Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Sample Collection
2.3. PCR Amplification
2.4. Illumina MiSeq Sequencing
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Taxonomy and Alpha Diversity of the Four Groups
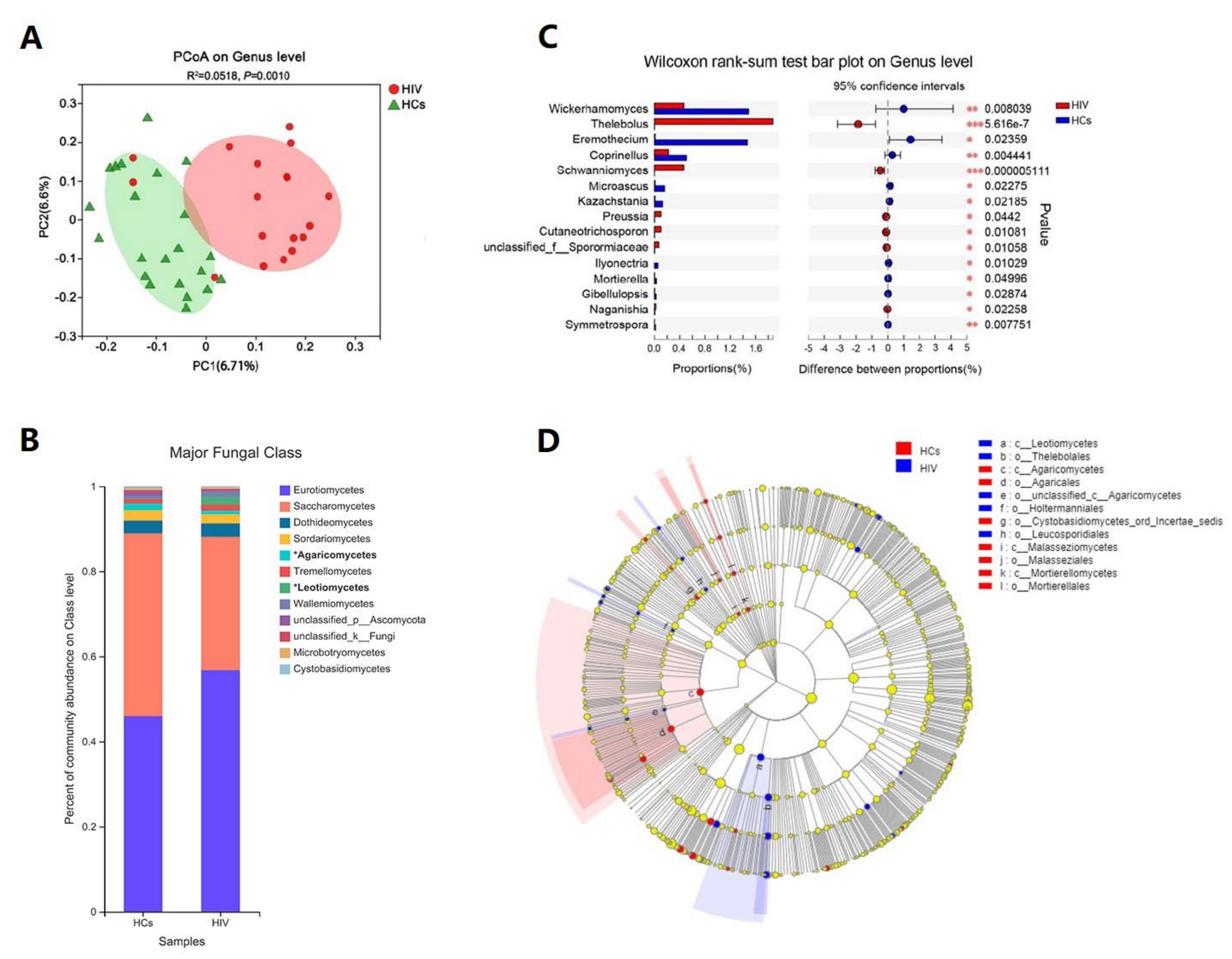
3.3. HIV Mono-Infected Patients Had an Altered Gut Mycobiome Compared to the HC Group
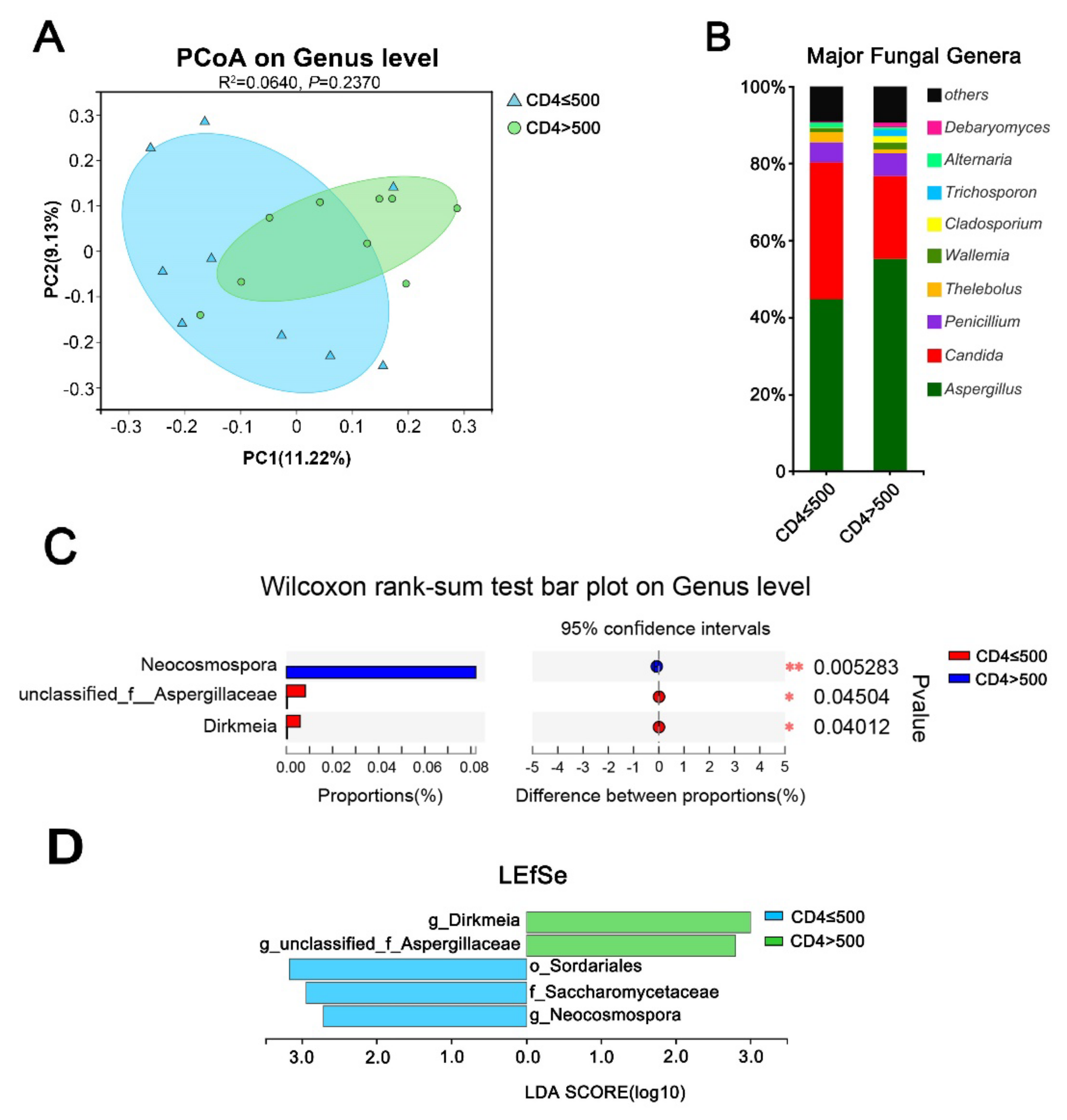
3.4. Immune Status Significantly Impacts the Gut Mycobiome of HIV Mono-Infected Patients
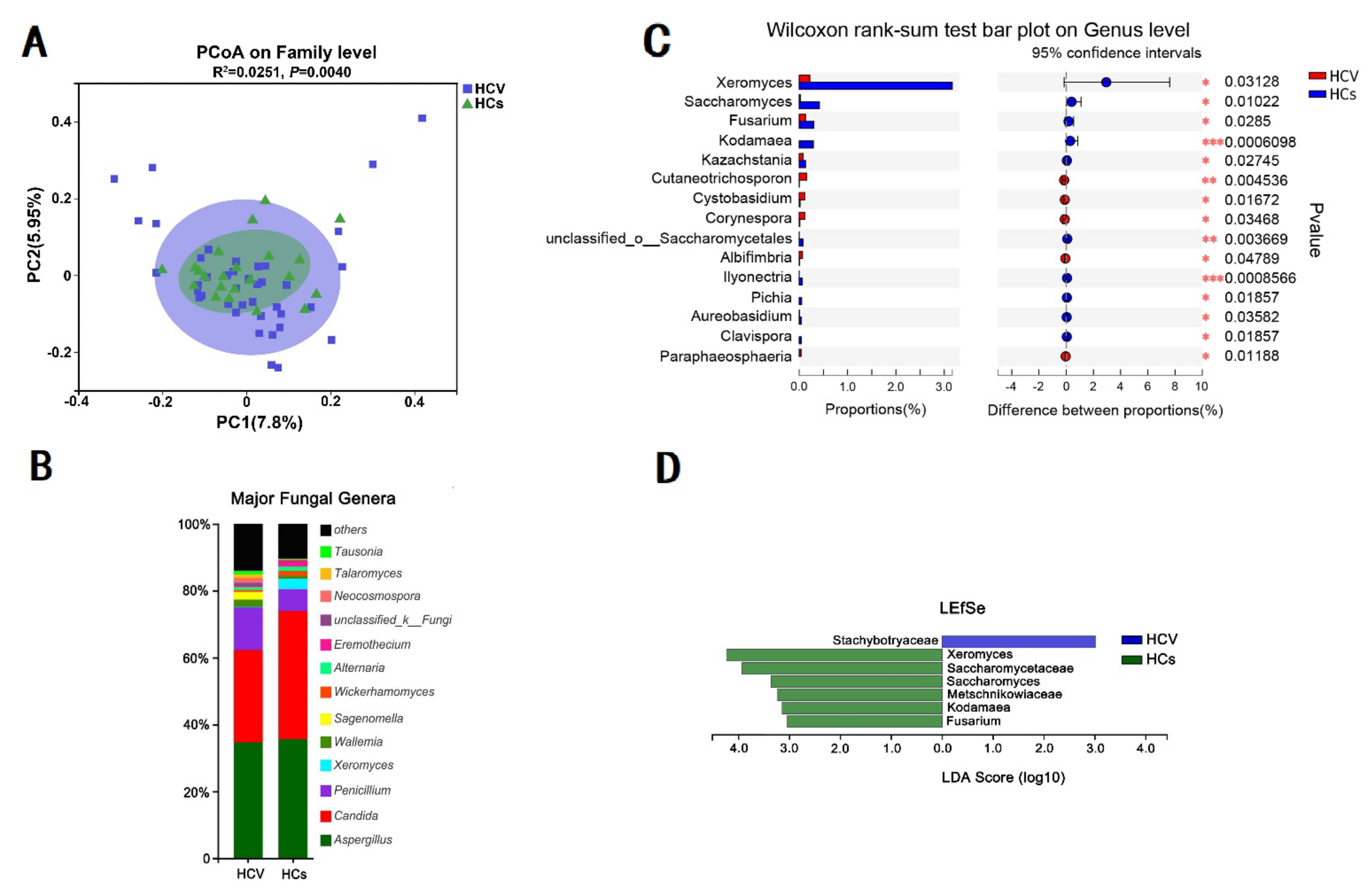
3.5. HCV Mono-Infected Individuals Had an Altered Gut Mycobiome Compared to the HC Group
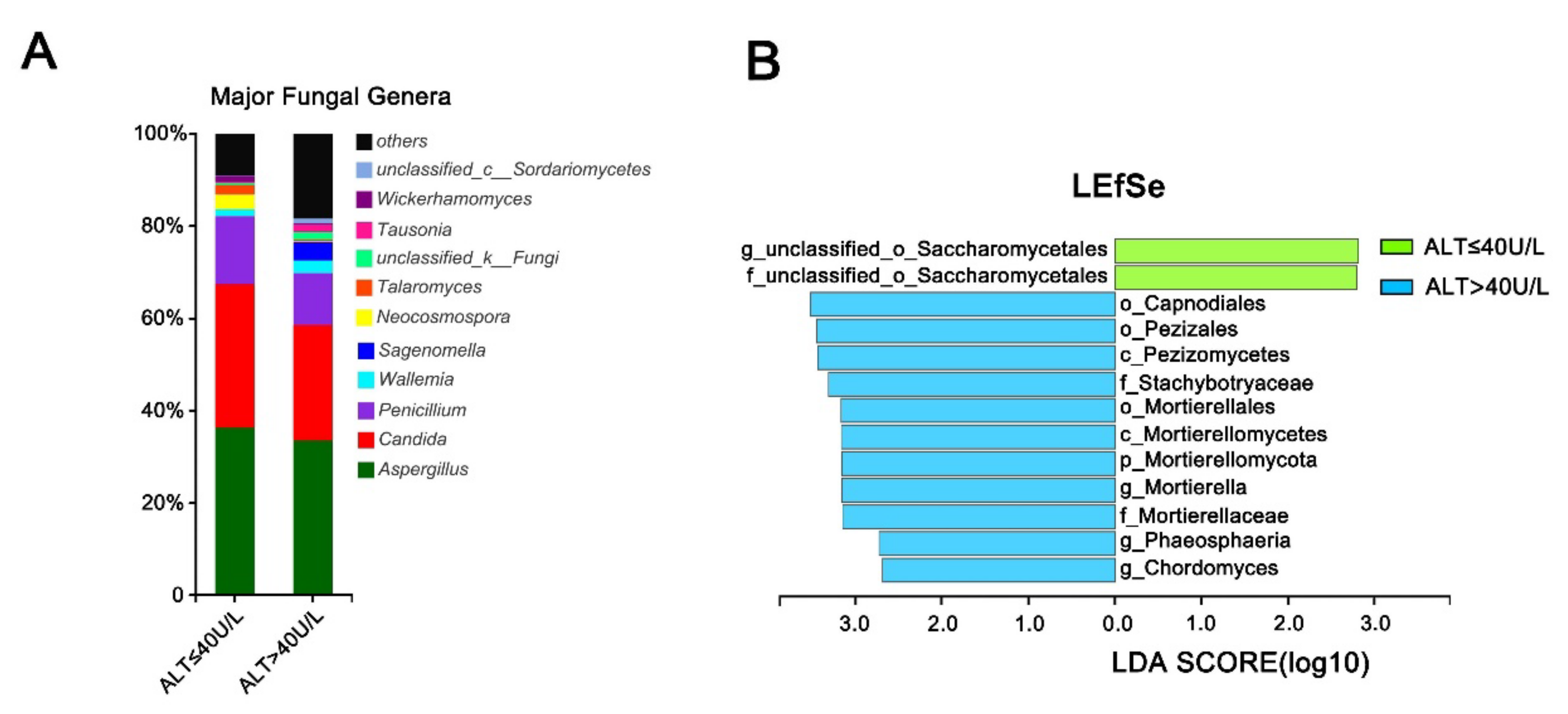
3.6. Effect of Alanine Aminotransferase on the Intestinal Fungal Profile of HCV Mono-Infected Patients
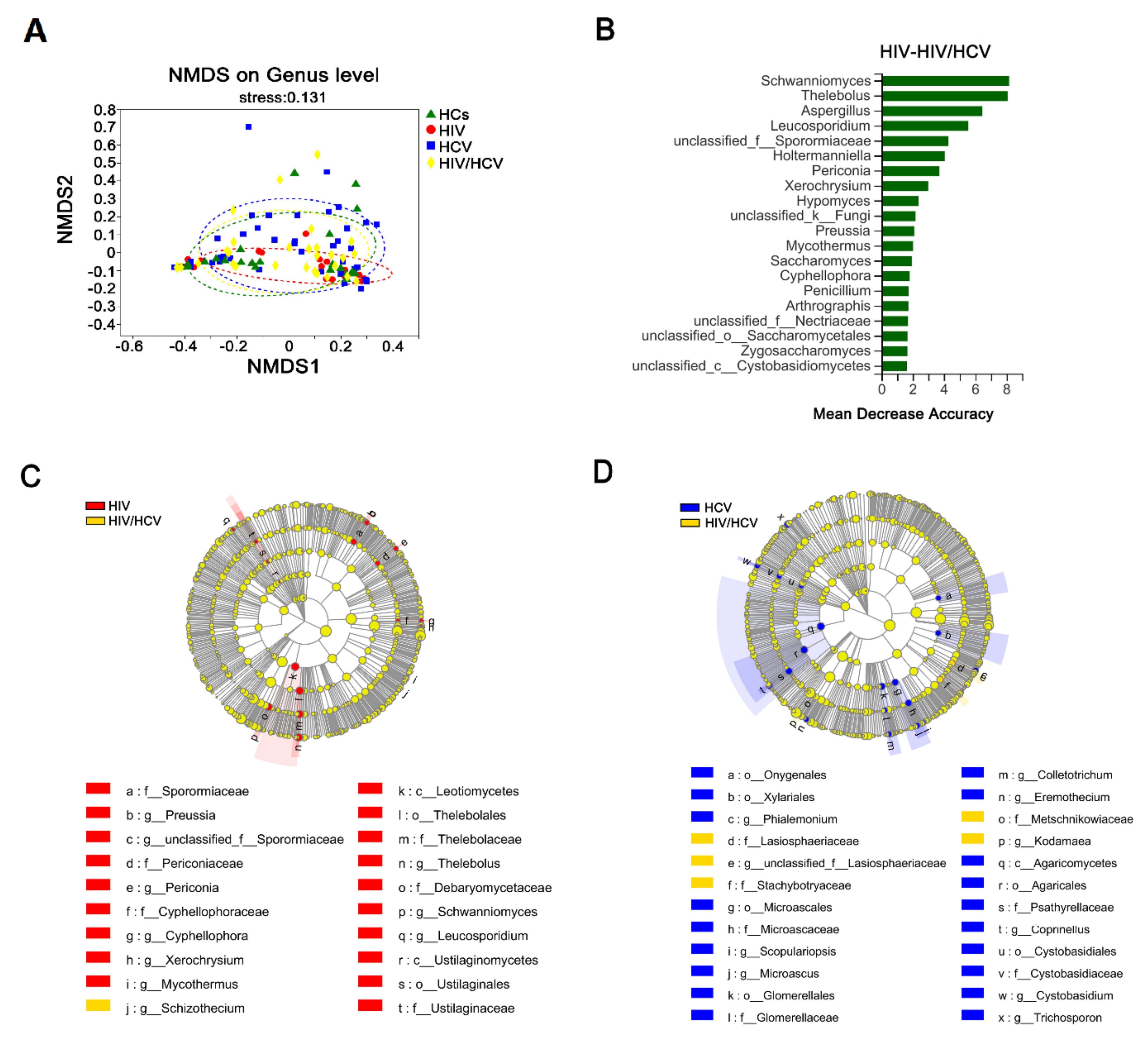
3.7. The Intestinal Fungal Profile Was Altered When HIV-Infected Patients Were Co-Infected with HCV
3.8. Fungal Inter-Correlations Differed between the Mono-Infection and Co-Infection Groups
4. Discussion
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial Translocation in the Pathogenesis of HIV Infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; de Oliveira, M.F.; Pérez-Santiago, J.; Zhang, Y.; Woods, S.P.; Finkelman, M.; Gianella, S. Correlation of (1→3)-β-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect. Dis. 2015, 3, Doc03. [Google Scholar] [CrossRef]
- Hoenigl, M.; Pérez-Santiago, J.; Nakazawa, M.; de Oliveira, M.F.; Zhang, Y.; Finkelman, M.A.; Letendre, S.; Smith, D.; Gianella, S. (1→3)-β-d-Glucan: A Biomarker for Microbial Translocation in Individuals with Acute or Early HIV Infection? Front. Immunol. 2016, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.; Retuerto, M.; Hager, C.L.; El Kamari, V.; Shan, L.; Sattar, A.; Kulkarni, M.; Funderburg, N.; Ghannoum, M.A.; Dirajlal-Fargo, S.; et al. Fungal Translocation Is Associated with Immune Activation and Systemic Inflammation in Treated HIV. AIDS Res. Hum. Retrovir. 2019, 35, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, V.; Ramendra, R.; Isnard, S.; Dupuy, F.P.; Ponte, R.; Chen, J.; Kema, I.; Jenabian, M.-A.; Costiniuk, C.T.; Lebouché, B.; et al. Circulating (1→3)-β-D-glucan Is Associated With Immune Activation During Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2019, 70, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef]
- Miranda, L.; van der Heijden, I.; Costa, S.; Sousa, A.; Sienra, R.; Gobara, S.; Santos, C.; Lobo, R.; Pessoa, V.; Levin, A. Candida colonisation as a source for candidaemia. J. Hosp. Infect. 2009, 72, 9–16. [Google Scholar] [CrossRef]
- Jayshree, R.S.; Shafiulla, M.; George, J.; David, J.K.; Bapsy, P.P.; Chakrabarti, A. Microscopic, cultural and molecular evidence of disseminated invasive aspergillosis involving the lungs and the gastrointestinal tract. J. Med Microbiol. 2006, 55, 961–964. [Google Scholar] [CrossRef]
- Krause, W.; Matheis, H.; Wulf, K. Fungaemia and funguria after oral administration of Candida albicans. Lancet 1969, 293, 598–599. [Google Scholar] [CrossRef]
- Colombo, A.L.; Branchini, M.L.; Geiger, D.D.C.P.; Schimidt, A.L.; Pignatari, A.C.C.; Fischman, O. Gastrointestinal translocation as a possible source of candidemia in an AIDS patient. Rev. Inst. Med. Trop. Sao Paulo 1996, 38, 197–200. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Jha, A.K.; Uppal, B.; Chadha, S.; Bhalla, P.; Ghosh, R.; Aggarwal, P.; Dewan, R. Clinical and Microbiological Profile of HIV/AIDS Cases with Diarrhea in North India. J. Pathog. 2012, 2012, 971958. [Google Scholar] [CrossRef]
- Janoff, E.N.; Smith, P.D. Perspectives on gastrointestinal infections in AIDS. Gastroenterol. Clin. N. Am. 1988, 17, 451–463. [Google Scholar] [CrossRef]
- Kashyap, B.; Bhalla, P.; Uppal, B. Enteric pathogens in HIV/AIDS from a tertiary care hospital. Indian J. Community Med. 2009, 34, 237–242. [Google Scholar] [CrossRef]
- E Mönkemüller, K.; Wilcox, C.M. Investigation of Diarrhea in AIDS. Can. J. Gastroenterol. 2000, 14, 933–940. [Google Scholar] [CrossRef]
- Siddiqui, U.; Bini, E.J.; Chandarana, K.; Leong, J.; Ramsetty, S.; Schiliro, D.; Poles, M. Prevalence and Impact of Diarrhea on Health-related Quality of Life in HIV-infected Patients in the Era of Highly Active Antiretroviral Therapy. J. Clin. Gastroenterol. 2007, 41, 484–490. [Google Scholar] [CrossRef]
- Awoyeni, A.; Olaniran, O.; Odetoyin, B.; Hassan-Olajokun, R.; Olopade, B.; Afolayan, D.; Adekunle, O. Isolation and evaluation of Candida species and their association with CD4+ T cells counts in HIV patients with diarrhoea. Afr. Health Sci. 2017, 17, 322–329. [Google Scholar] [CrossRef]
- Gouba, N.; Drancourt, M. Digestive tract mycobiota: A source of infection. Med. Mal. Infect. 2015, 45, 9–16. [Google Scholar] [CrossRef]
- Lee, M.-H.; Yang, H.-I.; Yuan, Y.; L’Italien, G.; Chen, C.-J. Epidemiology and natural history of hepatitis C virus infection. World J. Gastroenterol. 2014, 20, 9270–9280. [Google Scholar] [CrossRef]
- Hanafiah, K.M.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Márquez, M.C.; del Álamo, F.G.; Girón-González, J.A. Gut epithelial barrier dysfunction in human immunodeficiency virus-hepatitis C virus co-infected patients: Influence on innate and acquired immunity. World J. Gastroenterol. 2016, 22, 1433–1448. [Google Scholar] [CrossRef]
- Park, J.S.; Saraf, N.; Dieterich, D.T. HBV plus HCV, HCV plus HIV, HBV plus HIV. Curr. Gastroenterol. Rep. 2006, 8, 67–74. [Google Scholar] [CrossRef]
- Housset, C.; Lamas, E.; Bréchot, C. Detection of HIV1 RNA and p24 antigen in HIV1-infected human liver. Res. Virol. 1990, 141, 153–159. [Google Scholar] [CrossRef]
- Lin, W.; Weinberg, E.M.; Tai, A.W.; Peng, L.F.; Brockman, M.A.; Kim, K.A.; Kim, S.S.; Borges, C.B.; Shao, R.X.; Chung, R.T. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology 2008, 134, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Housset, C.; Boucher, O.; Girard, P.; Leibowitch, J.; Saimot, A.; Bréchot, C.; Marche, C. Immunohistochemical evidence for human immunodeficiency virus-1 infection of liver kupffer cells. Hum. Pathol. 1990, 21, 404–408. [Google Scholar] [CrossRef]
- Tuyama, A.C.; Hong, F.; Saiman, Y.; Wang, C.; Ozkok, D.; Mosoian, A.; Chen, P.; Chen, B.K.; Klotman, M.E.; Bansal, M.B. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: Implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010, 52, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Blackard, J.T.; Sherman, K.E. HCV/HIV co-infection: Time to re-evaluate the role of HIV in the liver? J. Viral. Hepat. 2008, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Blackard, J.T.; Hiasa, Y.; Smeaton, L.; Jamieson, D.J.; Rodriguez, I.; Mayer, K.H.; Chung, R.T. Compartmentalization of hepatitis C virus (HCV) during HCV/HIV co-infection. J. Infect. Dis. 2007, 195, 1765–1773. [Google Scholar] [CrossRef]
- Laufer, N.; Ojeda, D.; Polo, M.L.; Martinez, A.; Pérez, H.; Turk, G.; Cahn, P.; Zwirner, N.W.; Quarleri, J. CD4(+) T cells and natural killer cells: Biomarkers for hepatic fibrosis in human immunodeficiency virus/hepatitis C virus-co-infected patients. World J. Hepatol. 2017, 9, 1073–1080. [Google Scholar] [CrossRef]
- Goeser, F.; Glässner, A.; Kokordelis, P.; Wolter, F.; Lutz, P.; Kaczmarek, D.J.; Schwarze-Zander, C.; Boesecke, C.; Strassburg, C.P.; Rockstroh, J.K.; et al. HIV mono-infection is associated with an impaired anti-hepatitis C virus activity of natural killer cells. AIDS 2016, 30, 355–363. [Google Scholar]
- Glässner, A.; Eisenhardt, M.; Kokordelis, P.; Krämer, B.; Wolter, F.; Nischalke, H.D.; Boesecke, C.; Sauerbruch, T.; Rockstroh, J.K.; Spengler, U.; et al. Impaired CD4+ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J. Hepatol. 2013, 59, 427–433. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Goerges, S.; Mounier, J.; Rea, M.C.; Gelsomino, R.; Heise, V.; Beduhn, R.; Cogan, T.M.; Vancanneyt, M.; Scherer, S. Commercial Ripening Starter Microorganisms Inoculated into Cheese Milk Do Not Successfully Establish Themselves in the Resident Microbial Ripening Consortia of a South German Red Smear Cheese. Appl. Environ. Microbiol. 2008, 74, 2210–2217. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, C.; Chen, W.; Mou, Q.; Lu, X.; Han, Y.; Huang, J.; Liang, Z. The Enigmatic Thelebolaceae (Thelebolales, Leotiomycetes): One New Genus Solomyces and Five New Species. Front. Microbiol. 2020, 11, 572596. [Google Scholar] [CrossRef]
- Tolentino, G.N. Facts for Life in the Philippines. Hygie 1989, 8, 16–17. [Google Scholar]
- Yamasmith, E.; Chongtrakool, P.; Chayakulkeeree, M. Isolated pulmonary fusariosis caused by Neocosmospora pseudensiformis in a liver transplant recipient: A case report and review of the literature. Transpl. Infect. Dis. 2020, 22, e13344. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Crous, P. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Chang, S.; Guo, H.; Li, J.; Ji, Y.; Jiang, H.; Ruan, L.; Du, M. Comparative Analysis of Salivary Mycobiome Diversity in Human Immunodeficiency Virus-Infected Patients. Front. Cell. Infect. Microbiol. 2021, 11, 781246. [Google Scholar] [CrossRef]
- Ried, K.; Fakler, P. Rare Fungal Infection Linked to a Case of Juvenile Arthritis. Cureus 2018, 10, e3229. [Google Scholar] [CrossRef]
- Therese, K.L.; Lakshmipathy, M.; Lakshmipathy, D. First report of Mortierella wolfii causing fungal keratitis from a tertiary eye hospital in India. Indian J. Ophthalmol. 2020, 68, 2272–2274. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Tuohutaerbieke, M.; Feng, C.; Li, X.; Zhang, Y.; Xu, Q.; Tu, J.; Yang, E.; Zou, Q.; Shen, T. Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients. Viruses 2022, 14, 1811. https://doi.org/10.3390/v14081811
Yin Y, Tuohutaerbieke M, Feng C, Li X, Zhang Y, Xu Q, Tu J, Yang E, Zou Q, Shen T. Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients. Viruses. 2022; 14(8):1811. https://doi.org/10.3390/v14081811
Chicago/Turabian StyleYin, Yue, Maermaer Tuohutaerbieke, Chengjie Feng, Xinjie Li, Yuqi Zhang, Qiang Xu, Jing Tu, Ence Yang, Qinghua Zou, and Tao Shen. 2022. "Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients" Viruses 14, no. 8: 1811. https://doi.org/10.3390/v14081811
APA StyleYin, Y., Tuohutaerbieke, M., Feng, C., Li, X., Zhang, Y., Xu, Q., Tu, J., Yang, E., Zou, Q., & Shen, T. (2022). Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients. Viruses, 14(8), 1811. https://doi.org/10.3390/v14081811





