Obesity and Dysmetabolic Factors among Deceased COVID-19 Adults under 65 Years of Age in Italy: A Retrospective Case-Control Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Data Collection
2.2. Statistical Analysis
2.3. Ethics
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Factors Independently Associated with Death
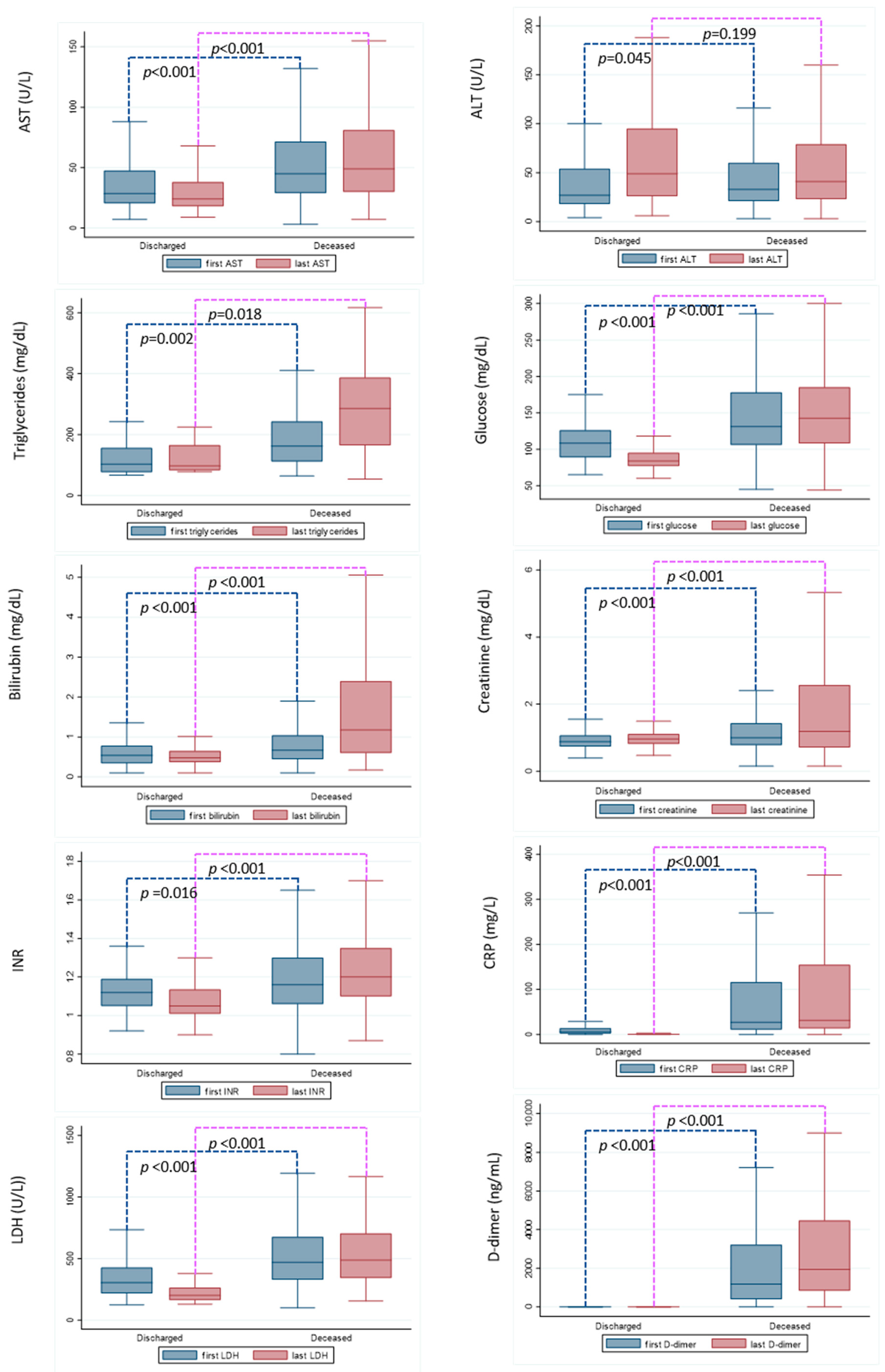
3.3. Laboratory Parameters at Hospital Admission and before Discharging
4. Discussion
5. Limits of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boccia, S.; Ricciardi, W.; Ioannidis, J.P.A. What Other Countries Can Learn from Italy During the COVID-19 Pandemic. JAMA Intern. Med. 2020, 180, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Qian, L.; Hong, V.; Wei, R.; Nadjafi, R.F.; Fischer, H.; Li, M.Z.; Shaw, D.S.F.; Caparosa, M.S.L.; Nau, C.L.; et al. Obesity and Mortality among Patients Diagnosed With COVID-19: Results from an Integrated Health Care Organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L. Body Mass Index and Risk for COVID-19–Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death—United States, March–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 355–361. [Google Scholar] [CrossRef]
- O’Hearn, M.; Liu, J.; Cudhea, F.; Micha, R.; Mozaffarian, D. Coronavirus Disease 2019 Hospitalizations Attributable to Cardiometabolic Conditions in the United States: A Comparative Risk Assessment Analysis. J. Am. Heart Assoc. 2021, 10, e019259. [Google Scholar] [CrossRef]
- Kass, D.A.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- COVID-19 Recommendations for Older Adults. 2021. Available online: https://www.cdc.gov/aging/covid19-guidance.html (accessed on 28 July 2022).
- Palmieri, L.; Vanacore, N.; Donfrancesco, C.; Lo Noce, C.; Canevelli, M.; Punzo, O.; Raparelli, V.; Pezzotti, P.; Riccardo, F.; Bella, A.; et al. Clinical Characteristics of Hospitalized Individuals Dying With COVID-19 by Age Group in Italy. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Palmer, K.; Lo Noce, C.; Meli, P.; Giuliano, M.; Floridia, M.; de Bella, M.T.; Piccioli, A.; Brusaferro, S.; Onder, G. Differences in the clinical characteristics of COVID-19 patients who died in hospital during different phases of the pandemic: National data from Italy. Aging Clin. Exp. Res. 2021, 33, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gazzetta Ufficiale, n.d. Available online: https://www.gazzettaufficiale.it/eli/id/2020/02/28/20A01348/SG (accessed on 28 July 2022).
- CDC. Obesity, Race/Ethnicity, and COVID-19. Centers for Disease Control and Prevention. 2022. Available online: https://www.cdc.gov/obesity/data/Having obesity increases risk of severe illness from COVID-19 (accessed on 28 July 2022).
- Rottoli, M.; Bernante, P.; Belvedere, A.; Balsamo, F.; Garelli, S.; Giannella, M.; Cascavilla, A.; Tedeschi, S.; Ianniruberto, S.; Del Turco, E.R.; et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur. J. Endocrinol. 2020, 183, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Klang, E.; Kassim, G.; Soffer, S.; Freeman, R.; Levin, M.A.; Reich, D.L. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity 2020, 28, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Piernas, C.; Astbury, N.M.; Hippisley-Cox, J.; O’Rahilly, S.; Aveyard, P.; Jebb, S.A. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021, 9, 350–359. [Google Scholar] [CrossRef]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Reitsma, M.B.; Murray, C.J.L. Health Effects of Overweight and Obesity in 195 Countries. N. Engl. J. Med. 2017, 377, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Diedisheim, M.; Dancoisne, E.; Gautier, J.-F.; Larger, E.; Cosson, E.; Fève, B.; Chanson, P.; Czernichow, S.; Tatulashvili, S.; Raffin-Sanson, M.-L.; et al. Diabetes Increases Severe COVID-19 Outcomes Primarily in Younger Adults. J. Clin. Endocrinol. Metab. 2021, 106, e3364–e3368. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.I.; Gao, F.; Wang, X.-B.; Sun, Q.-F.; Pan, K.-H.; Wang, T.-Y.; Ma, H.-L.; Chen, Y.-P.; Liu, W.-Y.; George, J.; et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism 2020, 108, 154244. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Deceased N = 593 n (%) | Discharged N = 914 n (%) | p-Value |
|---|---|---|---|
| Gender: | |||
| Males | 460 (77.6) | 303 (33.1) | <0.001 |
| Females | 133 (22.4) | 611 (66.9) | |
| Age distribution: | |||
| 20–45 year | 57 (9.6) | 257 (28.1) | <0.001 |
| 46–55 years | 149 (25.1) | 318 (34.8) | |
| 56–65 years | 387 (65.3) | 339 (37.1) | |
| Median Age (range) | 59 (22–65) | 52 (20–65) | <0.001 |
| BMI: | |||
| Underweight/Normal | 190 (32) | 603 (66) | <0.001 |
| Overweight | 151 (25.5) | 179 (19.6) | |
| Obese | 213 (35.9) | 130 (14.2) | |
| Not available | 39 (6.6) | 2 (0.2) | |
| Presence of comorbidities: | |||
| Diabetes | 203 (34.2) | 127 (13.9) | <0.001 |
| Dyslipidaemia | 62 (10.5) | 23 (2.5) | <0.001 |
| Hearth Disease | 141 (23.8) | 69 (7.5) | <0.001 |
| Hypertension | 272 (45.9) | 144 (15.7) | <0.001 |
| COPD | 67 (11.3) | 29 (3.2) | <0.001 |
| CKD | 85 (14.3) | 44 (4.8) | <0.001 |
| Cancer | 88 (14.8) | 107 (11.7) | 0.077 |
| Age 20–45 | Age 46–55 | Age 56–65 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deceased (n = 57; 9.6%) | Discharged (n = 257; 28.1%) | p | Deceased (n = 149; 25.1%) | Discharged (n = 318; 34.8%) | p | Deceased (n = 387; 65.3%) | Discharged (n = 339; 37.1%) | p | |
| Diabetes | 9 (15.8) | 13 (5.1) | 0.008 | 46 (30.9) | 49 (15.4) | <0.001 | 148 (38.2) | 65 (19.2) | <0.001 |
| Dyslipidaemia | 2 (3.5) | 3 (1.2) | 0.225 | 7 (4.7) | 7 (2.2) | 0.153 | 53 (13.7) | 13 (3.8) | <0.001 |
| Hearth Disease | 9 (15.8) | 5 (1.9) | <0.001 | 24 (16.1) | 25 (7.9) | 0.007 | 108 (27.9) | 39 (11.5) | <0.001 |
| Hypertension | 11 (19.3) | 21 (8.2) | 0.012 | 50 (33.6) | 49 (15.4) | <0.001 | 211 (54.5) | 74 (21.8) | <0.001 |
| Overweight Obese | 13 (25.5) 20 (39.2) | 29 (11.3) 32 (12.5) | <0.001 | 31 (25.3) 48 (36.1) | 66 (20.7) 51 (16.0) | <0.001 | 107 (28.9) 145 (39.2) | 84 (24.8) 47 (13.9) | <0.001 |
| COPD | 3 (5.3) | 4 (1.6) | 0.086 | 14 (9.4) | 10 (3.1) | 0.004 | 50 (12.9) | 15 (4.4) | <0.001 |
| CKD | 4 (7.0) | 9 (3.5) | 0.228 | 16 (10.7) | 18 (5.7) | 0.049 | 65 (16.8) | 17 (5.0) | <0.001 |
| Cancer | 11 (19.3) | 12 (4.5) | <0.001 | 27 (18.1) | 39 (12.3) | 0.090 | 50 (12.9) | 56 (16.5) | 0.171 |
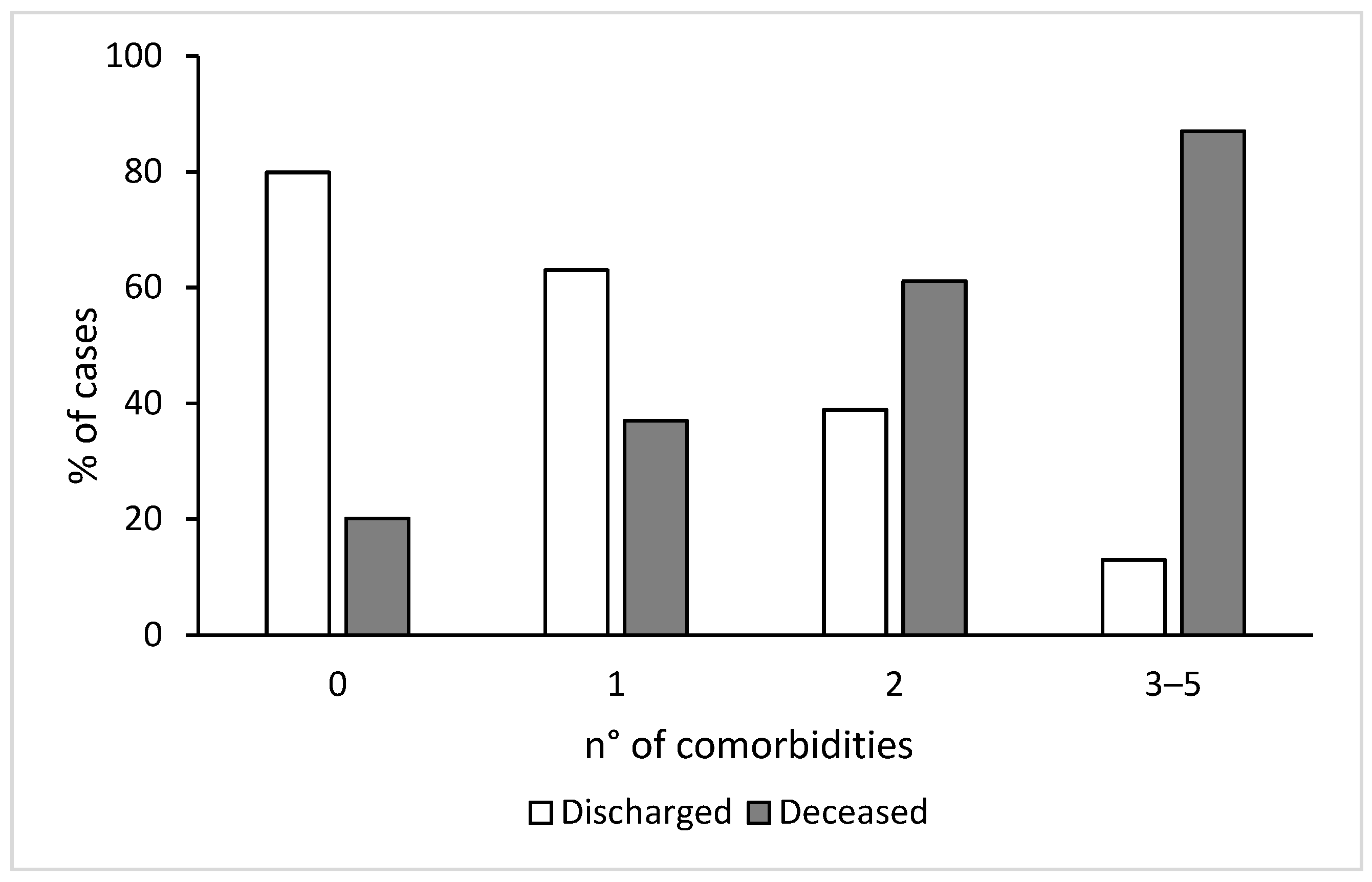
| N. of comorbidities related to metabolic impairment * | |||||||||
| 0 1 2 3 or more | 17 (33.3) 23 (45.1) 5 (9.8) 6 (11.8) | 197 (76.9) 45 (17.6) 13 (5.1) 1 (0.4) | <0.001 | 40 (30.1) 46 (34.6) 27 (20.3) 20 (15.0) | 187 (58.8) 94 (29.6) 27 (8.5) 10 (3.1) | <0.001 | 82 (22.2) 80 (21.6) 100 (27.0) 108 (29.2) | 170 (50.3) 115 (34.0) 44 (13.0) 9 (2.7) | <0.001 |
| Overall (n * = 1466) | Age 20–45 (n * = 307) | Age 46–55 (n * = 451) | Age 56–65 (n * = 708) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AdjOR | 95% CI | p | AdjOR | 95% CI | p | AdjOR | 95% CI | p | AdjOR | 95% CI | p | |
| Age | 1.05 | 1.03–1.07 | <0.001 | 1.05 | 0.98–1.11 | 0.138 | 1.07 | 0.98–1.17 | 0.117 | 1.09 | 1.02–1.17 | 0.008 |
| Gender (ref. female) | 6.92 | 5.22–9.17 | <0.001 | 5.24 | 2.30–11.94 | <0.001 | 5.41 | 3.33–8.79 | <0.001 | 9.00 | 6.03–13.45 | <0.001 |
| Overweight (ref. under-normalweight) | 2.19 | 1.57–3.05 | <0.001 | 5.53 | 2.07–14.76 | 0.001 | 1.49 | 0.81–2.72 | 0.196 | 2.36 | 1.49–3.74 | <0.001 |
| Obese (ref. under-normalweight) | 3.81 | 2.71–5.36 | <0.001 | 8.58 | 3.30–22.30 | <0.001 | 3.00 | 1.69–5.34 | <0.001 | 4.08 | 2.46–6.77 | <0.001 |
| Diabetes | 1.73 | 1.24–2.40 | 0.001 | 2.82 | 0.84–9.48 | 0.094 | 1.85 | 1.05–3.25 | 0.033 | 1.62 | 1.04–2.53 | 0.034 |
| Dyslipidaemia | 1.70 | 0.91–3.16 | 0.096 | 10.02 | 1.07–94.22 | 0.044 | 1.32 | 0.33–5.25 | 0.691 | 1.76 | 0.82–3.77 | 0.147 |
| Hearth Disease | 1.73 | 1.15–2.61 | 0.009 | 17.68 | 3.80–82.19 | <0.001 | 1.43 | 0.66–3.11 | 0.362 | 1.44 | 0.84–2.45 | 0.183 |
| Hypertension | 2.41 | 1.76–3.30 | <0.001 | 1.24 | 0.37–4.13 | 0.726 | 1.88 | 1.07–3.32 | 0.029 | 2.93 | 1.93–4.45 | <0.001 |
| COPD | 2.50 | 1.40–4.48 | 0.002 | 1.86 | 0.20–17.23 | 0.585 | 2.22 | 0.77–6.43 | 0.142 | 3.06 | 1.40–6.72 | 0.005 |
| Cancer | 1.72 | 1.18–2.51 | 0.005 | 13.28 | 4.25–41.51 | <0.001 | 2.05 | 1.10–3.83 | 0.023 | 1.10 | 0.65–1.86 | 0.731 |
| CKD | 2.33 | 1.43–3.80 | 0.001 | 0.29 | 0.06–1.56 | 0.150 | 2.62 | 1.15–5.97 | 0.022 | 3.23 | 1.58–6.60 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondili, L.A.; Quaranta, M.G.; Viganò, M.; Tata, X.; D’Angelo, F.; Lo Noce, C.; Palmieri, L.; Onder, G.; D’Amico, F.; Inglese, E.; et al. Obesity and Dysmetabolic Factors among Deceased COVID-19 Adults under 65 Years of Age in Italy: A Retrospective Case-Control Study. Viruses 2022, 14, 1981. https://doi.org/10.3390/v14091981
Kondili LA, Quaranta MG, Viganò M, Tata X, D’Angelo F, Lo Noce C, Palmieri L, Onder G, D’Amico F, Inglese E, et al. Obesity and Dysmetabolic Factors among Deceased COVID-19 Adults under 65 Years of Age in Italy: A Retrospective Case-Control Study. Viruses. 2022; 14(9):1981. https://doi.org/10.3390/v14091981
Chicago/Turabian StyleKondili, Loreta A., Maria Giovanna Quaranta, Mauro Viganò, Xhimi Tata, Franca D’Angelo, Cinzia Lo Noce, Luigi Palmieri, Graziano Onder, Federico D’Amico, Elvira Inglese, and et al. 2022. "Obesity and Dysmetabolic Factors among Deceased COVID-19 Adults under 65 Years of Age in Italy: A Retrospective Case-Control Study" Viruses 14, no. 9: 1981. https://doi.org/10.3390/v14091981
APA StyleKondili, L. A., Quaranta, M. G., Viganò, M., Tata, X., D’Angelo, F., Lo Noce, C., Palmieri, L., Onder, G., D’Amico, F., Inglese, E., Puoti, M., Aghemo, A., & Tosti, M. E. (2022). Obesity and Dysmetabolic Factors among Deceased COVID-19 Adults under 65 Years of Age in Italy: A Retrospective Case-Control Study. Viruses, 14(9), 1981. https://doi.org/10.3390/v14091981







