The Genetic Characterization of the First Detected Bat Coronaviruses in Poland Revealed SARS-Related Types and Alphacoronaviruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. RNA Extraction
2.3. Molecular Detection of BtCoVs and Sequencing
2.4. Phylogenetic Analysis
3. Results
3.1. Prevalence of BtCoVs in Different Bat Species in Poland
3.2. Phylogenetic Resemblance of Polish BtCoVs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciechanowski, M. Rząd: Nietoperze–Chiroptera (Order: Chiroptera (bats)–in Polish). In Tom 3 Część 3 Zoologia: Ssaki; Polskie Wydawnictwo Naukowe (PWN): Warsaw, Poland, 2020; pp. 236–304. [Google Scholar]
- Taylor, M. Bats: An Illustrated Guide to All Species; Smithsonian Books: Washington, DC, USA, 2019; ISBN 1588346471. [Google Scholar]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Eaton, B.T. Bats, civets and the emergence of SARS. Curr. Top. Microbiol. Immunol. 2007, 315, 325–344. [Google Scholar]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronaviruses. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [PubMed]
- Wong, A.C.P.; Li, X.; Lau, S.K.P.; Woo, P.C.L. Global Epidemiology of Bat Coronaviruses. Viruses 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.A. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Ben, H.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Nat. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Chia, W.N.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021, 12, 972. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Drosten, C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 2014, 101, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Beltz, L.A. Bats and Human Health: Ebola, SARS, Rabies and Beyond, 1st ed.; Wiley Blackwell: Hoboken, NJ, USA, 2018; p. 111. [Google Scholar]
- Fischer, K.; Zeus, V.; Kwasnitschka, L.; Kerth, G.; Haase, M.; Groshup, M.H.; Balkema-Buschmann, A. Insectivorous bats carry host specific astroviruses and coronaviruses across different regions in Germany. Inf. Genet. Evol. 2016, 37, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lazov, C.M.; Chriel, M.; Baagøe, H.J.; Fjederholt, E.; Deng, Y.; Kooi, E.A.; Belsham, G.J.; Bøtner, A.; Rasmussen, T.B. Detection and characterization of distinct alphacoronaviruses in five different bat species in Denmark. Viruses 2018, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Pir, J.B.; Loesch, C.; Sausy, A.; Snoeck, C.J.; Hübschen, J.M.; Muller, C.P. Novel alphacoronaviruses and paramyxoviruses circulate with type 1 and severe acute respiratory system (SARS)-related betacoronaviruses in synanthropic bats of Luxembourg. Appl. Envirom. Microb. 2017, 83, 18. [Google Scholar] [CrossRef]
- Kemenesi, G.; Dallos, B.; Görföl, T.; Boldoglh, S.; Estók, P.; Kurucz, K.; Kutas, A.; Földes, F.; Oldal, M.; Németh, V.; et al. Molecular survey of RNA viruses in Hungarian bats: Discovering novel astroviruses, coronaviruses, and caliciviruses. Vector Borne Zoonotic Dis. 2014, 14, 846–855. [Google Scholar] [CrossRef]
- Kivisto, I.; Tidenberg, E.M.; Lilley, T.; Suominen, K.; Forbes, K.M.; Vapalahti, O.; Huovilainen, A.; Sironen, T. First report of coronaviruses in Northern European bats. Vector Borne Zoonotic Dis. 2020, 20, 155–158. [Google Scholar] [CrossRef]
- Monchatre-Leroy, E.; Boué, F.; Boucher, J.M.; Renault, C.; Moutou, F.; Gouilch, M.A.; Umhang, G. Identification of alpha and beta coronaviruses in wildlife species in France: Bats, rodents, rabbits, and hedgehogs. Viruses 2017, 9, 364. [Google Scholar] [CrossRef]
- Gloza-Rausch, F.; Ipsen, A.; Seebens, A.; Göttsche, M.; Panning, M.; Drexler, J.F.; Petersen, N.; Annan, A.; Grywna, K.; Müller, M.; et al. Detection and prevalence pattern of group I coronaviruses in bats, Northern Germany. Emerg. Infect. Dis. 2008, 14, 626–631. [Google Scholar] [CrossRef]
- Lelli, D.; Papetti, A.; Sabelli, C.; Rosti, E.; Moreno, A.; Boniotti, M.B. Detection of coronaviruses in bats of various species in Italy. Viruses 2013, 5, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Goffard, A.; Damanche, C.; Arthur, L.; Pinçon, C.; Michaux, J.; Dubuisson, J. Alphacoronaviruses detected in French bats are phylogenetically linked to coronaviruses of European bats. Viruses 2015, 7, 6279–6290. [Google Scholar] [CrossRef]
- De Benedictis, P.; Marciano, S.; Scaravelli, D.; Priori, P.; Zecchin, B.; Capua, I.; Monne, I.; Cattoli, G. Alpha and lineage C betaCoV infection in Italian bats. Virus Genes 2014, 48, 366–371. [Google Scholar] [CrossRef]
- Rizzo, F.; Edenbourgh, K.M.; Toffoli, R.; Culasso, P.; Zoppi, S.; Dondo, A.; Robetto, S.; Rosati, S.; Lander, A.; Kurth, A.; et al. Coronavirus and paramyxovirus in bats from Northern Italy. BMC Vet. Res. 2017, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Goulh, M.A.; Puechmaille, J.S.; Diancourt, L.; Vandenbogaert, M.; Serra-Cobo, J.; Roïg, M.L.; Brown, P.; Moutou, F.; Caro, V.; Vabret, A.; et al. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology 2018, 517, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hardmeier, I.; Aeberhard, N.; Qi, W.; Schoenbaechler, K.; Kraettli, H.; Hatt, J.M.; Fraefel, C.; Kubacki, J. Metagenomic analysis of fecal and tissue samples from 18 endemic bat species in Switzerland revealed a diverse virus composition including potentially zoonotic viruses. PLoS ONE 2021, 16, e0252534. [Google Scholar] [CrossRef]
- Rihtarič, D.; Hostnik, P.; Steyer, P.; Grom, J.; Toplak, I. Identification of SARS-like coronaviruses in horseshoe bats (Rhinolophus hipposideros) in Slovenia. Arch. Virol. 2010, 155, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Palladini, A.; Bogliani, G.; Battiliani, M. Detection of a virus related to betacoronaviruses in Italian greater horseshoe bats. Epidemiol. Infect. 2011, 139, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Gloza-Rausch, F.; Glende, J.; Corman, V.J.; Muth, D.; Goettsche, M.; Seebens, A.; Niedrig, M.; Pfefferle, S.; Yordanov, S.; et al. Genomic characterization of severe acute respiratory syndrome–related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J. Virol. 2010, 84, 11336–11349. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.; Murphy, I.; Carter, D.; Pullan, S.; Carroll, M.; Vipond, R.; Cunningham, A.A.; Bell, D. Metagenomic identification of a new sarbecovirus from horseshoe bats in Europe. Sci. Rep. 2021, 11, 14723. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- De Souza-Luna, L.K.; Heiser, V.; Regamey, N.; Panning, M.; Drexler, J.F.; Mulangu, S.; Poon, L.; Baumgarte, S.; Haijema, B.J.; Kaiser, L.; et al. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription-PCR and nonfluorescent low-density microarray. J. Clin. Microbiol. 2007, 45, 1049–1052. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- The Virus Hunters Who Search Bat Caves to Predict the Next Pandemic. Available online: https://edition.cnn.com/2020/04/26/health/virus-hunters-bat-cave-coronavirus-hnk-intl/index.html (accessed on 30 June 2022).
- Ge, X.Y.; Li, J.L.; Yang, X.L.; Chmura, A.A.; Zhu, G.G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Latinne, A.; Hu, B.; Olival, K.J.; Zhu, G.; Zhang, L.; Li, H.; Chmura, A.A.; Field, H.E.; Zambrana-Torrelio, C.; Epstein, J.H.; et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020, 11, 4235. [Google Scholar] [CrossRef] [PubMed]
- The Detection and Isolation of Bat Sarbecoviruses from Several Locations in Japan. Available online: https://www.news-medical.net/news/20220519/The-detection-and-isolation-of-bat-sarbecoviruses-from-several-locations-in-Japan.aspx (accessed on 1 July 2022).
- Murakami, S.; Kitamura, T.; Suzuki, J.; Sato, R.; Aoi, T.; Fujii, M.; Matsugo, H.; Kamiki, H.; Ishida, H.; Takenaka-Uema, A.; et al. Detection and Characterization of Bat Sarbecovirus Phylogenetically Related to SARS-CoV-2, Japan. Emerg. Infect. Dis. 2020, 26, 3025–3029. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Kosicki, J.Z.; Tryjanowski, P.; Nowak, J.; Węgiel, J. Long-term population trends of the lesser horseshoe bat Rhinolophus hipposideros and the greater mouse-eared bat Myotis myotis in Poland. Eur. Zool. J. 2021, 88, 1189–1200. [Google Scholar] [CrossRef]
- Kohl, C.; Nitsche, A.; Kurth, A. Update on potentially zoonotic viruses of European bats. Vaccines 2021, 9, 690. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O.; Nill, D. Handbook of the Bats of Europe and Northwestern Africa: Biology, Identification and Conservation Status; Franckh-Kosmos Verlags GmbH & Co. KG: Stuttgart, Germany, 2007; ISBN 9781408105313. [Google Scholar]
- Drexler, J.F.; Corman, V.M.; Wegner, T.; Tateno, A.F.; Zerbinati, R.M.; Gloza-Rausch, F.; Seebens, A.; Müller, M.A.; Drosten, C. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011, 17, 449–456. [Google Scholar] [CrossRef]
- MacLean, O.A.; Lytras, S.; Weaver, S.; Singer, J.B.; Boni, M.F.; Lemey, P.; Kosakovsky Pond, S.L.; Robertson, D.L. Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen. PLoS Biol. 2021, 19, e3001115. [Google Scholar] [CrossRef]
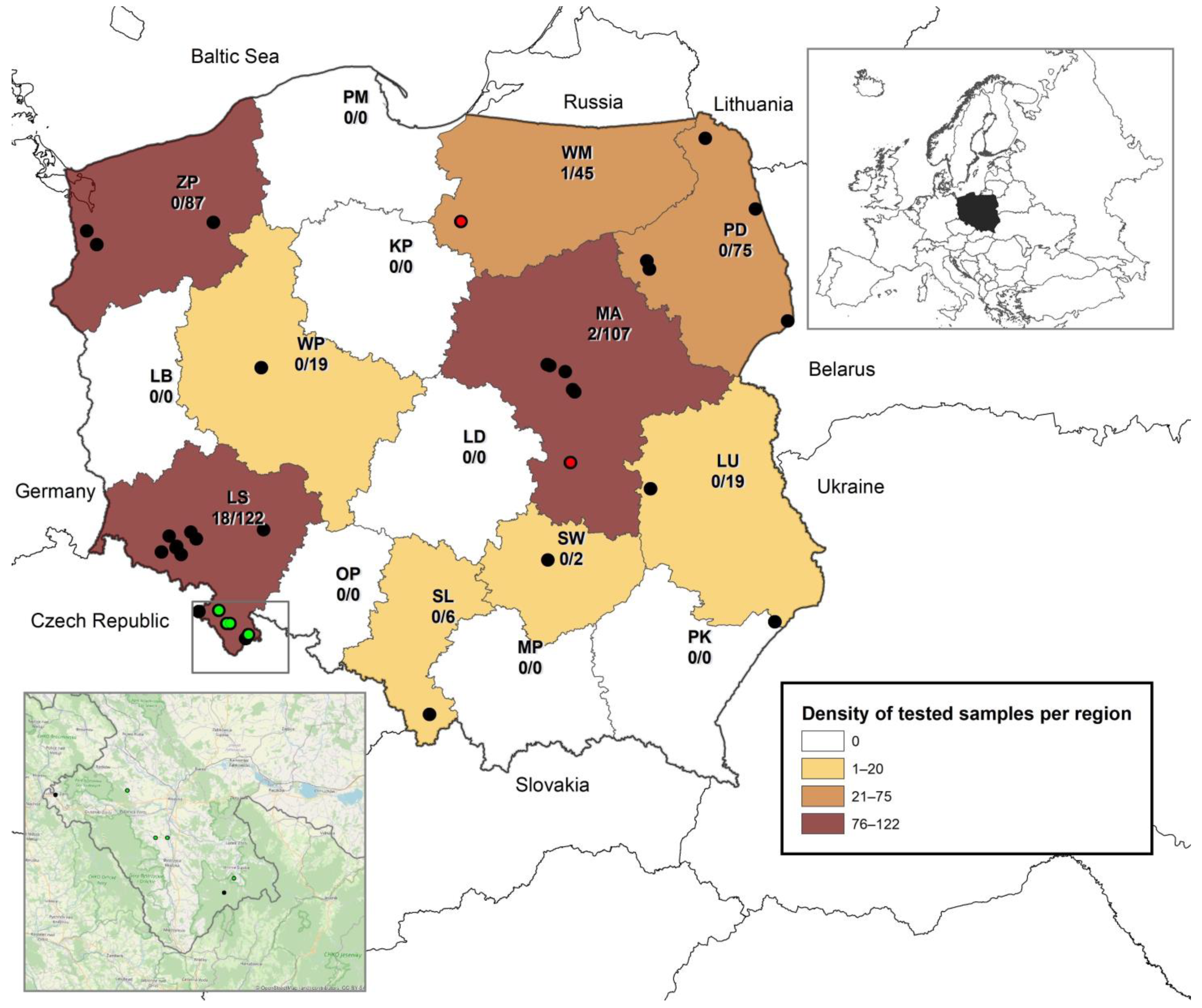
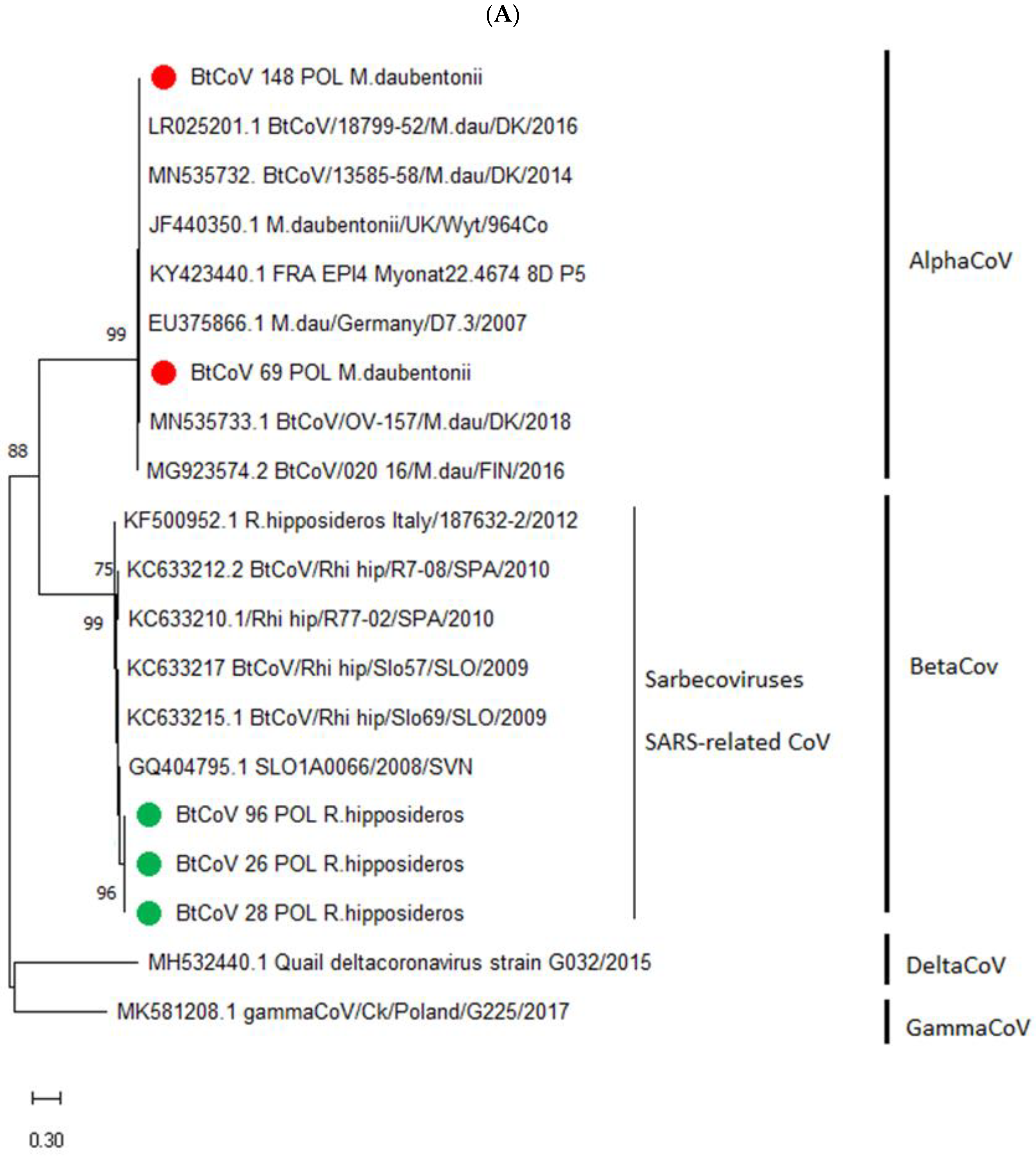
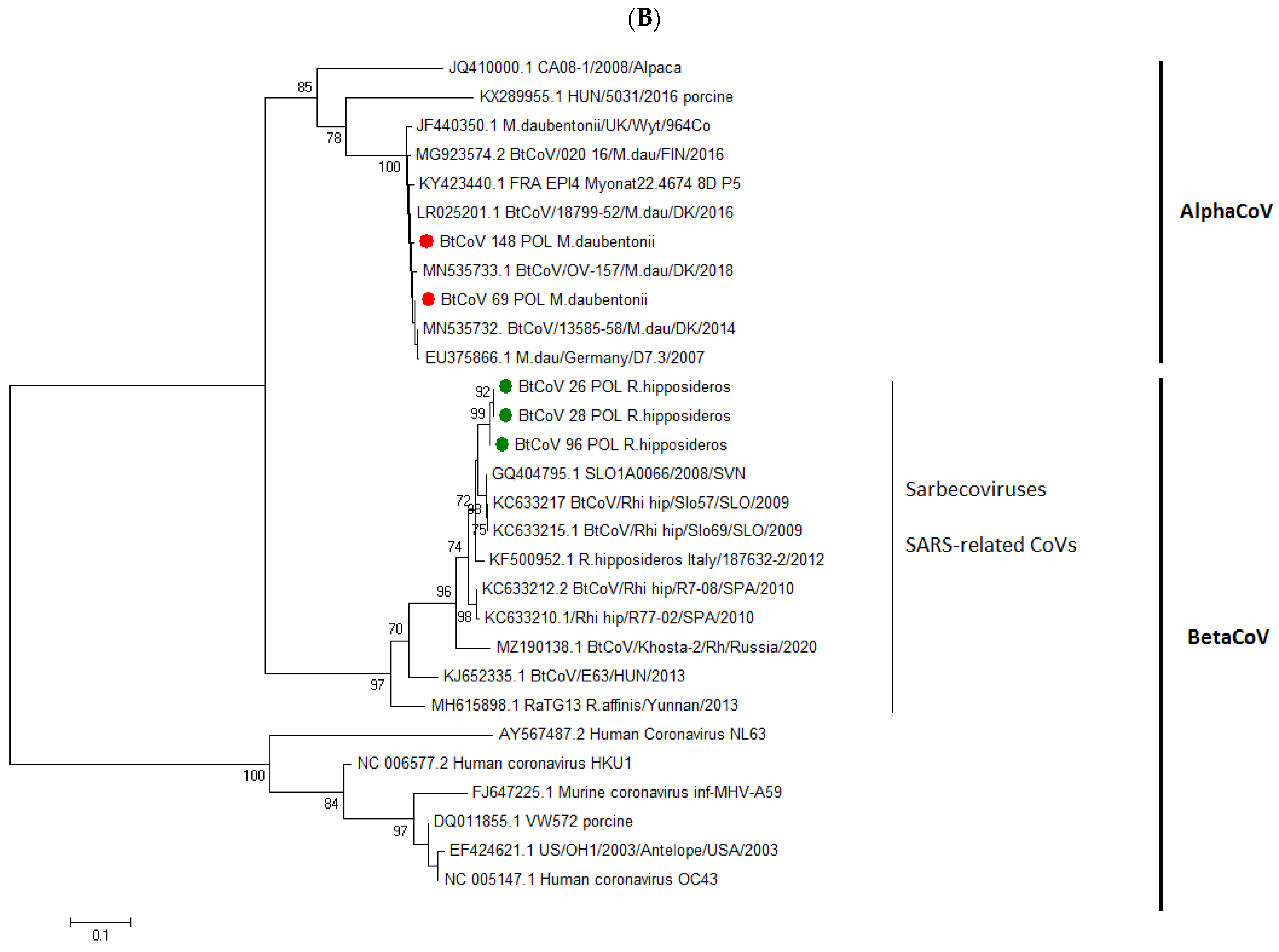
| Species | No of Samples | No of Positives rtRT-qPCR | No of Positives nRT-PCR | Total No of Positives | Positives % (95% CI) |
|---|---|---|---|---|---|
| Eptesicus serotinus | 57 | 0 | 0 | 0 | 0% (0.0–6.3) |
| Nyctalus noctula | 79 | 0 | 0 | 0 | 0% (0.0–4.6) |
| Nyctalus leisleri | 3 | 0 | 0 | 0 | 0% (0.0–56.1) |
| Barbastella barbastellus | 55 | 0 | 0 | 0 | 0% (0.0–6.5) |
| Plecotus auritus | 30 | 0 | 0 | 0 | 0% (0.0–11.4) |
| Vespertillo murinus | 12 | 0 | 0 | 0 | 0% (0.0–24.2) |
| Pipistrellus pygmaeus | 29 | 0 | 0 | 0 | 0% (0–11.7) |
| Pipistrellus pipistrellus | 10 | 0 | 0 | 0 | 0% (0–27.8) |
| Pipistrellus spp. | 6 | 0 | 0 | 0 | 0% (0.0–39.0) |
| Pipistrellus nathusii | 19 | 0 | 0 | 0 | 0% (0.0–16.8) |
| Myotis dasycneme | 3 | 0 | 0 | 0 | 0% (0.0–56.1) |
| Myotis daubentonii | 55 | 0 | 2 (F) | 2 (F) | 3.6% (0.6–12.3) |
| Myotis myotis | 4 | 0 | 0 | 0 | 0% (0–49.0) |
| Myotis mystacinus | 6 | 0 | 0 | 0 | 0% (0–39.0) |
| Myotis nattereri | 22 | 0 | 0 | 0 | 0% (0–14.9) |
| Myotis bechsteinii | 8 | 0 | 0 | 0 | 0% (0–32.4) |
| Myotis alcathoe | 1 | 0 | 0 | 0 | 0% (0–94.9) |
| Myotis brandtii | 7 | 0 | 0 | 0 | 0% (0–35.4) |
| Myotis emarginatus | 1 | 0 | 0 | 0 | 0% (0–94.9) |
| Rhinolophus hipposideros | 58 | 18 (14 O; 4 F) | 3 (F) | 18 (14 O; 4 F) | 31% (20.6–43.8) |
| unidentified | 38 | 0 | 0 | 0 | 0% (0.0–9.2) |
| Total | 503 | 20 (14 O; 6 F) | 4.0% (2.6–6.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orłowska, A.; Smreczak, M.; Thor, K.; Niedbalska, M.; Pawelec, D.; Trębas, P.; Rola, J. The Genetic Characterization of the First Detected Bat Coronaviruses in Poland Revealed SARS-Related Types and Alphacoronaviruses. Viruses 2022, 14, 1914. https://doi.org/10.3390/v14091914
Orłowska A, Smreczak M, Thor K, Niedbalska M, Pawelec D, Trębas P, Rola J. The Genetic Characterization of the First Detected Bat Coronaviruses in Poland Revealed SARS-Related Types and Alphacoronaviruses. Viruses. 2022; 14(9):1914. https://doi.org/10.3390/v14091914
Chicago/Turabian StyleOrłowska, Anna, Marcin Smreczak, Katarzyna Thor, Magda Niedbalska, Dominika Pawelec, Paweł Trębas, and Jerzy Rola. 2022. "The Genetic Characterization of the First Detected Bat Coronaviruses in Poland Revealed SARS-Related Types and Alphacoronaviruses" Viruses 14, no. 9: 1914. https://doi.org/10.3390/v14091914
APA StyleOrłowska, A., Smreczak, M., Thor, K., Niedbalska, M., Pawelec, D., Trębas, P., & Rola, J. (2022). The Genetic Characterization of the First Detected Bat Coronaviruses in Poland Revealed SARS-Related Types and Alphacoronaviruses. Viruses, 14(9), 1914. https://doi.org/10.3390/v14091914






