Abstract
Formulating termination of isolation (de-isolation) policies requires up-to-date knowledge about viral shedding dynamics. However, current de-isolation policies are largely based on viral load data obtained before the emergence of Omicron variant. In this retrospective cohort study involving adult patients hospitalised for COVID-19 between January and February 2022, we sought to determine SARS-CoV-2 viral shedding kinetics and to investigate the risk factors associated with slow viral decline during the 2022 Omicron wave. A total of 104 patients were included. The viral load was highest (Ct value was lowest) on days 1 post-symptom-onset (PSO) and gradually declined. Older age, hypertension, hyperlipidaemia and chronic kidney disease were associated with slow viral decline in the univariate analysis on both day 7 and day 10 PSO, while incomplete or no vaccination was associated with slow viral decline on day 7 PSO only. However, older age was the only risk factor that remained statistically significant in the multivariate analysis. In conclusion, older age is an independent risk factor associated with slow viral decline in this study conducted during the Omicron-dominant 2022 COVID-19 wave. Transmission-based precaution guidelines should take age into consideration when determining the timing of de-isolation.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of the most contagious respiratory viruses [1]. The Omicron variant, first emerged in South Africa in November 2021, is particularly transmissible, with an estimated growth rate 3.5 times faster than that of Delta variant [2]. In particular, BA.2 sub-lineage is more transmissible than BA.1 [3,4]. In Hong Kong, the Omicron variant has caused the fifth wave of COVID-19, and the sub-lineage BA.2.2 is the predominant lineage since late January 2022 [3,5].
Home isolation is an important public health measure to reduce community spread, while cohort or single-room isolation can prevent nosocomial transmission within healthcare facilities. Symptom-based approach is currently used in most places for the determination of the duration of isolation. The recommended duration of home isolation in many countries is often 5–7 days [6,7,8,9,10]. In hospital settings, the US Centers for Disease Control and Prevention and the UK Health Security Agency recommend that a COVID-19 patient should be isolated in a single-person room for at least ten days, until fever subsides and symptoms are improving, except for immunocompromised patients for whom a test-based strategy is required for ending isolation [11,12].
Although symptom-based isolation policy is convenient, it does not take into account other factors which have been shown to affect the duration of high viral load shedding [13,14,15,16,17,18]. Furthermore, since current isolation policies are mainly based on viral load dynamics data collected before the emergence of the Omicron variant [11], these may not be applicable to patients infected by the Omicron variant. Moreover, previous studies on each host factor were often analysed individually without performing multivariate analysis to exclude potential confounding factors. Here, we address these deficiencies by assessing the viral load kinetics among COVID-19 patients during the 2022 Omicron wave and used univariate and multivariate analyses to determine risk factors affecting viral decline.
2. Materials and Methods
2.1. Patients
This is a retrospective cohort study involving adult patients admitted to Queen Mary Hospital for laboratory-confirmed COVID-19 between 20 January 2022 and 25 February 2022. In Hong Kong, the cycle threshold (Ct) value was used as one of the discharge criteria during this period (Table 1). Therefore, even patients who were asymptomatic or had clinically improved were required to be hospitalised until the Ct values met the discharge criteria. In our hospital, saliva was used for monitoring viral load because saliva is a non-invasive type of specimen, and it has been shown to demonstrate less variation in human RNase P Ct value than nasopharyngeal swabs [19].

Table 1.
Evolution of discharge criteria for laboratory-confirmed COVID-19 patients in Hong Kong.
Patients were included if (i) aged 18 years or above; (ii) at least one saliva specimen tested positive for SARS-CoV-2 by real-time reverse transcription-polymerase chain reaction (RT-PCR), and (iii) at least one saliva specimen was available on or after day 7. Patients were excluded if clinical information was not available or if COVID-19 vaccination status was unknown. The clinical details of each patient, including the demographics, chronic comorbidities, COVID-19 vaccination details, severity of infection and treatment received, were retrieved from the electronic patient record. We defined severe disease as the need for supplemental oxygen. The study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 22-052). Since patients were recruited retrospectively and archived specimens were used, written informed consent was waived.
2.2. Definitions
Patients were considered to be fully vaccinated if they have received at least 2 doses of COVID-19 vaccines at least 14 days prior to symptom onset for symptomatic patients or the first positive SARS-CoV-2 test for asymptomatic patients. For the purpose of assessing viral kinetics, day 0 was taken as the day of the first positive SARS-CoV-2 test for asymptomatic patients.
A patient was considered to have slow viral decline (SVD) on day 7 PSO if the Ct value was <30 for any specimen collected on or after day 7 PSO (SVD-7). Patients were considered to have rapid viral decline (RVD) on day 7 PSO if they did not fulfil the criteria for SVD. The Ct value cut-off of 30 was chosen to differentiate SVD and RVD because a previous study has shown that transmission was lower for patients with Ct values of >30 than those with Ct < 30 [20]. Furthermore, several studies have shown that specimens with Ct values of >30 are unlikely to yield live virus [21].
We assessed SVD and RVD both on day 7 and day 10 PSO because the de-isolation criteria for hospitalised patients vary from day 7 or 10 according to different guidelines and situations. The criteria for classifying a patient as SVD or RVD for day 10 PSO were similar to those of day 7 PSO, except that (i) all patients with RVD on day 7 PSO were considered to have RVD on day 10 PSO; and (ii) a patient was excluded from day 10 PSO analysis if no specimens were collected on or after day 10 PSO.
2.3. Saliva Specimens SARS-CoV-2 RT-PCR and Whole Genome Sequencing
Saliva specimens were collected as described previously [22]. Patients were instructed to submit around 1 mL of saliva directly into a sterile bottle. Upon arrival at the laboratory, phosphate buffered saline was added to the specimen to top up the volume to 2 mL. During the study period, all saliva specimens submitted to our laboratory for urgent SARS-CoV-2 RT-PCR were tested by the by the Xpert Xpress SARS-CoV-2 assay (Cepheid, Sunnyvale, CA, USA) which targeted the E and N genes of SARS-CoV-2, according to manufacturer’s instructions. Briefly, 300 μL of each specimen was directly added into the Xpert cartridge, which was loaded into the GeneXpert XVI system (Cepheid, Sunnyvale, CA, USA). Routine specimens (those not tagged as “urgent”) were tested by a commercial SARS-CoV-2 real-time RT-PCR targeting the E gene (TIB Molbiol, Berlin, Germany). The SARS-CoV-2 lineage was determined by whole genome sequencing using the Oxford Nanopore MinION device (Oxford Nanopore Technologies, Oxford, UK) as described previously [3].
2.4. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 28.0.1.0 or PRISM version 9.1.2 (GraphPad Software, San Diego, CA, USA). Categorical variables were compared using Fisher’s exact test and continuous variables compared using Mann–Whitney U test. Multivariable logistic regression models were built using backward stepwise elimination (likelihood ratio) method with a p value of <0.1 required for inclusion. A 2-sided p value of <0.05 was considered statistically significant. Graphs were created using PRISM.
3. Results
3.1. Patient Characteristics
During the study period, there were a total of 211 hospitalised adult patients with at least one saliva specimens tested positive for SARS-CoV-2, and 104 fulfilled the inclusion and exclusion criteria and had sufficient specimens available to determine the viral decline on day 7 PSO (Table 2). The median age was 68 years (interquartile range: 47–76), and 44.2% (46/104) were female. Chronic medical illness was present in 70.2% (73/104) of patients, and 49% (51/104) were fully vaccinated. Remdesivir was given to 27.9% (29/104) of patients, respectively, and 2.9% (3/104) required oxygen supplementation. Whole viral genome sequencing showed that 90.4% (94/104) and 9.6% (10/104) were infected with the Omicron and Delta variants, respectively.

Table 2.
Comparison between patients with slow viral decline (SVD) and rapid viral clearance (RVD) on day 7 PSO.
Figure 1 shows the overall trend of SARS-CoV-2 RNA shedding in saliva in the first 10 days PSO. The viral load was highest (Ct value was lowest) on day 1 PSO, and gradually decreased (Figure 1A). There was no difference in the median peak viral loads between older and younger individuals (Figure 1B) and between fully vaccinated and non-fully vaccinated patients (Figure 1C). However, on or after day 2 PSO, the viral load was generally lower in younger adults (<60 years old) than older adults (≥60 years old) and in fully vaccinated than non-fully vaccinated individuals.
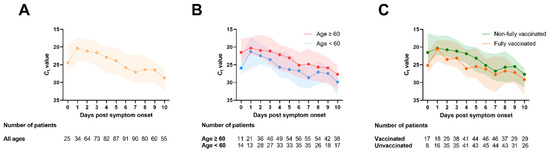
Figure 1.
Serial changes of Ct value in saliva specimens. (A) All patients; (B) according to age; (C) according to vaccination status. Each dot represents the median viral load, and the shading represents the interquartile range.
3.2. Risk Factors Associated with Slow Viral Decline in Saliva
To assess for risk factors associated with prolonged high-level RNA shedding, we classified patients into two groups, SVD and RVD (see methods for the criteria). Univariate analysis showed that older age, presence of chronic comorbidities, hypertension, hyperlipidaemia, and chronic kidney disease were statistically significantly associated with SVD on both day 7 and 10 PSO (Table 2), while connective tissue disease was significantly associated with SVD on day 10 PSO only. The proportion of fully vaccinated patients was statistically significantly lower in the SVD than RVD group, but only reached statistical significance on day 7 PSO (44% (37/84) for SVD vs. 70% (14/20) for RVD; p = 0.047). Notably, disease severity (symptomatic or oxygen requirement) and treatment (remdesivir) were not significantly associated with viral decline on either day 7 or 10 PSO.
Since previous studies demonstrated that the neutralising antibody levels were lower in patients who received CoronaVac vaccine compared with those who received BNT162b2 vaccine [23], we performed sub-group analysis for the fully-vaccinated patients. There was no significant difference in the proportion of patients receiving BNT162b2 between the SVD and RVD group on either day 7 (p = 0.525) or day 10 PSO (p = 0.547).
In order to determine independent host factors associated with slower viral clearance, we performed a multivariate analysis. Only older age remained statistically significantly associated with SVD on both day 7 and day 10 PSO (Day 7: p = 0.016; Day 10: p = 0.018).
4. Discussion
Viral shedding dynamics is a major consideration when formulating policies on de-isolation of COVID-19 patients. Patients with higher viral loads (lower Ct values) are more likely to transmit the virus to others [24]. In this study, older age was the only independent host factor significantly associated with slower viral decline. Aging is associated with immunosenescence, which affects both the innate and the adaptive immune systems [25]. In our previous in vivo study, aged mice showed higher viral loads in the nasal turbinate and in the lung, but weaker interferon and antibody response, than young mice (6–8 weeks old) [26]. Aging is also associated with T cell exhaustion [27], but the role of T cell in viral clearance during SARS-CoV-2 infection remains to be determined [28].
In a large study involving over 10,000 individuals, Levine-Tiefenbrun et al. found that fully vaccinated individuals had lower viral loads than non-vaccinated individuals [17]. Another cohort study of healthcare workers by Jung J et al. found that fully vaccinated individuals had a shorter duration of viable viral shedding [29]. In our current study, although there was a tendency towards lower viral load among fully vaccinated individuals, multivariate analysis revealed that vaccination status was not an independent risk factor associated with SVD. Our results concurred with a recent study by Boucau J et al., which showed no significant difference in the time to PCR conversion or culture conversion between non-vaccinated and vaccinated individuals infected with Delta or Omicron variants [30]. The apparent contradictory findings may be related to the different settings. First, our cohort had a median age of 68 years old, which was much older than those in the studies by Levine-Tiefenbrun et al. (median age, 42 years) and Jung J et al. (median age, 47 years). Hence, we were able to evaluate viral decline in these older adults. Second, our study was conducted during the Omicron wave. The neutralizing antibody titers against the Omicron variant were much lower than those against the ancestral virus due to immune escape [31,32,33]. As a result, vaccine effectiveness seems to be much reduced against the Omicron variant than against previous strains.
We chose a Ct value of 30 as the cut-off for SVD and RVD because the main aim of this study was to provide data for guiding de-isolation policy. In the study by Kim et al., which assessed serial specimens from hospitalised patients, only specimens with a Ct of 28.4 were culturable [22]. Takahashi et al. showed that for Omicron patients, only those with Ct of <30 can be cultured [34].
We did not find any differences between patients with prior CoronaVac and BNT162b2 vaccination. Previous studies showed that the neutralizing antibody titers elicited by BNT162b2 were much higher than by CoronaVac [23]. Therefore, viral clearance in the upper respiratory tract may not correlate with serum neutralizing antibody titers, but it depends on a complex interplay between humoral and cell-mediated immune pathways. Whether the intranasal vaccine, which elicits better mucosal immune response in the airway, can hasten the decline in viral load in the upper respiratory tract remains to be determined [35].
There are several limitations in this study. First, we could not determine the effect of three doses of vaccine as there were only seven patients who had received booster dose vaccine. Multiple studies have demonstrated that a booster dose of vaccine can significantly increase the neutralization titers against the Omicron variant [36], which may accelerate the decline in viral load. Second, none of the patients received the oral antivirals molnupiravir or nirmatrelvir-ritonavir as these were not available in Hong Kong during the study period. Previous studies suggest that viral load at day 5 of treatment decreased by 0.3 log10 copies/mL for molnupiravir and 0.87 log10 copies/mL for nirmatrelvir-ritonavir compared with placebo [37,38]. Further studies should be conducted to evaluate the reduction in viral load for these oral drugs in real-life situations. Third, there were too few severe cases in our cohort to determine whether patients with severe disease require a longer duration of isolation. Similarly, many of the comparisons in the univariate analysis were limited by small sample size, such as immunocompromised state and connective tissue disease, which may limit the power to detect significant association. Fourth, we selected two time points, 7 and 10 days PSO, to assess the viral shedding since most guidelines stipulated an isolation duration of 7 to 10 days. However, both intermittent viral shedding and prolonged detection of viral RNA have been reported in COVID-19 patients [22]. More frequent collection of specimens for extended duration is required to better delineate the temporal profile. Finally, we only included patients with at least one saliva specimen available on or after day 7. Since some patients may have been discharged before day 7 by meeting the serological and Ct value criteria (Table 1), this may cause selection bias in the cohort of patients included in the analysis.
In conclusion, after adjusting for confounding factors, only older age remained to be an independent risk factor associated with SVD. Viral load monitoring by real-time RT-PCR or antigen test may be useful in guiding decision for de-isolation among older adults.
Author Contributions
Conceptualization, X.L. and K.K.-W.T.; methodology, A.R.T., W.-M.C. (Wing-Ming Chu), W.-M.C. (Wan-Mui Chan), J.D.I., A.W.-H.C., S.M.U.A., C.C.-Y.Y., K.-H.C. and S.S.-Y.W.; writing—original draft preparation, X.L., S.S.-Y.W. and K.K.-W.T.; writing—review and editing, V.C.-C.C., K.-Y.Y. and I.F.-N.H.; supervision, K.K.-W.T.; funding acquisition, K.-Y.Y. and K.K.-W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for Department of Health of the Hong Kong; the Theme-Based Research Scheme (T11/707/15) of the Research Grants Council, Hong Kong Special Administrative Region; Emerging Collaborative Project of Guangzhou Laboratory (EKPG22-01); Emergency COVID-19 Project (2021YFC0866100), Major Projects on Public Security, National Key Research and Development Program; and the donations of the Shaw Foundation Hong Kong, Richard Yu and Carol Yu, May Tam Mak Mei Yin, Michael Seak-Kan Tong, Respiratory Viral Research Foundation Limited, Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited, Chan Yin Chuen Memorial Charitable Foundation, and Marina Man-Wai Lee. The funding sources had no role in study design, data collection, analysis, interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 22-052).
Informed Consent Statement
Patient consent was waived due to the use of archived specimens.
Data Availability Statement
Data are contained within the article. Further enquires can be directed to corresponding author.
Conflicts of Interest
K.Y.-Y., K.K.-W.T. and I.F.-N.H. report collaboration with Sinovac and Sinopharm. I.F.-N.H. received payment from M.S.D. for speaking at the COVID-19 Regional Expert Input Forum 2021; is on the advisory board of Pfizer on COVID-19 management in 2022; and was on the advisory board of Gilead on evolving treatment landscape in COVID-19 in 2021. Other authors declare no conflict of interest.
References
- Standl, F.; Jockel, K.H.; Brune, B.; Schmidt, B.; Stang, A. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet. Infect. Dis. 2021, 21, e77. [Google Scholar] [CrossRef]
- Elliott, P.; Bodinier, B.; Eales, O.; Wang, H.; Haw, D.; Elliott, J.; Whitaker, M.; Jonnerby, J.; Tang, D.; Walters, C.E.; et al. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science 2022, 375, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Ip, J.D.; Chu, A.W.; Tam, A.R.; Chan, W.M.; Abdullah, S.M.U.; Chan, B.P.; Wong, S.C.; Kwan, M.Y.; Chua, G.T.; et al. Rapid spread of SARS-CoV-2 Omicron subvariant BA.2 in a single-source community outbreak. Clin. Infect. Dis. 2022, ciac203. [Google Scholar] [CrossRef] [PubMed]
- Lyngse, F.P.; Kirkeby, C.T.; Denwood, M.; Christiansen, L.E.; Mølbak, K.; Møller, C.H.; Skov, R.L.; Krause, T.G.; Rasmussen, M.; Sieber, R.N.; et al. Transmission of SARS-CoV-2 Omicron VOC subvariants BA.1 and BA.2: Evidence from Danish Households. medRxiv 2022, 1, 28. [Google Scholar] [CrossRef]
- Chen, L.-L.; Abdullah, S.M.U.; Chan, W.-M.; Chan, B.P.-C.; Ip, J.D.; Chu, A.W.-H.; Lu, L.; Zhang, X.; Zhao, Y.; Chuang, V.W.-M.; et al. Contribution of low population immunity to the severe Omicron BA.2 outbreak in Hong Kong. Nat. Commun. 2022, 13, 3618. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Quarantine and Isolation. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html#iso (accessed on 20 June 2022).
- Department of Health of the Australian Government. If You Have COVID-19. Available online: https://www.health.gov.au/health-alerts/covid-19/testing-positive?gclsrc=aw.ds&gclid=CjwKCAjw-8qVBhANEiwAfjXLrvtWVfA6QGZkFfvH-KnY8c0f2n32Q_vQf1GaReQz5MdnZthO2aVvRRoCwEQQAvD_BwE (accessed on 27 June 2022).
- New Zealand Government. If You Have COVID. Available online: https://covid19.govt.nz/isolation-and-care/if-you-have-covid-19/#:~:text=You%20must%20self%2Disolate%20for,in%20the%20last%203%20months (accessed on 27 June 2022).
- Health and Safety Executive. Self-Isolation (Stay in Your Room). Available online: https://www2.hse.ie/conditions/covid19/restricted-movements/how-to-self-isolate/ (accessed on 27 June 2022).
- Clinical Excellence Commission of the NSW Government. COVID-19 Infection Prevention and Control Manual. Available online: https://www.cec.health.nsw.gov.au/keep-patients-safe/COVID-19/COVID-19-IPAC-manual (accessed on 27 June 2022).
- Centers for Disease Control and Prevention. Ending Isolation and Precautions for People with COVID-19: Interim Guidance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed on 20 June 2022).
- UK Health Security Agency. COVID-19: Information and Advice for Health and Care Professionals; Published on 27 May 2022. Available online: https://www.gov.uk/guidance/covid-19-information-and-advice-for-health-and-care-professionals (accessed on 20 June 2022).
- Munker, D.; Osterman, A.; Stubbe, H.; Muenchhoff, M.; Veit, T.; Weinberger, T.; Barnikel, M.; Mumm, J.N.; Milger, K.; Khatamzas, E.; et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur. Respir. J. 2021, 58, 2002724. [Google Scholar] [CrossRef]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Han, P.; Zhu, R.; Bai, T.; Yi, J.; Zhao, X.; Tao, M.; Quan, R.; Chen, C.; Zhang, Y.; et al. Risk factors for viral RNA shedding in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001190. [Google Scholar] [CrossRef]
- Kang, M.; Xin, H.; Yuan, J.; Ali, S.T.; Liang, Z.; Zhang, J.; Hu, T.; Lau, E.H.; Zhang, Y.; Zhang, M.; et al. Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Eurosurveill 2022, 27, 2100815. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef]
- Lingas, G.; Neant, N.; Gaymard, A.; Belhadi, D.; Peytavin, G.; Hites, M.; Staub, T.; Greil, R.; Paiva, J.A.; Poissy, J.; et al. Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: A modelling analysis of the randomized, controlled, open-label DisCoVeRy trial. J. Antimicrob. Chemother. 2022, 77, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Al Bayat, S.; Mundodan, J.; Hasnain, S.; Sallam, M.; Khogali, H.; Ali, D.; Alateeg, S.; Osama, M.; Elberdiny, A.; Al-Romaihi, H.; et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? J. Infect. Public Health 2021, 14, 1201–1205. [Google Scholar] [CrossRef]
- Kim, M.C.; Cui, C.; Shin, K.R.; Bae, J.Y.; Kweon, O.J.; Lee, M.K.; Choi, S.H.; Jung, S.Y.; Park, M.S.; Chung, J.W. Duration of Culturable SARS-CoV-2 in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 671–673. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.K.; Wang, H.; et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine 2022, 77, 103904. [Google Scholar] [CrossRef]
- Jajou, R.; Mutsaers-van Oudheusden, A.; Verweij, J.J.; Rietveld, A.; Murk, J.L. SARS-CoV-2 transmitters have more than three times higher viral loads than non-transmitters—Practical use of viral load for disease control. J. Clin. Virol. 2022, 150–151, 105131. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Liu, F.; Ye, Z.; Song, W.; Lee, A.C.Y.; Shuai, H.; Lu, L.; To, K.K.; Chan, J.F.; et al. Age-associated SARS-CoV-2 breakthrough infection and changes in immune response in a mouse model. Emerg. Microbes Infect. 2022, 11, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Mogilenko, D.A.; Shchukina, I.; Artyomov, M.N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 2021, 22, 484–498. [Google Scholar] [CrossRef]
- Kent, S.J.; Khoury, D.S.; Reynaldi, A.; Juno, J.A.; Wheatley, A.K.; Stadler, E.; John Wherry, E.; Triccas, J.; Sasson, S.C.; Cromer, D.; et al. Disentangling the relative importance of T cell responses in COVID-19: Leading actors or supporting cast? Nat. Rev. Immunol. 2022, 22, 387–397. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.Y.; Park, H.; Park, S.; Lim, J.S.; Lim, S.Y.; Bae, S.; Lim, Y.J.; Kim, E.O.; Kim, J.; et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw. Open 2022, 5, e2213606. [Google Scholar] [CrossRef] [PubMed]
- Boucau, J.; Marino, C.; Regan, J.; Uddin, R.; Choudhary, M.C.; Flynn, J.P.; Chen, G.; Stuckwisch, A.M.; Mathews, J.; Liew, M.Y.; et al. Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection. N. Engl. J. Med. 2022, 387, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mok, B.W.; Chen, L.L.; Chan, J.M.; Tsang, O.T.; Lam, B.H.; Chuang, V.W.; Chu, A.W.; Chan, W.M.; Ip, J.D.; et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021, ciab1041. [Google Scholar] [CrossRef]
- Chen, L.L.; Chu, A.W.; Zhang, R.R.; Hung, I.F.; To, K.K. Serum neutralisation of the SARS-CoV-2 omicron sublineage BA.2. Lancet Microbe 2022, 3, e404. [Google Scholar] [CrossRef]
- Chen, L.L.; Chua, G.T.; Lu, L.; Chan, B.P.; Wong, J.S.; Chow, C.C.; Yu, T.C.; Leung, A.S.; Lam, S.Y.; Wong, T.W.; et al. Omicron variant susceptibility to neutralizing antibodies induced in children by natural SARS-CoV-2 infection or COVID-19 vaccine. Emerg. Microbes Infect. 2022, 11, 543–547. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikane, M.; Ujiie, M.; Iwamoto, N.; Okumura, N.; Sato, T.; Nagashima, M.; Moriya, A.; Suzuki, M.; Hojo, M.; et al. Duration of Infectious Virus Shedding by SARS-CoV-2 Omicron Variant-Infected Vaccinees. Emerg. Infect. Dis. 2022, 28, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Ong, C.P.; Tang, K.; Fan, Y.; Luo, C.; Zhou, R.; Luo, P.; Cheng, Y.; Gray, V.S.; Wang, P.; et al. Intranasal administration of a single dose of a candidate live attenuated vaccine derived from an NSP16-deficient SARS-CoV-2 strain confers sterilizing immunity in animals. Cell. Mol. Immunol. 2022, 19, 588–601. [Google Scholar] [CrossRef]
- Khong, K.W.; Liu, D.; Leung, K.Y.; Lu, L.; Lam, H.Y.; Chen, L.; Chan, P.C.; Lam, H.M.; Xie, X.; Zhang, R.; et al. Antibody Response of Combination of BNT162b2 and CoronaVac Platforms of COVID-19 Vaccines against Omicron Variant. Vaccines 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).