Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
2.4. Institutional Review Board Approval
3. Results
3.1. Baseline Characteristics
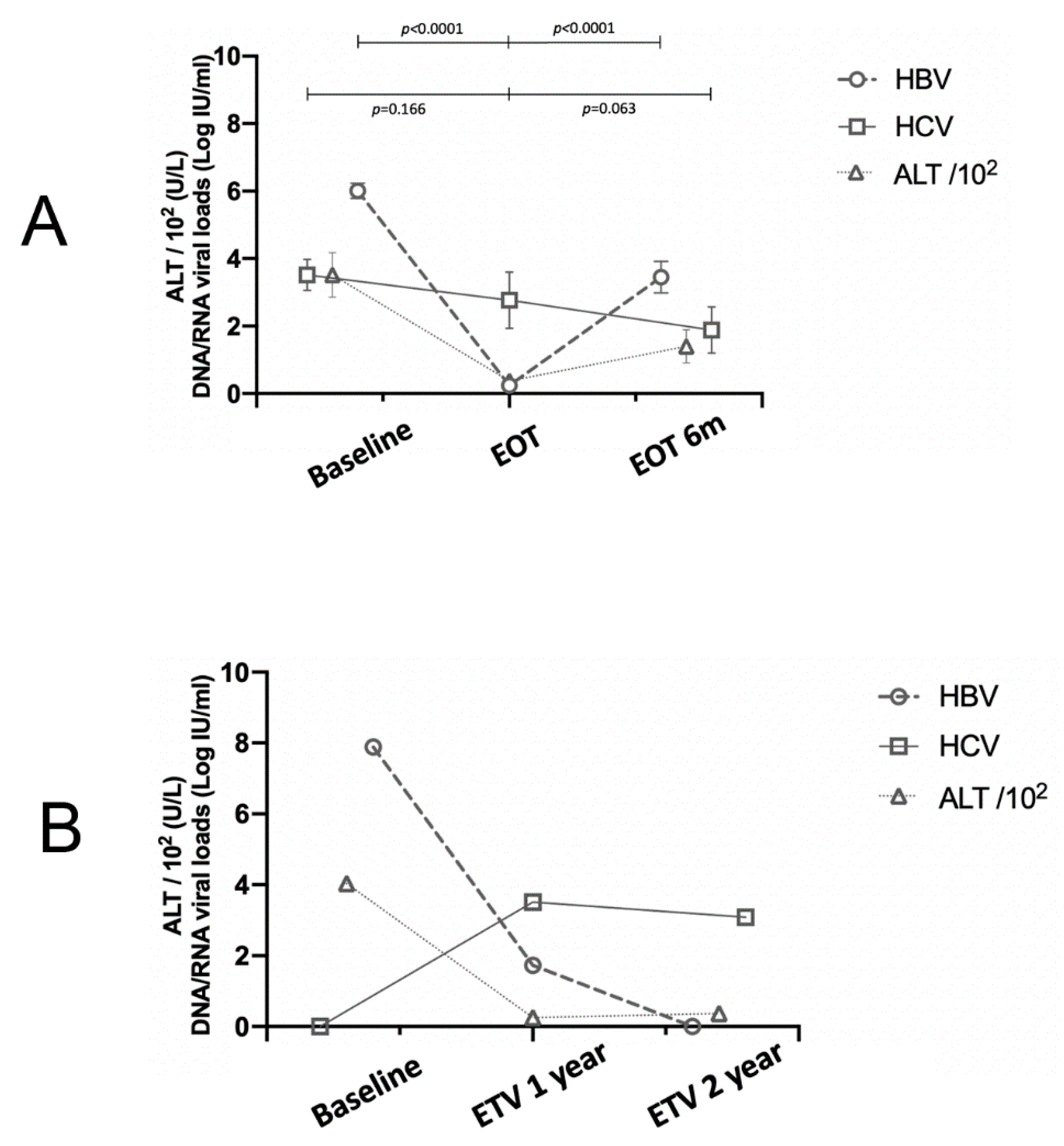
3.2. The Evolutionary Trends of HBV and HCV Viral Loads
3.3. Long-Term Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shih, Y.-F.; Liu, C.-J. Hepatitis C Virus and Hepatitis B Virus Co-Infection. Viruses 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Wu, G.Y. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J. Clin. Transl. Hepatol. 2018, 6, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaw, Y.F. Hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. J. Gastroenterol. 2002, 37 (Suppl. S13), 65–68. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F. Role of hepatitis C virus in dual and triple hepatitis virus infection. Hepatology 1995, 22, 1101–1108. [Google Scholar] [PubMed]
- Chien, R.-N.; Kao, J.-H.; Peng, C.-Y.; Chen, C.-H.; Liu, C.-J.; Huang, Y.-H.; Hu, T.-H.; Yang, H.-I.; Lu, S.-N.; Ni, Y.-H.; et al. Taiwan consensus statement on the management of chronic hepatitis B. J. Formos. Med. Assoc. 2019, 118, 7–38. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Kao, C.-F.; Chen, C.-M.; Shih, C.-M.; Hsu, M.-J.; Chao, C.-H.; Wang, S.-H.; You, L.-R.; Lee, Y.-H.W. Mechanisms for Inhibition of Hepatitis B Virus Gene Expression and Replication by Hepatitis C Virus Core Protein. J. Biol. Chem. 2003, 278, 591–607. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.F.; Yeh, C.T.; Tsai, S.L. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am. J. Gastroenterol. 2000, 95, 2978–2980. [Google Scholar] [CrossRef]
- Mücke, M.M.; Backus, L.I.; Mücke, V.T.; Coppola, N.; Preda, C.M.; Yeh, M.-L.; Tang, L.S.Y.; Belperio, P.S.; Wilson, E.M.; Yu, M.-L.; et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 172–180. [Google Scholar] [CrossRef]
- Pisaturo, M.; Macera, M.; Alessio, L.; Calò, F.; Coppola, N. Hepatitis B Virus (HBV) Reactivation Following Pharmacological Eradication of Hepatitis C Virus (HCV). Viruses 2019, 11, 850. [Google Scholar] [CrossRef] [Green Version]
- Liaw, Y.-F.; Chien, R.-N.; Lin, S.-M.; Yeh, C.-T.; Tsai, S.-L.; Sheen, I.-S.; Chu, C.-M. Response of Patients with Dual Hepatitis B Virus and C Virus Infection to Interferon Therapy. J. Interf. Cytokine Res. 1997, 17, 449–452. [Google Scholar] [CrossRef]
- Chang, M.-L.; Chang, S.-W.; Chen, S.-C.; Chien, R.-N.; Hsu, C.-L.; Chang, M.-Y.; Fann, C. Genetic Association of Hepatitis C-Related Mixed Cryoglobulinemia: A 10-Year Prospective Study of Asians Treated with Antivirals. Viruses 2021, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; Hosry, J.; Mahale, P.; Economides, M.P.; Jiang, Y.; Lok, A.S. Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study. Hepatology 2018, 67, 36–47. [Google Scholar] [CrossRef]
- Chang, M.-L.; Cheng, J.-S.; Chien, R.-N.; Liaw, Y.-F. Hepatitis Flares Are Associated With Better Outcomes Than No Flare in Patients With Decompensated Cirrhosis and Chronic Hepatitis B Virus Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2064–2072.e2. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-L.; Liaw, Y.-F. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J. Hepatol. 2014, 61, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-L.; Jeng, W.-J.; Liaw, Y.-F. Clinical Events After Cessation of Lamivudine Therapy in Patients Recovered From Hepatitis B Flare With Hepatic Decompensation. Clin. Gastroenterol. Hepatol. 2015, 13, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.-F.; Kao, J.-H.; Piratvisuth, T.; Chan, H.L.Y.; Chien, R.-N.; Liu, C.-J.; Gane, E.; Locarnini, S.; Lim, S.-G.; Han, K.-H.; et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol. Int. 2012, 6, 531–561. [Google Scholar] [CrossRef]
- Airewele, N.E.; Shiffman, M.L. Chronic Hepatitis B Virus in Patients with Chronic Hepatitis C Virus. Clin. Liver Dis. 2021, 25, 817–829. [Google Scholar] [CrossRef]
- Pockros, P.J. Black Box Warning for Possible HBV Reactivation During DAA Therapy for Chronic HCV Infection. Gastroenterol. Hepatol. 2017, 13, 536–540. [Google Scholar]
- Joyce, M.A.; Berry-Wynne, K.M.; dos Santos, T.; Addison, W.R.; McFarlane, N.; Hobman, T.; Tyrrell, D.L. HCV and flaviviruses hijack cellular mechanisms for nuclear STAT2 degradation: Up-regulation of PDLIM2 suppresses the innate immune response. PLoS Pathog. 2019, 15, e1007949. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, T.; Naganuma, A.; Tateyama, Y.; Yoshinari, F.; Hoshino, T.; Sato, K.; Hmwe, S.S.; Aizaki, H.; Wakita, T.; Kakizaki, S.; et al. Ledipasvir and Sofosbuvir for Acute Hepatitis C Virus Monoinfection Associated with a High Risk of Acute Liver Failure. Intern. Med. 2019, 58, 2969–2975. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, T.; Yan, Y. Sofosbuvir and ribavirin in acute hepatitis C–infected patient with decompensated cirrhosis. Medicine 2016, 95, e5555. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Takaki, A.; Yagi, T.; Ikeda, F.; Yasunaka, T.; Koike, K.; Miyake, Y.; Iwasaki, Y.; Nouso, K.; Sadamori, H.; et al. A case of fulminant liver failure associated with hepatitis C virus. Clin. J. Gastroenterol. 2014, 7, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, E.; Zuckerman, T.; Douer, D.; Qian, D.; Levine, A.M. Liver dysfunction in patients infected with hepatitis C virus undergoing chemotherapy for hematologic malignancies. Cancer 1998, 15, 1224–1230. [Google Scholar] [CrossRef]
- Liaw, Y.-F.; Tsai, S.-L.; Chang, J.-J.; Sheen, I.-S.; Chien, R.-N.; Lin, D.-Y.; Chu, C.-M. Displacement of hepatitis B virus by hepatitis C virus as the cause of continuing chronic hepatitis. Gastroenterology 1994, 106, 1048–1053. [Google Scholar] [CrossRef]
- Schüttler, C.G.; Fiedler, N.; Schmidt, K.; Repp, R.; Gerlich, W.H.; Schaefer, S. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J. Hepatol. 2002, 37, 855–862. [Google Scholar] [CrossRef]
- Bellecave, P.; Gouttenoire, J.; Gajer, M.; Brass, V.; Koutsoudakis, G.; Blum, H.E.; Bartenschlager, R.; Nassal, M.; Moradpour, D. Hepatitis B and C virus coinfection: A novel model system reveals the absence of direct viral interference. Hepatology 2009, 50, 46–55. [Google Scholar] [CrossRef]
- Eyre, N.S.; Phillips, R.J.; Bowden, S.; Yip, E.; Dewar, B.; Locarnini, S.A.; Beard, M.R. Hepatitis B virus and hepatitis C virus interaction in Huh-7 cells. J. Hepatol. 2009, 51, 446–457. [Google Scholar] [CrossRef]
- Wiegand, S.; Jaroszewicz, J.; Potthoff, A.; zu Siederdissen, C.H.; Maasoumy, B.; Deterding, K.; Manns, M.; Wedemeyer, H.; Cornberg, M. Dominance of hepatitis C virus (HCV) is associated with lower quantitative hepatitis B surface antigen and higher serum interferon-γ-induced protein 10 levels in HBV/HCV-coinfected patients. Clin. Microbiol. Infect. 2015, 21, 710.e1–710.e9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Uchida, T.; Xia, Y.; Umarova, R.; Liu, C.-J.; Chen, P.-J.; Gaggar, A.; Suri, V.; Mücke, M.M.; Vermehren, J.; et al. Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection. J. Clin. Investig. 2020, 130, 3205–3220. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-L.; Liaw, Y.-F. Hepatitis B Flare in Hepatitis B e Antigen-Negative Patients: A Complicated Cascade of Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2022, 23, 1552. [Google Scholar] [CrossRef]
- Mutz, P.; Metz, P.; Lempp, F.A.; Bender, S.; Qu, B.; Schöneweis, K.; Seitz, S.; Tu, T.; Restuccia, A.; Frankish, J.; et al. HBV Bypasses the Innate Immune Response and Does Not Protect HCV From Antiviral Activity of Interferon. Gastroenterology 2018, 154, 1791–1804.e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.-R.; Kao, J.-H.; Wu, H.-L.; Tseng, T.-C.; Liu, C.-H.; Yang, H.-C.; Su, T.-H.; Chen, P.-J.; Chen, D.-S.; Liu, C.-J. Clinical significance of circulating miR-122 in patients with dual chronic hepatitis B and C virus infection. Hepatol. Int. 2015, 9, 35–42. [Google Scholar] [CrossRef]
- Squadrito, G.; Orlando, M.E.; Pollicino, T.; Raffa, G.; Restuccia, T.; Cacciola, I.; Di Marco, V.; Picciotto, A.; Colucci, G.; Craxì, A.; et al. Virological profiles in patients with chronic hepatitis C and overt or occult HBV infection. Am. J. Gastroenterol. 2002, 97, 1518–1523. [Google Scholar] [CrossRef]
- Coffin, C.S.; Mulrooney-Cousins, P.M.; Lee, S.S.; Michalak, T.I.; Swain, M.G. Profound suppression of chronic hepatitis C following superinfection with hepatitis B virus. Liver Int. 2007, 27, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Chen, P.-J. Elimination of Hepatitis B in Highly Endemic Settings: Lessons Learned in Taiwan and Challenges Ahead. Viruses 2020, 12, 815. [Google Scholar] [CrossRef]
- Liu, C.; Chen, P. Changing epidemiology of liver disease in Asia: Dual infection of HBV and HCV. Liver Int. 2021, 42, 1945–1954. [Google Scholar] [CrossRef]
- Adland, E.; Jesuthasan, G.; Downs, L.; Wharton, V.; Wilde, G.; McNaughton, A.L.; Collier, J.; Barnes, E.; Klenerman, P.; Andersson, M.; et al. Hepatitis virus (HCV) diagnosis and access to treatment in a UK cohort. BMC Infect. Dis. 2018, 18, 461. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, H.; Tamura, I.; Yamada, O.; Takezaki, E.-I.; Koda, T.; Kurimura, O.; Kurimura, T. Hepatitis C virus rna and hepatitis C virus antibody in the serum of patients with abnormal liver function. J. Infect. 1992, 25, 47–53. [Google Scholar] [CrossRef]
- Liu, C.-J.; Kao, J.-H. Global Perspective on the Natural History of Chronic Hepatitis B: Role of Hepatitis B Virus Genotypes A to J. Semin. Liver Dis. 2013, 33, 97–102. [Google Scholar] [CrossRef]
- Castillo, I.; Bartolomé, J.; Quiroga, J.A.; Barril, G.; Carreño, V. Presence of HCV-RNA after ultracentrifugation of serum samples during the follow-up of chronic hepatitis C patients with a sustained virological response may predict reactivation of hepatitis C virus infection. Aliment. Pharmacol. Ther. 2009, 30, 477–486. [Google Scholar] [CrossRef]
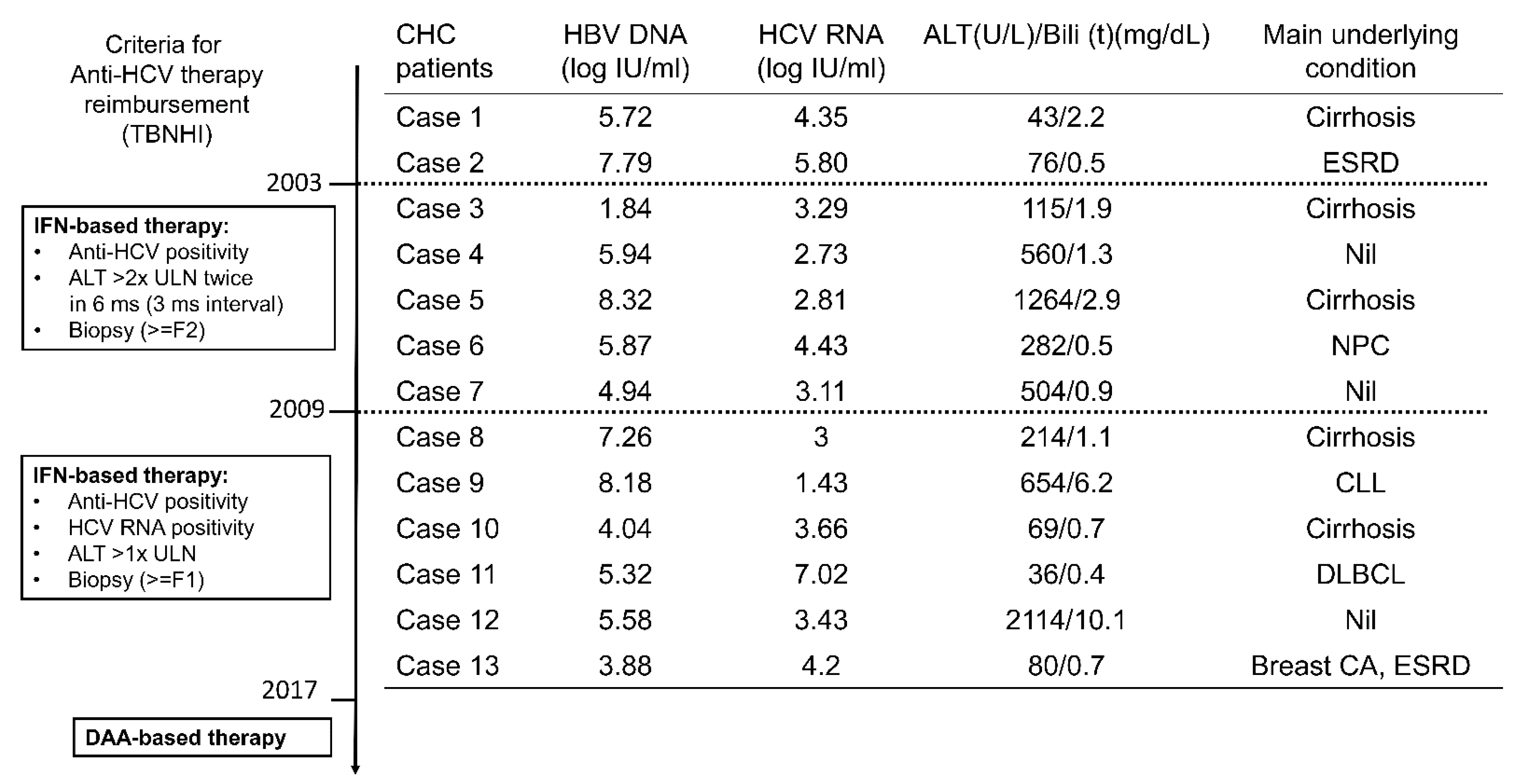
| All (n = 66) | CHC (n = 13) | Resolved Past HCV Infection (n = 53) | p Values | |
|---|---|---|---|---|
| Age (years) * | 52.1 ± 10.9 (51.5; 32–76) | 52.5 ± 14.0 (53; 35–71) | 51.9 ± 10.2 (51; 32–76) | 0.8796 |
| Male, n (%) | 40 (60.6) | 7 (53.8) | 33 (62.3) | 0.7526 |
| HBV genotype, n (%) | ||||
| B | 51 (77.3) | 13 (100) | 38 (71.7) | 0.0298 |
| C | 15 (22.7) | 0 | 15 (28.3) | 0.0298 |
| HBeAg positivity, n (%) | 15 (22.7) | 3 (23.1) | 12 (22.6) | >0.9999 |
| Cirrhosis, n (%) | 33 (50.0) | 5 (38.5) | 28 (52.8) | 0.5372 |
| Decompensation, n (%) | 2 (3.0) | 0 (0) | 2 (3.8) | 0.4769 |
| HBV DNA (log IU/mL) * | 6.05 ± 1.88 (5.94; 1.84–9.82) | 5.75 ± 1.86 (5.72; 1.84–8.32) | 6.13 ± 1.89 (6.2; 2.04–9.82) | 0.5155 |
| HCV RNA (log IU/mL) * | 0.75 ± 1.64 (0; 0–7.02) | 3.79 ± 1.43 (3.42; 1.43–7.02) | undetectable | <0.0001 |
| ALT (U/L) * | 362.3 ± 545.5 (142; 19–2735) | 494.7 ± 624 (248; 36–2114) | 332.4 ± 528.2 (129; 19–2735) | 0.3561 |
| Bili (t) (mg/dL) * | 2.2 ± 3.57 (0.95; 0.4–22) | 2.39 ± 2.92 (1.2; 0.4–10.1) | 2.15 ± 3.73 (0.9; 0.4–22) | 0.8371 |
| INR * | 1.20 ± 0.23 (1.2, 1–2.1) | 1.17 ± 0.21 (1.1; 1–1.4) | 1.2 ± 0.23 (1.2; 1–2.1) | 0.7951 |
| Albumin (g/dL) * | 3.92 ± 0.68 (4.05; 2.3–4.9) | 3.97 ± 0.59 (4.2; 2.7–4.8) | 3.91 ± 0.71 (4; 2.3–4.9) | 0.8033 |
| AFP (ng/mL) * | 66.6 ± 380.1 (5; 2–3031) | 56.9 ± 157.7 (5; 3–556) | 68.7 ± 415.4 (5.3; 2–3031) | 0.9232 |
| Platelet (103/uL) * | 175 ± 64.9 (174.5; 38–342) | 178.4 ± 64.9 (189; 85–279) | 174.3 ± 65.5 (174; 38–342) | 0.8558 |
| Creatinine (mg/dl) * | 1.02 ± 0.87 (0.86; 0.5–7) | 1.05 ± 0.40 (0.99; 0.54–2) | 1.01 ± 0.95 (0.8; 0.5–7) | 0.8975 |
| Nucs, n (%) | ||||
| Ldt | 1 (1.5) | 0 (0) | 1 (1.9) | >0.9999 |
| LAM | 15 (22.7) | 7 (53.8) | 8 (15.1) | 0.0065 |
| ETV | 40 (60.6) | 5 (38.5) | 35 (66.0) | 0.1115 |
| TDF | 8 (12.1) | 1 (7.7) | 7 (13.2) | >0.9999 |
| TAF | 2 (3.0) | 0 (0) | 2 (3.8) | >0.9999 |
| Baseline | EOT | 6mEOT | Underlying Conditions | ||
|---|---|---|---|---|---|
| Case 1 | HCV RNA (logIU/mL) | 3.42 | 5.85 | 5.31 | Dyslipidemia |
| HBV DNA (logIU/mL) | 5.58 | undetectable | undetectable | ||
| ALT (U/L) | 2114 | 49 | 62 | ||
| Case 2 | HCV RNA (logIU/mL) | 2.81 | 5.20 | 3.59 | Cirrhosis |
| HBV DNA (logIU/mL) | 8.32 | undetectable | 2.68 | ||
| ALT (U/L) | 1264 | 27 | 28 |
| All (n = 66) | CHC (n = 13) | Resolved Past HCV Infection (n = 53) | p Values | |
|---|---|---|---|---|
| Follow-up duration (m) * | 84.8 ± 53.6 (79.5; 8–213) | 99.0 ± 66.4 (79; 25–213) | 81.3 ± 50.1(80; 8–193) | 0.2903 |
| Treatment duration (m) * | 46.2 ± 42.1 (36; 5–177) | 37.5 ± 51.9 (14; 5–177) | 48.3 ± 39.7(36; 8–161) | 0.4117 |
| Outcomes | ||||
| Mortality, n (%) | 7 (10.6) | 4 (30.8) | 3 (5.7) | 0.0239 |
| HCC, n (%) | 6 (9.1) | 2 (15.4) | 4 (7.5) | 0.3367 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-T.; Chang, M.-L.; Chien, R.-N.; Liaw, Y.-F. Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies. Viruses 2022, 14, 1858. https://doi.org/10.3390/v14091858
Su Y-T, Chang M-L, Chien R-N, Liaw Y-F. Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies. Viruses. 2022; 14(9):1858. https://doi.org/10.3390/v14091858
Chicago/Turabian StyleSu, Yi-Tse, Ming-Ling Chang, Rong-Nan Chien, and Yun-Fan Liaw. 2022. "Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies" Viruses 14, no. 9: 1858. https://doi.org/10.3390/v14091858
APA StyleSu, Y.-T., Chang, M.-L., Chien, R.-N., & Liaw, Y.-F. (2022). Hepatitis C Virus Reactivation in Anti-HCV Antibody-Positive Patients with Chronic Hepatitis B Following Anti-HBV Therapies. Viruses, 14(9), 1858. https://doi.org/10.3390/v14091858







