Correlating IgG Levels with Neutralising Antibody Levels to Indicate Clinical Protection in Healthcare Workers at Risk during a Measles Outbreak
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Cell Lines
2.3. IgG Testing
2.4. Production of Vesicular Stomatitis Virus (Measles Virus) Pseudotypes (VSV (MeV))
2.5. Pseudotype-Based Virus Neutralisation Assay
2.6. Statistical Analysis
3. Results
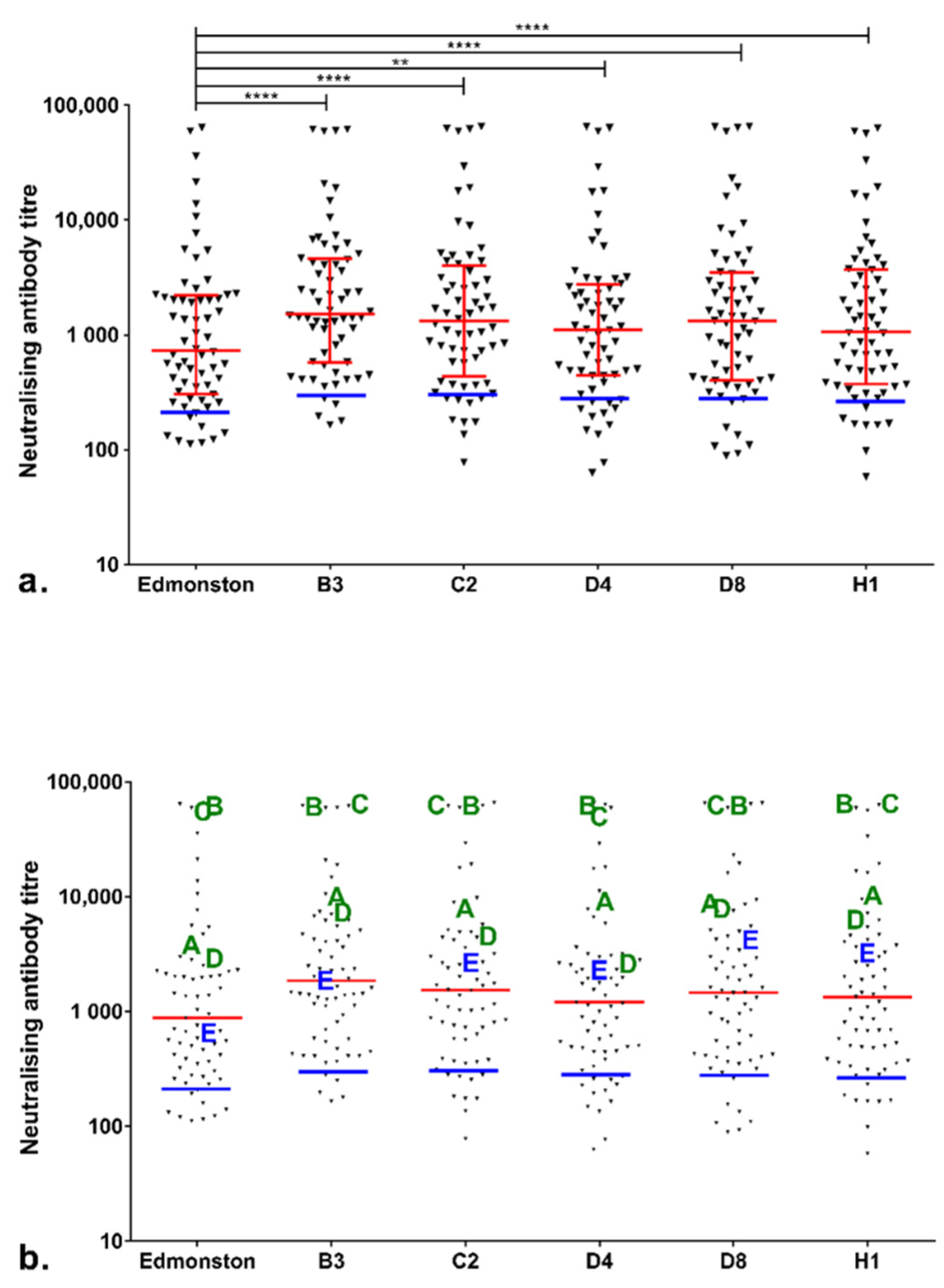
3.1. Immune Status Check in Sheffield HCWs during the Sheffield Measles Outbreak
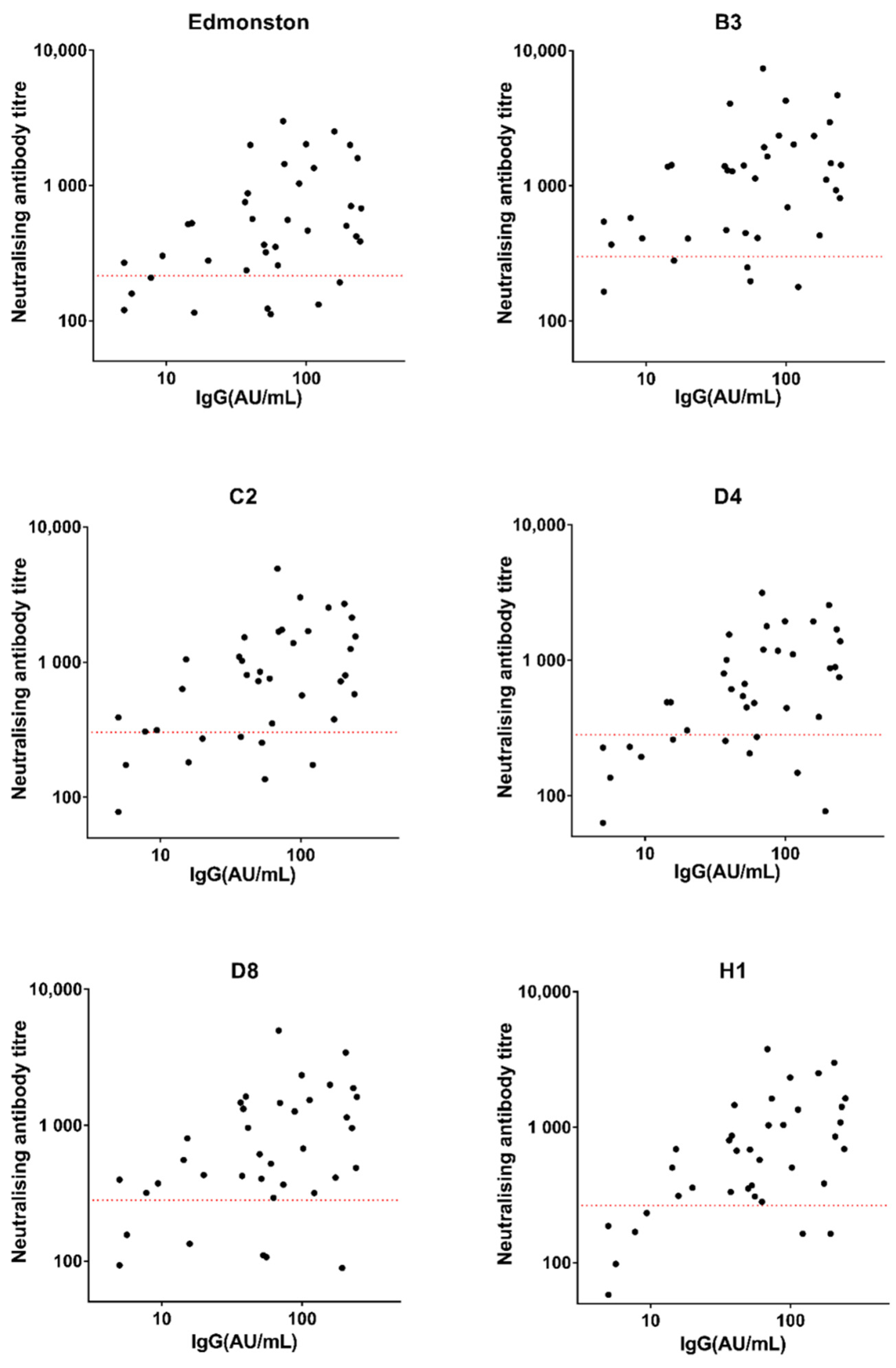
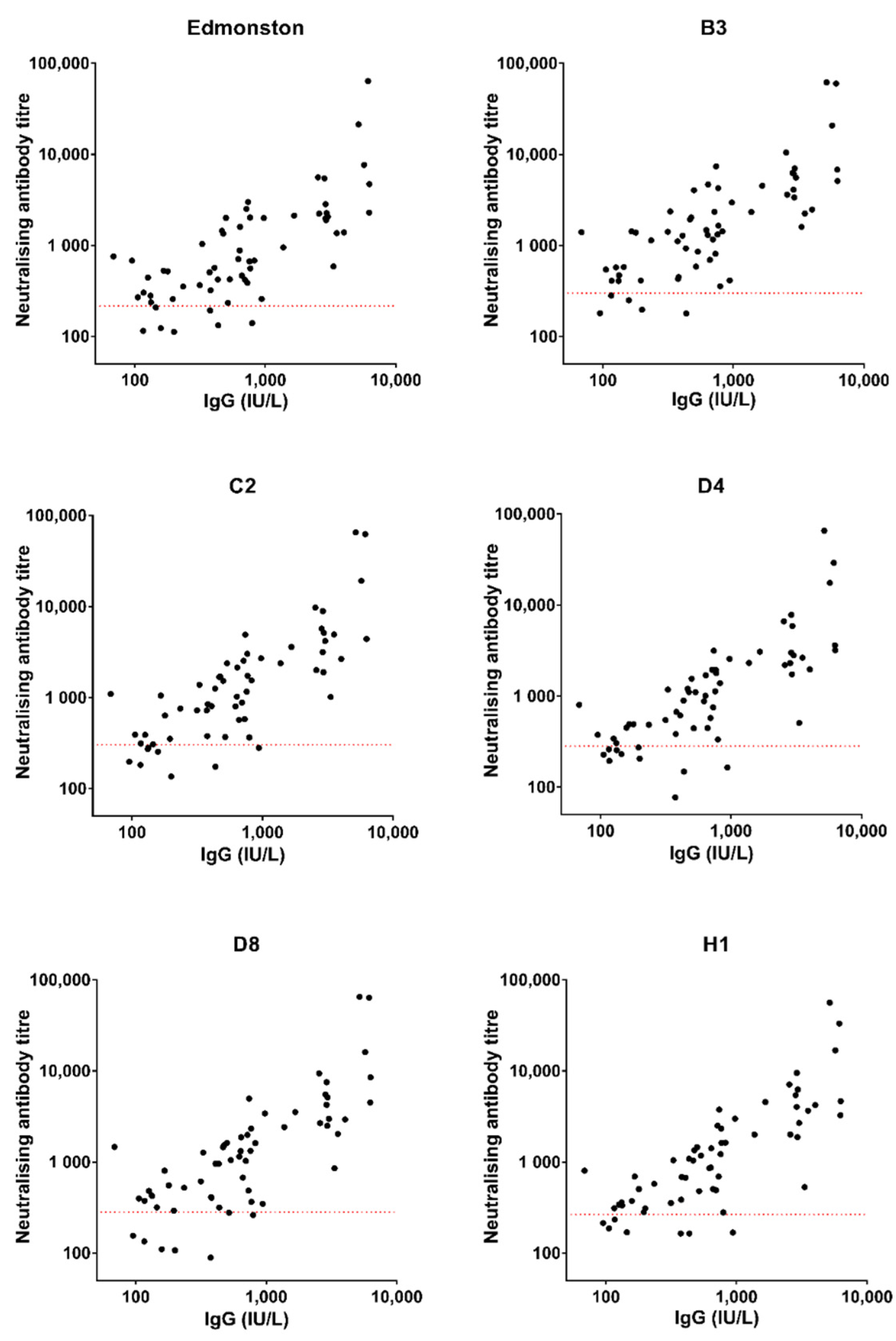
3.2. Correlation between Measles-Specific IgG Level and Neutralising Antibody Titre: Relationship with Clinical Protection Status
4. Discussion
4.1. Vaccinated Individuals Could Still Be Infected with Measles
4.2. Correlation between IgG Levels and Neutralising Antibody Titres in Healthy Individuals Might Be Used to Predict Clinical Protection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The Basic Reproduction Number (R0) of Measles: A Systematic Review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the Basic Reproduction Number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S. Interrupting the Transmission of Respiratory Tract Infections: Theory and Practice. Clin. Infect. Dis. 1999, 28, 200–204. [Google Scholar] [CrossRef][Green Version]
- Leonard, V.H.J.; Sinn, P.L.; Hodge, G.; Miest, T.; Devaux, P.; Oezguen, N.; Braun, W.; McCray, P.B.; McChesney, M.B.; Cattaneo, R. Measles Virus Blind to Its Epithelial Cell Receptor Remains Virulent in Rhesus Monkeys but Cannot Cross the Airway Epithelium and Is Not Shed. J. Clin. Investig. 2008, 118, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens Junction Protein Nectin-4 Is the Epithelial Receptor for Measles Virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Fact Sheet: Measles. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/measles (accessed on 22 May 2022).
- Public Health England. UK Measles and Rubella Elimination Strategy 2019. 2019. Available online: https://www.gov.uk/government/publications/measles-and-rubella-elimination-uk-strategy (accessed on 22 May 2022).
- European Centre for Disease Prevention and Control. Measles and Rubella Surveillance for 2017. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/annual-measles-and-rubella-monitoring-report-2017 (accessed on 12 October 2021).
- European Centre for Disease Prevention and Control. Measles—Annual Epidemiological Report for 2018. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/measles-annual-epidemiological-report-2018 (accessed on 12 October 2021).
- European Centre for Disease Prevention and Control. Measles—Annual Epidemiological Report for 2019. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/measles-annual-epidemiological-report-2019 (accessed on 12 October 2021).
- World Health Organisation. WHO EpiBrief, No. 1/2021. 2021. Available online: https://www.who.int/europe/publications/m/item/who-epibrief-no.-1-2021 (accessed on 26 July 2022).
- World Health Organisation. WHO EpiBrief, No. 2/2021. 2021. Available online: https://www.who.int/europe/publications/m/item/who-epibrief--no.-2-2021 (accessed on 6 July 2022).
- Currie, J.; Davies, L.; Mccarthy, J.; Perry, M.; Moore, C.; Cottrell, S.; Bowley, M.; Williams, C.; Shankar, A.G.; Stiff, R. Measles Outbreak Linked to European B3 Outbreaks, Wales, United Kingdom. Eurosurveillance 2017, 22, 17-00673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirolos, A.; Waugh, C.; Templeton, K.; McCormick, D.; Othieno, R.; Willocks, L.J.; Stevenson, J. Imported Case of Measles in a University Setting Leading to an Outbreak of Measles in Edinburgh, Scotland from September to December 2016. Epidemiol. Infect. 2018, 146, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Steingart, K.R.; Thomas, A.R.; Dykewicz, C.A.; Redd, S.C. Transmission of Measles Virus in Healthcare Settings During a Communitywide Outbreak. Infect. Control Hosp. Epidemiol. 1999, 20, 115–119. [Google Scholar] [CrossRef]
- Cherry, J.D.; Zahn, M. Clinical Characteristics of Measles in Previously Vaccinated and Unvaccinated Patients in California. Clin. Infect. Dis. 2018, 67, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, K.; Seto, J.; Tanaka, S.; Suzuki, Y.; Ikeda, T.; Onuki, N.; Yamada, K.; Ahiko, T.; Ishikawa, H.; Mizuta, K. The Largest Measles Outbreak, Including 38 Modified Measles and 22 Typical Measles Cases in Its Elimination Era in Yamagata, Japan, 2017. Jpn. J. Infect. Dis. 2018, 71, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gibney, K.; Attwood, L.; Nicholson, S.; Tran, T.; Druce, J.; Healy, J.; Strachan, J.; Franklin, L.; Hall, R.; Cross, G. Emergence of Attenuated Measles Illness among IgG Positive/IgM Negative Measles Cases, Victoria, Australia 2008–2017. Clin. Infect. Dis. 2020, 70, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Weber, J.; Jessamine, A.; O’Shaughnessy, M. Comparison of Measles Antihemolysin Test, Enzyme-Linked Immunosorbent Assay, and Hemagglutination Inhibition Test with Neutralization Test for Determination of Immune Status. J. Clin. Microbiol. 1985, 22, 296–298. [Google Scholar] [CrossRef]
- Hiebert, J.; Zubach, V.; Charlton, C.L.; Fenton, J.; Tipples, G.A.; Fonseca, K.; Severini, A. Evaluation of Diagnostic Accuracy of Eight Commercial Assays for the Detection of Measles Virus-Specific IgM Antibodies. J. Clin. Microbiol. 2021, 59, e03161-20. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Cohen, B.; Hand, J.; Nokes, D.J. A Simplified and Standardized Neutralization Enzyme Immunoassay for the Quantification of Measles Neutralizing Antibody. J. Virol. Methods 1999, 78, 209–217. [Google Scholar] [CrossRef]
- Lee, M.S.; Nokes, D.J.; Hsu, H.M.; Lu, C.F. Protective Titres of Measles Neutralising Antibody. J. Med. Virol. 2000, 62, 511–517. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Marianov, B.; Wicker, S.; Allwinn, R. Comparison of the Neutralizing and ELISA Antibody Titres to Measles Virus in Human Sera and in Gamma Globulin Preparations. Med. Microbiol. Immunol. 2007, 196, 151–155. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Weekly Epidemiological Record, 13 November 2020, Vol. 95, 46. Wkly. Epidemiol. Rec. 2020, 95, 557–572. [Google Scholar]
- World Health Organisation. Immunological Basis for Immunization Series: Module 7: Measles: Update 2020. 2020. Available online: https://www.who.int/publications/i/item/9789241516655 (accessed on 23 May 2022).
- DuBridge, R.; Tang, P.; Hsia, H.; Leong, P.; Miller, J.; Calos, M. Analysis of Mutation in Human Cells by Using an Epstein-Barr Virus Shuttle System. Mol. Cell. Biol. 1987, 7, 379–387. [Google Scholar] [CrossRef]
- Russell, W.C.; Graham, F.L.; Smiley, J.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef]
- Logan, N.; McMonagle, E.; Drew, A.A.; Takahashi, E.; McDonald, M.; Baron, M.D.; Gilbert, M.; Cleaveland, S.; Haydon, D.T.; Hosie, M.J.; et al. Efficient Generation of Vesicular Stomatitis Virus (VSV)-Pseudotypes Bearing Morbilliviral Glycoproteins and Their Use in Quantifying Virus Neutralising Antibodies. Vaccine 2016, 34, 814–822. [Google Scholar] [CrossRef]
- Hu, S.; Logan, N.; Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y.; Willett, B.J.; Hosie, M.J. Evaluation of the Effect of Maternally Derived Antibody on Response to MMR Vaccine in Thai Infants. Vaccine 2022, 40, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.T.; Wright, E.; Scott, S.D.; Temperton, N.J. Lyophilisation of Influenza, Rabies and Marburg Lentiviral Pseudotype Viruses for the Development and Distribution of a Neutralisation -Assay-Based Diagnostic Kit. J. Virol. Methods 2014, 210, 51–58. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72. [Google Scholar] [CrossRef]
- Public Health England. National Measles Guidelines. 2019. Available online: https://www.gov.uk/government/publications/national-measles-guidelines (accessed on 23 May 2022).
- Vivancos, R.; Keenan, A.; Farmer, S.; Atkinson, J.; Coffey, E.; Dardamissis, E.; Dillon, J.; Drew, R.J.; Fallon, M.; Huyton, R.; et al. An Ongoing Large Outbreak of Measles in Merseyside, England, January to June 2012. Eurosurveillance 2012, 17, 20226. [Google Scholar] [CrossRef]
- Nic Lochlainn, L.; Mandal, S.; de Sousa, R.; Paranthaman, K.; van Binnendijk, R.; Ramsay, M.; Hahné, S.; Brown, K.E. A Unique Measles B3 Cluster in the United Kingdom and the Netherlands Linked to Air Travel and Transit at a Large International Airport, February to April 2014. Eurosurveillance 2016, 21, 30177. [Google Scholar] [CrossRef][Green Version]
- Hickman, C.J.; Hyde, T.B.; Sowers, S.B.; Mercader, S.; McGrew, M.; Williams, N.J.; Beeler, J.A.; Audet, S.; Kiehl, B.; Nandy, R.; et al. Laboratory Characterization of Measles Virus Infection in Previously Vaccinated and Unvaccinated Individuals. J. Infect. Dis. 2011, 204 (Suppl. 1), S549–S558. [Google Scholar] [CrossRef] [PubMed]
- Rota, J.S.; Hickman, C.J.; Sowers, S.B.; Rota, P.A.; Mercader, S.; Bellini, W.J. Two Case Studies of Modified Measles in Vaccinated Physicians Exposed to Primary Measles Cases: High Risk of Infection But Low Risk of Transmission. J. Infect. Dis. 2011, 204 (Suppl. 1), S559–S563. [Google Scholar] [CrossRef] [PubMed]
- Hahné, S.J.M.; Lochlainn, L.M.N.; Van Burgel, N.D.; Kerkhof, J.; Sane, J.; Yap, K.B.; Van Binnendijk, R.S. Measles Outbreak among Previously Immunized Healthcare Workers, the Netherlands, 2014. J. Infect. Dis. 2016, 214, 1980–1986. [Google Scholar] [CrossRef]
- Bianchi, S.; Gori, M.; Fappani, C.; Ciceri, G.; Canuti, M.; Colzani, D.; Dura, M.; Terraneo, M.; Lamberti, A.; Baggieri, M.; et al. Characterization of Vaccine Breakthrough Cases during Measles Outbreaks in Milan and Surrounding Areas, Italy, 2017–2021. Viruses 2022, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Measles Vaccines: WHO Position Paper—April 2017. Wkly. Epidemiol. Rec. Epidemiol. Rec. 2017, 92, 205–227. [Google Scholar]
- Bianchi, S.; Canuti, M.; Ciceri, G.; Gori, M.; Colzani, D.; Dura, M.; Pennati, B.; Baggieri, M.; Magurano, F.; Tanzi, E.; et al. Molecular Epidemiology of B3 and D8 Measles Viruses through Hemagglutinin Phylogenetic History. Int. J. Mol. Sci. 2020, 21, 4435. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, S.; Gadag, V.; West, R.; Burris, J.; Oates, E.; Stead, F.; Bouilianne, N. Comparison of Commercial Enzyme Immunoassay Kits with Plaque Reduction Neutralization Test for Detection of Measles Virus Antibody. J. Clin. Microbiol. 1995, 33, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.J.; Parry, R.P.; Doblas, D.; Samuel, D.; Warrener, L.; Andrews, N.; Brown, D. Measles Immunity Testing: Comparison of Two Measles IgG ELISAs with Plaque Reduction Neutralisation Assay. J. Virol. Methods 2006, 131, 209–212. [Google Scholar] [CrossRef] [PubMed]
| Genotypes | Percentage Protected (95%CI) |
|---|---|
| Edmonston | 87.3 (76.6–93.7) |
| B3 | 93.6 (84.3–98.0) |
| C2 | 87.3 (76.6–93.7) |
| D4 | 82.5 (71.2–90.1) |
| D8 | 88.9 (78.5–94.8) |
| H1 | 88.9 (78.5–94.8) |
| Genotypes | Rs | 95%CI | p Value |
|---|---|---|---|
| Edmonston | 0.42 | 0.098–0.66 | 0.0101 |
| B3 | 0.41 | 0.094–0.66 | 0.0109 |
| C2 | 0.49 | 0.19–0.71 | 0.0021 |
| D4 | 0.51 | 0.21–0.72 | 0.0013 |
| D8 | 0.40 | 0.075–0.65 | 0.0146 |
| H1 | 0.55 | 0.26–0.74 | 0.0005 |
| Genotypes | Rs | 95%CI | p Value |
|---|---|---|---|
| Edmonston | 0.71 | 0.55–0.82 | <0.0001 |
| B3 | 0.75 | 0.61–0.84 | <0.0001 |
| C2 | 0.78 | 0.66–0.87 | <0.0001 |
| D4 | 0.79 | 0.67–0.87 | <0.0001 |
| D8 | 0.76 | 0.62–0.85 | <0.0001 |
| H1 | 0.79 | 0.66–0.87 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Logan, N.; Coleman, S.; Evans, C.; Willett, B.J.; Hosie, M.J. Correlating IgG Levels with Neutralising Antibody Levels to Indicate Clinical Protection in Healthcare Workers at Risk during a Measles Outbreak. Viruses 2022, 14, 1716. https://doi.org/10.3390/v14081716
Hu S, Logan N, Coleman S, Evans C, Willett BJ, Hosie MJ. Correlating IgG Levels with Neutralising Antibody Levels to Indicate Clinical Protection in Healthcare Workers at Risk during a Measles Outbreak. Viruses. 2022; 14(8):1716. https://doi.org/10.3390/v14081716
Chicago/Turabian StyleHu, Siyuan, Nicola Logan, Sarah Coleman, Cariad Evans, Brian J. Willett, and Margaret J. Hosie. 2022. "Correlating IgG Levels with Neutralising Antibody Levels to Indicate Clinical Protection in Healthcare Workers at Risk during a Measles Outbreak" Viruses 14, no. 8: 1716. https://doi.org/10.3390/v14081716
APA StyleHu, S., Logan, N., Coleman, S., Evans, C., Willett, B. J., & Hosie, M. J. (2022). Correlating IgG Levels with Neutralising Antibody Levels to Indicate Clinical Protection in Healthcare Workers at Risk during a Measles Outbreak. Viruses, 14(8), 1716. https://doi.org/10.3390/v14081716







