Emergence of New Immunopathogenic Factors in Human Yellow Fever: Polarisation of the M1/M2 Macrophage Response in the Renal Parenchyma
Abstract
1. Introduction
2. Methods
2.1. Real-Time PCR for Yellow Fever Diagnosis
2.2. Immunohistochemistry Technique for M1 and M2 Macrophage Markers
2.3. Quantitative Analysis
2.4. Statistical Analysis
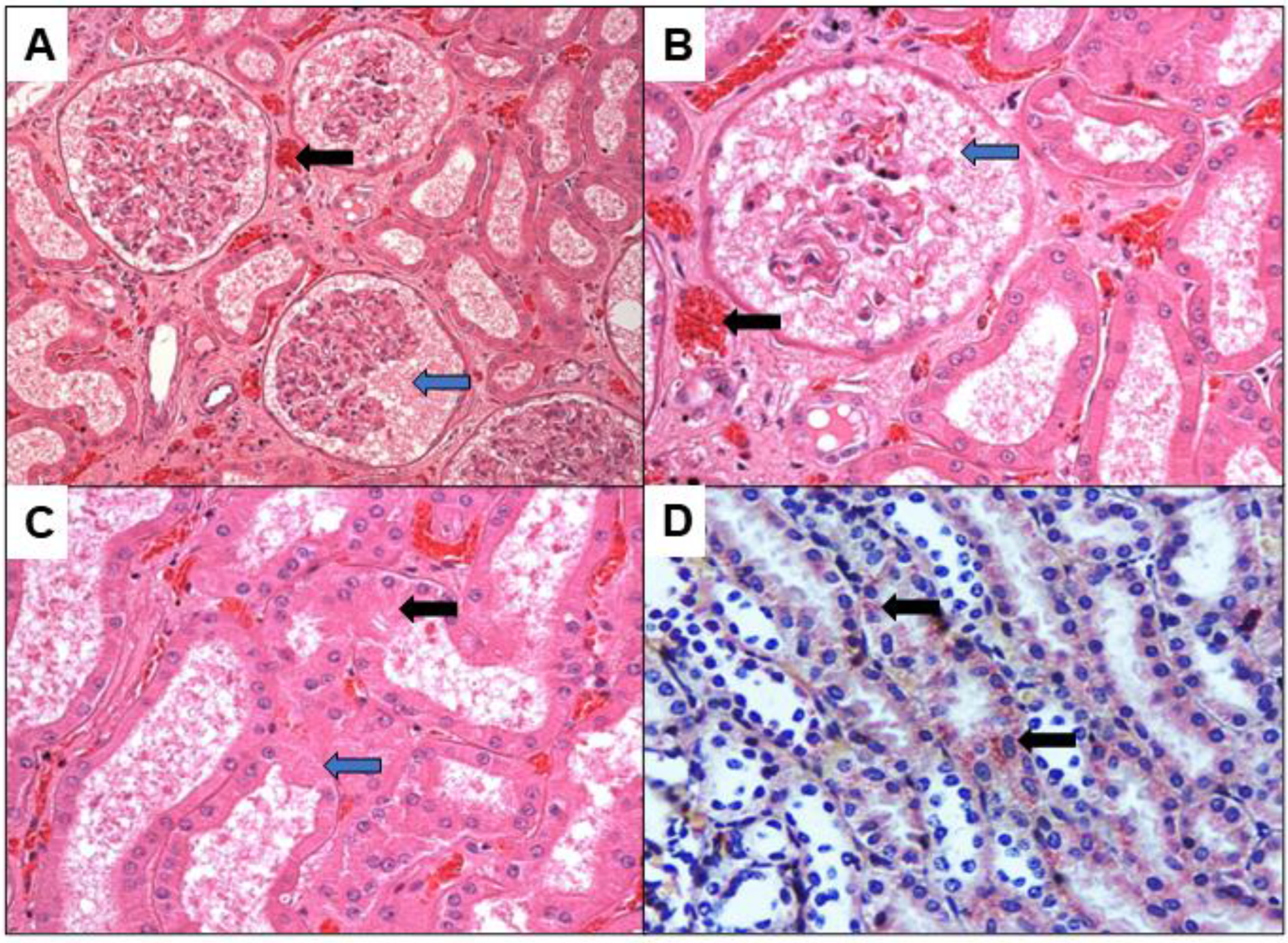
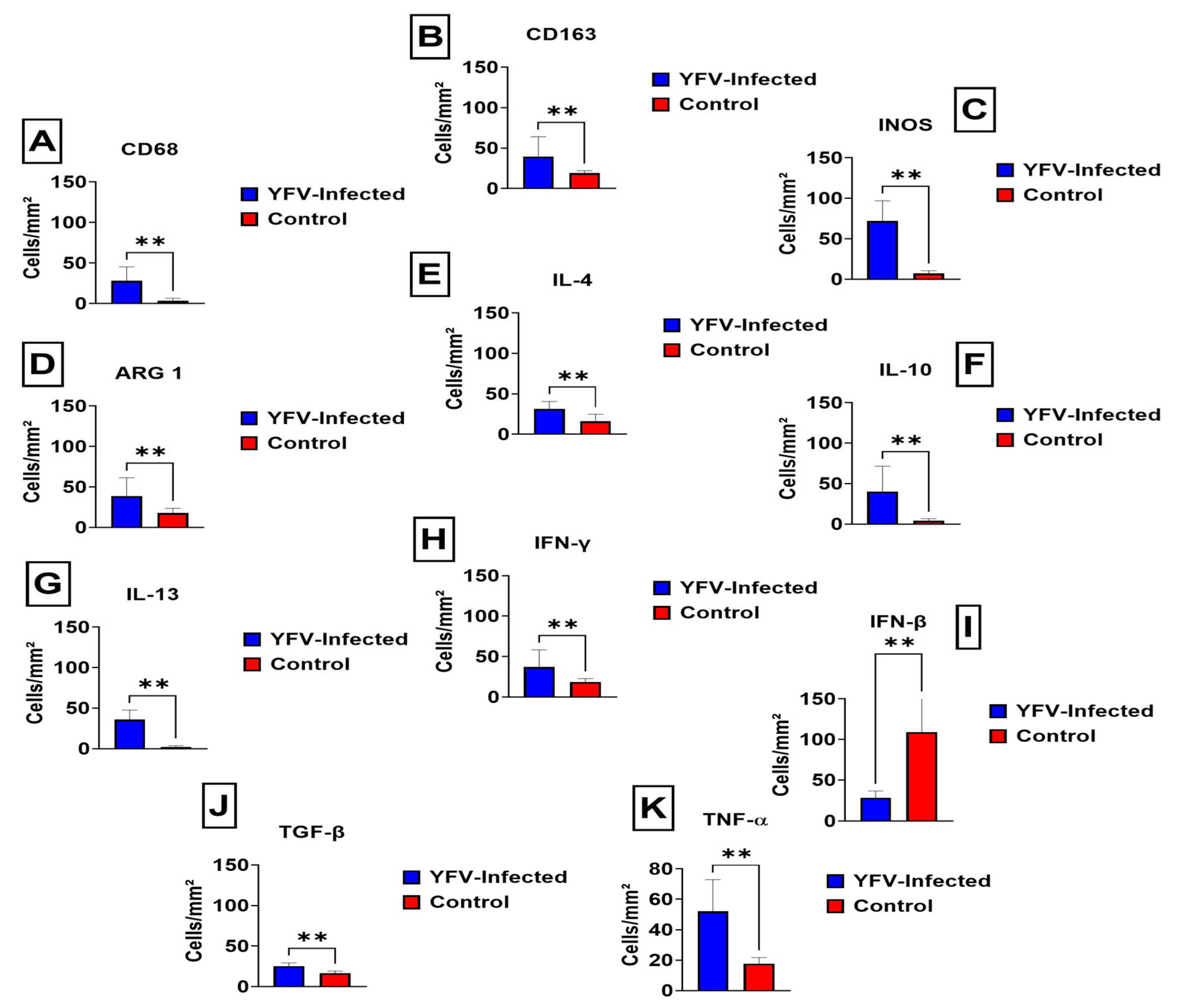
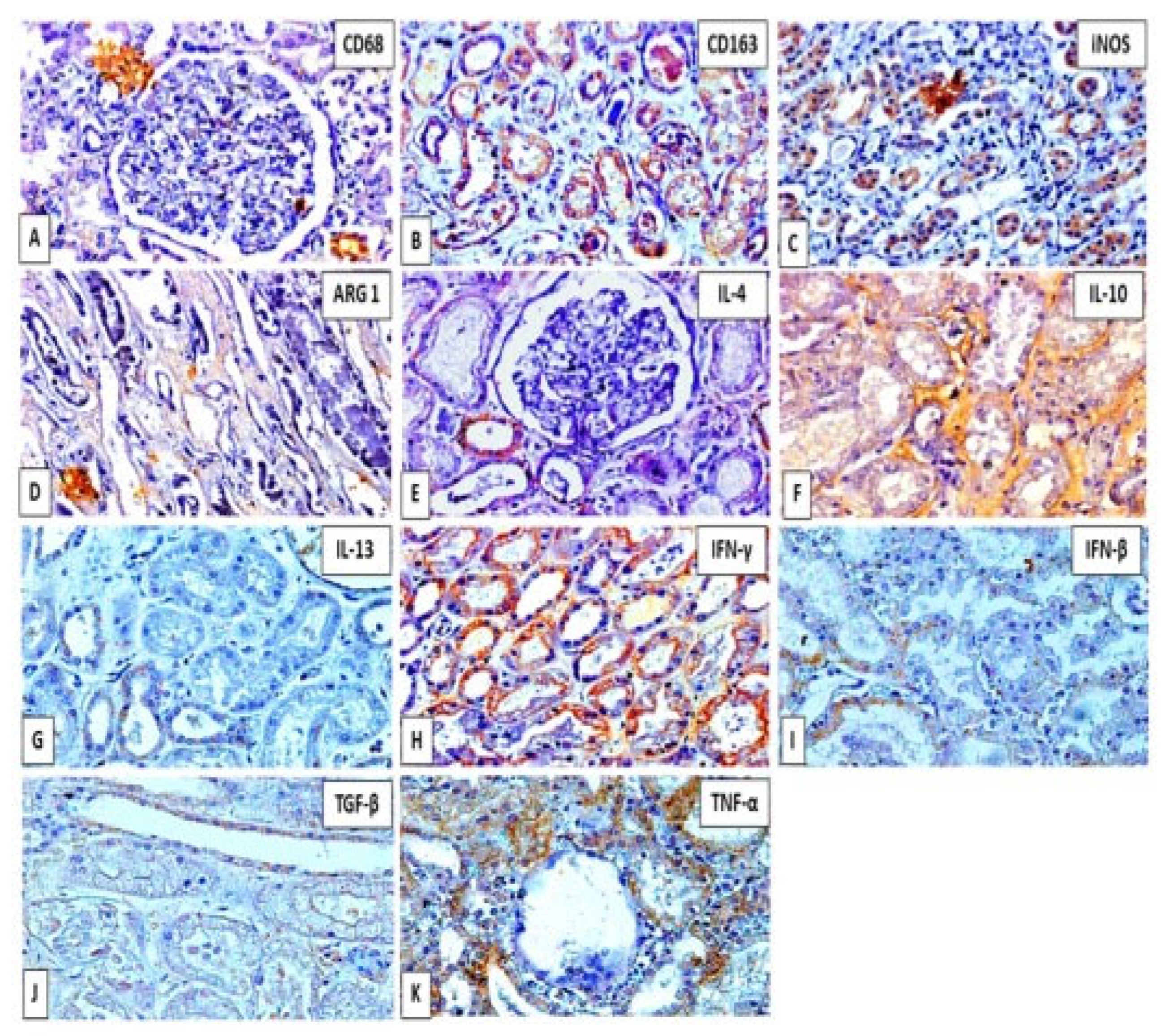
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, H.Y.; Leow CYAbdul Majeed, A.B.; Leow, C.H. Flavivirus infection-A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Jentes, E.S.; Poumerol, G.; Gershman, M.D.; Hill, D.R.; Lemarchand, J.; Lewis, R.F.; Staples, J.E.; Tomori, O.; Wilder-Smith, A.; Monath, T.P. Informal WHO Working Group on Geographic Risk for Yellow Fever. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011, 11, 622–632, Erratum in Lancet Infect. Dis. 2012, 12, 98. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Basler, C.F. Immunological features underlying viral hemorrhagic fevers. Curr. Opin. Immunol. 2015, 36, 38–46. [Google Scholar] [CrossRef]
- Burdmann, E.A. Flaviviruses and Kidney Diseases. Adv. Chronic Kidney Dis. 2019, 26, 198–206. [Google Scholar] [CrossRef]
- Arantes, M.F.; Seabra, V.F.; Lins, P.R.G.; Rodrigues, C.E.; Reichert, B.V.; Silveira, M.A.D.; Li, H.Y.; Malbouisson, L.M.; Andrade, L. Risk Factors for Acute Kidney Injury and Death in Patients Infected With the Yellow Fever Virus During the 2018 Outbreak in São Paulo, Brazil. Kidney Int. Rep. 2021, 7, 601–609. [Google Scholar] [CrossRef]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef]
- Menting, S.; Thai, K.T.; Nga, T.T.; Phuong, H.L.; Klatser, P.; Wolthers, K.C.; Binh, T.Q.; de Vries, P.J.; Beld, M. Internally controlled, generic real-time PCR for quantification and multiplex real-time PCR with serotype-specific probes for serotyping of dengue virus infections. Adv. Virol. 2011, 2011, 514681. [Google Scholar] [CrossRef][Green Version]
- Robertson, S.E.; Hull, B.P.; Tomori, O.; Bele, O.; LeDuc, J.W.; Esteves, K. Yellow fever: A decade of reemergence. JAMA 1996, 276, 1157–1162. [Google Scholar] [CrossRef]
- ter Meulen, J.; Sakho, M.; Koulemou, K.; Magassouba, N.; Bah, A.; Preiser, W.; Daffis, S.; Klewitz, C.; Bae, H.G.; Niedrig, M.; et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis. 2004, 190, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Duarte, M.I.; Vasconcelos, P.F. Midzonal lesions in yellow fever: A specific pattern of liver injury caused by direct virus action and in situ inflammatory response. Med. Hypotheses 2006, 67, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Rizvanov, A.A.; Holbrook, M.R.; St Jeor, S. Yellow fever virus strains Asibi and 17D-204 infect human umbilical cord endothelial cells and induce novel changes in gene expression. Virology 2005, 342, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Barrett, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [CrossRef]
- Gardner, C.L.; Ryman, K.D. Yellow fever: A reemerging threat. Clin. Lab. Med. 2010, 30, 237–260. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Di Paola, N.; Cunha, M.P.; Rodrigues-Jesus, M.J.; Araujo, D.B.; Silveira, V.B.; Leal, F.B.; Mesquita, F.S.; Botosso, V.F.; Zanotto, P.M.A.; et al. Yellow Fever Virus RNA in Urine and Semen of Convalescent Patient, Brazil. Emerg. Infect. Dis. 2018, 24, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Reusken, C.B.E.M.; Knoester, M.; GeurtsvanKessel, C.; Koopmans, M.; Knapen, D.G.; Bierman, W.F.W.; Pas, S. Urine as Sample Type for Molecular Diagnosis of Natural Yellow Fever Virus Infections. J. Clin. Microbiol. 2017, 55, 3294–3296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.N.; Lapage, J.; Hirschberg, R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J. Am. Soc. Nephrol. 2001, 12, 2392–2399. [Google Scholar] [CrossRef]
- Beasley, D.W.; McAuley, A.J.; Bente, D.A. Yellow fever virus: Genetic and phenotypic diversity and implications for detection, prevention and therapy. Antiviral Res. 2015, 115, 48–70. [Google Scholar] [CrossRef]
- Cao, Q.; Harris, D.C.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 2015, 30, 183–194. [Google Scholar] [CrossRef]
- Harris, R.C.; Neilson, E.G. Toward a unified theory of renal progression. Annu. Rev. Med. 2006, 57, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Mechanisms of progression of chronic kidney disease. Pediatric Nephrol. 2007, 22, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.C.; Theilgaard-Mönch, K.; Christensen, E.I.; Borregaard, N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood 2007, 109, 3084–3087. [Google Scholar] [CrossRef]
- Markowitz, C.E. Interferon-beta: Mechanism of action and dosing issues. Neurology 2007, 68 (Suppl. 4), S8–S11. [Google Scholar] [CrossRef]
- Kasper, L.H.; Reder, A.T. Immunomodulatory activity of interferon-beta. Ann. Clin. Transl. Neurol. 2014, 1, 622–631. [Google Scholar] [CrossRef]
- Goodbourn, S.; Didcock, L.; Randall, R.E. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000, 81, 2341–2364. [Google Scholar] [CrossRef]
- Pagliari, C.; Quaresma, J.A.S.; Galo, L.K.; Vitoria, W.O.; Silva, W.L.F.; Penny, R.; Vasconcelos, B.C.B.; Vasconcelos, P.F.C.; Duarte, M.I.S. Human kidney damage in fatal dengue hemorrhagic fever results of glomeruli injury mainly induced by IL17. J. Clin. Virol. 2016, 75, 16–20. [Google Scholar] [CrossRef]
- Domingo, C.; Charrel, R.N.; Schmidt-Chanasit, J.; Zeller, H.; Reusken, C. Yellow fever in the diagnostics laboratory. Emerg. Microbes Infect. 2018, 7, 129. [Google Scholar] [CrossRef]
| Case | State | Sex | Age | I.T. (Days) | Patient | Year |
|---|---|---|---|---|---|---|
| 1 | TO | M | 30 | N.I. | 494/00 | 2000 |
| 2 | GO | M | 48 | - | 255/00 | 2000 |
| 3 | GO | M | 23 | N.I. | 074/07 | 2007 |
| 4 | GO | F | 63 | 2 | 043/08 | 2008 |
| 5 | DF | M | 55 | - | 088/08 | 2008 |
| 6 | GO | M | 42 | N.I. | 095/08 | 2008 |
| 7 | DF | M | 35 | N.I. | 154/08 | 2008 |
| 8 | GO | M | 35 | N.I. | 062/16 | 2016 |
| 9 | PB | M | - | N.I. | 102/16 | 2016 |
| 10 | GO | M | 15 | 7 | 346/16 | 2016 |
| 11 | GO | M | 27 | 1 | 369/16 | 2016 |
| Primers or Probe | Sequence (5′-3′) | Position |
| YFallF | 5′-GCTAATTGAGGTGYATTGGTCTGC-3′ | 15–38 |
| YFallR | 5′-CTGCTAATCGCTCAAMGAACG-3′ | 83–103 |
| YFallP | 5′-FAM-ATCGAGTTGCTAGGCAATAAACAC-TMR-3′ | 41–64 |
| Primers or Probe | Sequence (5′-3′) | Position |
| MS2-F | 5′-ATCAAGTTAGATGGCCGTCTGT-3′ | 841–862 |
| MS2-R | 5′-TAGAGACGACAACCATGCCAAAC-3′ | 963–941 |
| MS2 probe | 5′-VIC-TCCAGACAACGTGCAACATATCGCGACGTATCGTGATATGG-BHQ1-3′ | 881–921 |
| Antibody | Mark/Code | Animal | Batch | Work Dilution |
|---|---|---|---|---|
| TNF-α | Abcam 6671 | rabbit | GR235155-32 | 1:100 |
| IL-4 | Abcam9622 | rabbit | GR3174920-9 | 1:100 |
| IL-13 | Abcam 9576 | rabbit | GR10654-33 | 1:100 |
| IL-10 | Abcam 34843 | rabbit | GR200618-33 | 1:100 |
| IFN-γ | biorbyt/orb 10878 | rabbit | 676 | 1:100 |
| INOS | NOVUS QG18859 | rabbit | QG218859 | 1:100 |
| CD 163 | NBP2-36494 | mouse | A-2 | 1:100 |
| ARGINASE I | NBP1-87455 | rabbit | A63844 | 1:100 |
| CD 68 | MO814 | mouse | 00012544 | 1:100 |
| IFN-β | Abcam140211 | rabbit | GR3208814-1 | 1:100 |
| TGF-beta | Abcam 190503 | mouse | GR3183728-7 | 1:100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, J.M.; Falcão, L.F.M.; da Ponte, L.C.T.; Silva, C.C.; Martins, L.C.; Chiang, J.O.; Martins Filho, A.J.; Franco, E.C.S.; Duarte, M.I.S.; Sousa, J.R.d.; et al. Emergence of New Immunopathogenic Factors in Human Yellow Fever: Polarisation of the M1/M2 Macrophage Response in the Renal Parenchyma. Viruses 2022, 14, 1725. https://doi.org/10.3390/v14081725
Melo JM, Falcão LFM, da Ponte LCT, Silva CC, Martins LC, Chiang JO, Martins Filho AJ, Franco ECS, Duarte MIS, Sousa JRd, et al. Emergence of New Immunopathogenic Factors in Human Yellow Fever: Polarisation of the M1/M2 Macrophage Response in the Renal Parenchyma. Viruses. 2022; 14(8):1725. https://doi.org/10.3390/v14081725
Chicago/Turabian StyleMelo, Juliana Marinho, Luiz Fabio Magno Falcão, Lucas Coutinho Tuma da Ponte, Camilla Costa Silva, Livia Caricio Martins, Jannifer Oliveira Chiang, Arnaldo Jorge Martins Filho, Edna Cristina Santos Franco, Maria Irma Seixas Duarte, Jorge Rodrigues de Sousa, and et al. 2022. "Emergence of New Immunopathogenic Factors in Human Yellow Fever: Polarisation of the M1/M2 Macrophage Response in the Renal Parenchyma" Viruses 14, no. 8: 1725. https://doi.org/10.3390/v14081725
APA StyleMelo, J. M., Falcão, L. F. M., da Ponte, L. C. T., Silva, C. C., Martins, L. C., Chiang, J. O., Martins Filho, A. J., Franco, E. C. S., Duarte, M. I. S., Sousa, J. R. d., Vasconcelos, P. F. d. C., & Quaresma, J. A. S. (2022). Emergence of New Immunopathogenic Factors in Human Yellow Fever: Polarisation of the M1/M2 Macrophage Response in the Renal Parenchyma. Viruses, 14(8), 1725. https://doi.org/10.3390/v14081725








