018). Viruses. 2022; 14(9):2013. https://doi.org/10.3390/v14092013
Chicago/Turabian StyleFourie, Isabel, Jumari Snyman, June Williams, Arshad Ismail, Petrus Jansen van Vuren, and Marietjie Venter. 2022. "Epidemiological and Genomic Characterisation of Middelburg and Sindbis Alphaviruses Identified in Horses with Febrile and Neurological Infections, South Africa (2014–2018)" Viruses 14, no. 9: 2013. https://doi.org/10.3390/v14092013
APA StyleFourie, I., Snyman, J., Williams, J., Ismail, A., Jansen van Vuren, P., & Venter, M. (2022). Epidemiological and Genomic Characterisation of Middelburg and Sindbis Alphaviruses Identified in Horses with Febrile and Neurological Infections, South Africa (2014–2018). Viruses, 14(9), 2013. https://doi.org/10.3390/v14092013








