Molecular Epidemiology of HIV-1 among Prisoners in Central Brazil and Evidence of Transmission Clusters
Abstract
1. Introduction
2. Materials and Methods
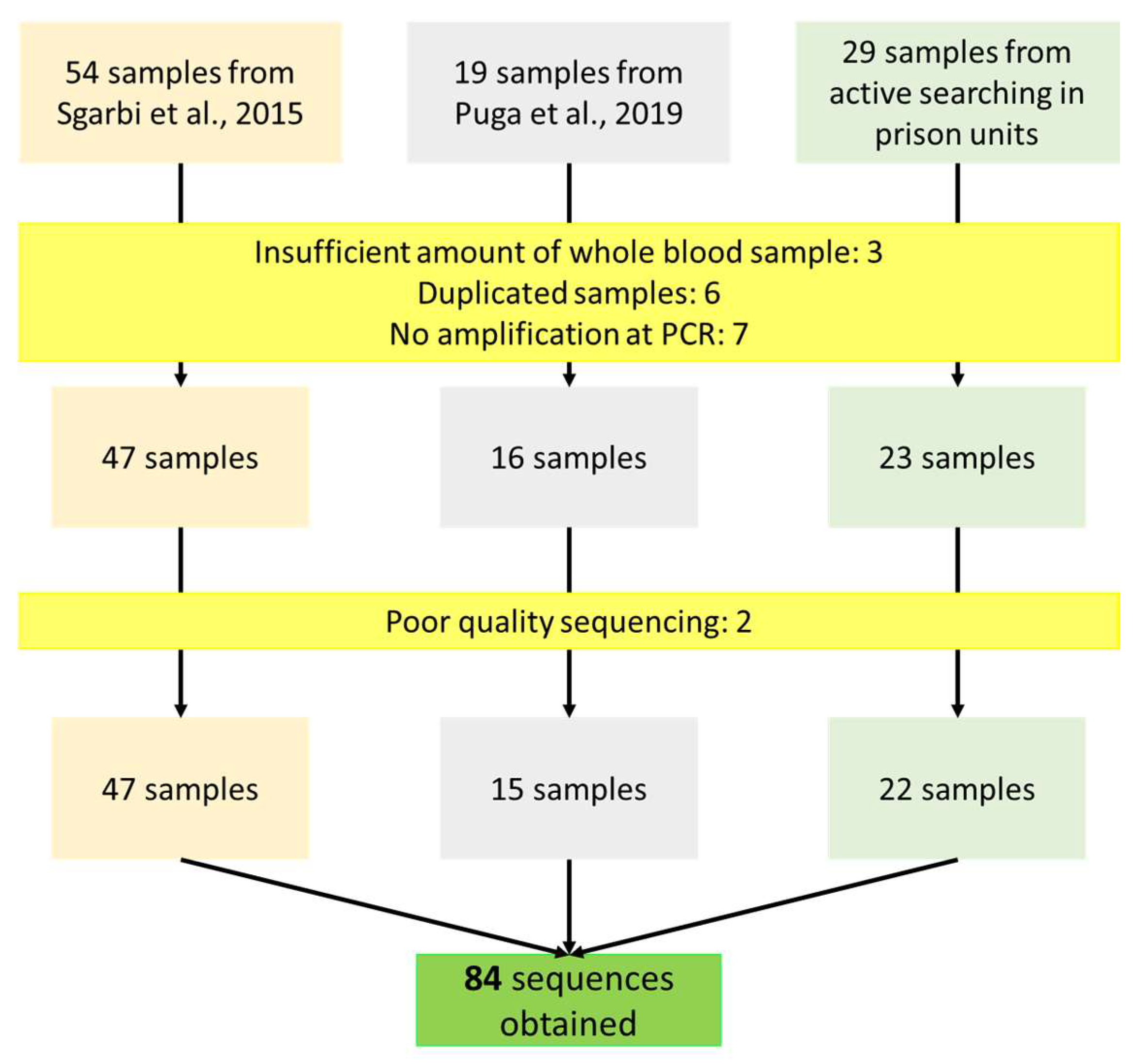
2.1. Subjects and Study Design
2.2. Amplification of HIV-1 PR/RT Region
2.3. Sequence Analysis
2.4. Genotypic Analysis of HIV-1 Drug Resistance
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime (UNODC). HIV Prevention, Testing, Treatment, Care and Support in Prisons and Other Closed Settings: A Comprehensive Package of Interventions. Technical Brief 2020 Update. 2020. Available online: unodc.org/documents/hiv-aids/publications/Prisons_and_other_closed_settings/20-06330_HIV_update_eBook.pdf (accessed on 14 January 2022).
- Walmsley, R. World Prison Population List, 12th ed.; Institute for Criminal Policy Research, University of London: Birkbeck, UK, 2018; Available online: www.prisonstudies.org/sites/default/files/resources/downloads/wppl_12.pdf (accessed on 28 June 2022).
- Agepen. Available online: https://www.agepen.ms.gov.br/wp-content/uploads/2021/12/MAPA-UNICO-NOVEMBRO-2021-FECH.-30-11-2021.pdf (accessed on 28 June 2022).
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). HIV and People in Prisons and Other Closed Settings Human Rights. Fact Sheet Series 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/06-hiv-human-rights-factsheet-prisons_en.pdf (accessed on 28 June 2022).
- Bet, G.M.D.S.; Souza, G.H.D.A.D.; Croda, J.; Correa, M.E.; De Sales, R.O.; Santos, R.A.D.S.; Sgarbi, R.V.E.; Yassuda, R.T.S.; Motta-Castro, A.R.C.; Pompílio, M.A.; et al. Treatment outcomes of brazilian inmates with treponema pallidum and human immunodeficiency virus infection: A prospective cohort study. Am. J. Trop. Med. Hyg. 2018, 98, 1603–1608. [Google Scholar] [CrossRef]
- Sgarbi, R.V.E.; Carbone, A.D.S.S.; Paião, D.S.G.; Lemos, E.F.; Simionatto, S.; Puga, M.A.M.; Motta-Castro, A.R.; Pompilio, M.A.; Urrego, J.; Ko, A.; et al. A cross-sectional survey of HIV testing and prevalence in twelve Brazilian correctional facilities. PLoS ONE 2015, 10, e0139487. [Google Scholar] [CrossRef]
- Prellwitz, I.M.; Alves, B.M.; Ikeda, M.L.R.; Kuhleis, D.; Picon, P.D.; Jarczewski, C.A.; Osório, M.R.; Sánchez, A.; Seuánez, H.N.; Larouzé, B.; et al. HIV behind Bars: Human Immunodeficiency Virus Cluster Analysis and Drug Resistance in a Reference Correctional Unit from Southern Brazil. PLoS ONE 2013, 8, e69033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das. Ministério Da Saúde. Secr. Vigilância Em Saúde. Dep. Vigilância Prevenção E Control. Das Infecções Sex. Transm. Do Hiv/Aids E Das Hepatites Virais 2018, 410. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_clinico_manejo_hiv_adultos.pdfc (accessed on 5 June 2022).
- Osti, N.M.; Pestana De Castro, A.F.; Ricci, L.C. Human immunodeficiency virus seroprevalence among inmates of the penitentiary complex of the region of campinas, State of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 1999, 94, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Burattini, M.N.; Massad, E.; Rozman, M.; Azevedo, R.S.; Carvalho, H.B. Correlation between HIV and HCV in Brazilian prisoners: Evidence for parenteral transmission inside prison. Rev. Saude Publica 2000, 34, 431–436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catalan-Soares, B.C.; Almeida, R.T.; Carneiro-Proietti, A.B. Prevalence of HIV-1/2, HTLV-I/II, hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and Trypanosoma cruzi among prison inmates at Manhuaçu, Minas Gerais State, Brazil. Rev. Soc. Bras. Med. Trop. 2000, 33, 27–30. [Google Scholar] [CrossRef]
- Marins, J.R.; Page-Shafer, K.; De Azevedo Barros, M.B.; Hudes, E.S.; Chen, S.; Hearst, N. Seroprevalence and risk factors for HIV infection among incarcerated men in Sorocaba, Brazil. AIDS Behav. 2000, 4, 121–128. [Google Scholar] [CrossRef]
- Berra JA, P.; Bacetti, L.B.; Buzo, A.A. Soroprevalência de HIV, sífilis, hepatite B e C em mulheres do Centro de Ressocialização Feminino, Rio Claro, São Paulo. Rev. Inst. Adolfo Lutz. 2006, 65, 133–136. [Google Scholar]
- Coelho, H.C.; Perdoná, G.C.; Neves, F.R.; Passos, A.D.C. HIV prevalence and risk factors in a Brazilian penitentiary. Cad. Saude Publica. 2007, 23, 2197–2204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabe, C.; Lara, G.M. Prevalência de anti-HCV, anti-HIV e co-infecção HCV/HIV em um presídio feminino do Estado do Rio Grande do Sul. Rev. Bras. Análises ClíNicas 2008, 40, 87–89. [Google Scholar]
- De Albuquerque, A.C.C.; Da Silva, D.M.; Rabelo, D.C.C.; De Lucena, W.A.T.; De Lima, P.C.S.; Coêlho, M.R.C.D.; Tiago, G.G.D.B. Seroprevalence and factors associated with human immunodeficiency virus (HIV) and syphilis in inmates in the state of Pernambuco, Brazil. Cien. Saude Colet. 2014, 19, 2125–2132. [Google Scholar] [CrossRef]
- El Maerrawi, I.; Carvalho, H.B. Prevalence and risk factors associated with HIV infection, hepatitis and syphilis in a state prison of São Paulo. Int. J. STD AIDS. 2015, 26, 120–127. [Google Scholar] [CrossRef]
- de Sousa, K.A.A.; de Araújo, T.M.E.; Teles, S.A.; Rangel, E.M.L.; Nery, I.S. Factors associated with HIV prevalence in a prison population. Rev. da Esc. Enferm. 2017, 51, e03274. [Google Scholar] [CrossRef]
- Ferreto, L.E.D.; Guedes, S.; Pauli, F.B.; Rovani, S.S.; Follador, F.A.C.; Vieira, A.P.; Torres, R.H.; Coelho, H.C.; Wendt, G.W. Seroprevalence and associated factors of hiv and hepatitis c in brazilian high-security prisons: A state-wide epidemiological study. PLoS ONE 2021, 16, e0255173. [Google Scholar] [CrossRef]
- Massad, E.; Rozman, M.; Azevedo, R.; Silveira, A.; Takey, K.; Yamamoto, Y.; Strazza, L.; Ferreira, M.; Burattini, M.N.; Burattini, M.N.; et al. Seroprevalence of HIV, HCV and syphilis in Brazilian prisoners: Preponderance of parenteral transmission. Eur. J. Epidemiol. 1999, 15, 439–445. [Google Scholar] [CrossRef]
- Brenner, B.G.; Ibanescu, R.-I.; Osman, N.; Cuadra-Foy, E.; Oliveira, M.; Chaillon, A.; Stephens, D.; Hardy, I.; Routy, J.-P.; Thomas, R.; et al. The role of phylogenetics in unravelling patterns of HIV transmission towards epidemic control: The Quebec experience (2002–2020). Viruses 2021, 13, 1643. [Google Scholar] [CrossRef]
- Grabowski, M.K.; Herbeck, J.T.; Poon, A.F.Y. Genetic Cluster Analysis for HIV Prevention. Curr. HIV/AIDS 2018, 15, 182–189. [Google Scholar] [CrossRef]
- Gräf, T.; Delatorre, E.; Bello, G. Phylogenetics applied to the human immunodeficiency virus type 1 (HIV-1): From the cross-species transmissions to the contact network inferences. Mem. Inst. Oswaldo Cruz. 2020, 115, e190461. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.A.M.; Bandeira, L.M.; Pompilio, M.A.; De Rezende, G.R.; Soares, L.S.; Castro, V.D.O.L.D.; Tanaka, T.S.O.; Cesar, G.A.; Oliveira, S.; Teles, S.A.; et al. Screening for HBV, HCV, HIV and syphilis infections among bacteriologically confirmed tuberculosis prisoners: An urgent action required. PLoS ONE 2019, 14, e0221265. [Google Scholar] [CrossRef]
- Delatorre, E.; Silva-de-Jesus, C.; Couto-Fernandez, J.C.; Pilotto, J.H.; Morgado, M.G. High HIV-1 diversity and prevalence of transmitted drug resistance among antiretroviral-Naive HIV-infected pregnant women from Rio de Janeiro, Brazil. AIDS Res. Hum. Retrovir. 2017, 33, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Peña, A.-C.; Faria, N.R.; Imbrechts, S.; Libin, P.; Abecasis, A.B.; Deforche, K.; Gómez-López, A.; Camacho, R.J.; de Oliveira, T.; Vandamme, A.-M. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infect. Genet. Evol. 2013, 19, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Struck, D.; Lawyer, G.; Ternes, A.M.; Schmit, J.C.; Bercoff, D.P. COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2014, 42, e144. [Google Scholar] [CrossRef]
- Nei, M.; Saitou, N. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Mehtods to Estimate Maximum-Likelihood Phylogenies: Asessing the Performance of PhyML 2.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, a. rate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- Gifford, R.J.; Liu, T.F.; Rhee, S.-Y.; Kiuchi, M.; Hue, S.; Pillay, D.; Shafer, R.W. The calibrated population resistance tool: Standardized genotypic estimation of transmitted HIV-1 drug resistance. Bioinformatics 2009, 25, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e4724. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.W. Rationale and uses of a public HIV drug-resistance database. J. Infect. Dis. 2006, 194 (Suppl. 1), S51–S58. [Google Scholar] [CrossRef]
- Cardoso LP, V.; Da Silveira, A.A.; Francisco RB, L.; Da Guarda Reis, M.N.; De Araújo Stefani, M.M. Molecular characteristics of HIV type 1 infection among prisoners from central western Brazil. AIDS Res. Hum. Retrovir. 2011, 27, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, A.A.; Cardoso, L.P.V.; Francisco, R.B.L.; De Araújo Stefani, M.M. HIV type 1 molecular epidemiology in pol and gp41 genes among naive patients from mato Grosso do sul State, Central Western Brazil. AIDS Res. Hum. Retrovir. 2012, 28, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.S.O.; Leite, T.F.; Freitas, S.Z.; Cesar, G.A.; de Rezende, G.R.; Lindenberg, A.D.S.C.; Guimarães, M.L.; Motta-Castro, A.R.C. HIV-1 molecular epidemiology, transmission clusters and transmitted drug resistance mutations in central Brazil. Front. Microbiol. 2019, 10, 20. [Google Scholar] [CrossRef]
- Stefani, M.M.; Pereira, G.A.; Lins, J.A.; Alcantara, K.C.; Silveira, A.A.; Viegas, A.A.; Maya, N.C.; Mussi, A.H. Molecular screening shows extensive HIV-1 genetic diversity in Central West Brazil. J. Clin. Virol. 2007, 39, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Mabud, T.S.; Alves, M.D.L.D.; Ko, A.I.; Basu, S.; Walter, K.S.; Cohen, T.; Mathema, B.; Colijn, C.; Lemos, E.; Croda, J.; et al. Evaluating strategies for control of tuberculosis in prisons and prevention of spillover into communities: An observational and modeling study from Brazil. PLoS Med. 2019, 16, e1002737. [Google Scholar] [CrossRef]
- Goodman, D.D.; Zhou, Y.; A Margot, N.; McColl, D.J.; Zhong, L.; Borroto-Esoda, K.; Miller, M.D.; Svarovskaia, E.S. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS 2011, 25, 325–333. [Google Scholar] [CrossRef]

| Cluster | aLRT | Sequences | Distance | Subcluster | aLRT | Distance | Cluster Confirmation |
|---|---|---|---|---|---|---|---|
| HIV-1B | |||||||
| 1 | 0.99 | BRMS02.JF342274 | 0.034 | - | - | - | Confirmed |
| BRMS46.JF342297 | |||||||
| HET801.MF545297 | |||||||
| HET462.MF545270 | |||||||
| HET631.MF545285 | |||||||
| RT74 | |||||||
| 2 | 0.94 | PSD1689 | 0.054 | 2A | 0.939 | 0.053 | Not confirmed |
| BRMS53.JF342303 | |||||||
| BREPM1028.EF637053 | |||||||
| 07CE140556.KY581465 | |||||||
| PSD668 | 2B | 0.71 | 0.049 | Not confirmed | |||
| HET830.MF545301 | |||||||
| PSD3419 | 2C | 0.968 | 0.039 | Confirmed | |||
| HET640.MF545286 | |||||||
| 3 | 0.92 | HSH874.MF545336 | 0.036 | Confirmed | |||
| HET814.MF545299 | |||||||
| RT53 | |||||||
| HSH881.MF545340 | |||||||
| HET249.MF545255 | |||||||
| 4 | 0.90 | HET622.MF545284 | 0.052 | ||||
| HET850.MF545302 | |||||||
| PTB204 | 4A | 0.91 | 0.027 | Confirmed | |||
| BRMS99.HQ831455 | |||||||
| BRMS97.HQ831454 | |||||||
| HSH871.MF545333 | |||||||
| PTB178 | |||||||
| PSD3034 | 4B | 0.96 | 0.043 | Confirmed | |||
| HSH408.MF545314 | |||||||
| HSH862.MF545331 | |||||||
| HET738.MF545293 | |||||||
| HET521.MF545276 | |||||||
| HET323.MF545260 | |||||||
| HSH719.MF545326 | |||||||
| HET437.MF545267 | |||||||
| HSH717.MF545325 | 4C | 1 | 0.039 | Confirmed | |||
| MF545324.HSH716 | |||||||
| PSD2529 | |||||||
| HSH875.MF545337 | |||||||
| MF545332.HSH867 | |||||||
| HET757.MF545294 | |||||||
| 5 | 0.91 | BREPM1084.FJ195088 | 0.051 | 5A | 0.91 | 0.051 | Not confirmed |
| PTB16 | |||||||
| HET219.MF545251 | 5B | 0.784 | 0.052 | Not confirmed | |||
| PSD1190 | |||||||
| 6 | 0.91 | RT58 | 0.074 | - | - | - | Not confirmed |
| 02BR002.DQ358805 | |||||||
| 7 | 0.97 | PSD1678 | 0.044 | - | - | - | Confirmed |
| BRMS117.HQ831457 | |||||||
| HSH859.MF545329 | |||||||
| 8 | 1 | PSD2207 | 0.044 | - | - | - | Confirmed |
| HET362.MF545261 | |||||||
| 9 | 0.93 | PTB92 | 0.057 | 9A | 0.095 | 0.057 | Not confirmed |
| HET446.MF545269 | |||||||
| PTB229 | |||||||
| HET522.MF545277 | |||||||
| PSD700 | 9B | 0.73 | 0.044 | Not confirmed | |||
| HET479.MF545271 | |||||||
| 10 | 0.98 | RT56 | 0.072 | - | - | - | Not confirmed |
| BRTO12 83.GU214093 | |||||||
| 11 | 0.912 | RT51 | 0.054 | - | - | - | Not confirmed |
| RT70 | |||||||
| 12 | 1 | RT52 | 0.037 | - | - | - | Confirmed |
| BRMS27.JF342282 | |||||||
| 13 | 0.92 | RT68 | 0.037 | - | - | - | Confirmed |
| HET501.MF545272 | |||||||
| 14 | 0.91 | RT73 | 0.036 | - | - | - | Confirmed |
| PSD3041 | |||||||
| HET671.MF545288 | |||||||
| PSD27 | |||||||
| HET438.MF545268 | |||||||
| 15 | 0.97 | PSD124 | 0.042 | - | - | - | Confirmed |
| PSD140 | |||||||
| 16 | 0.93 | RT71 | 0.031 | - | - | - | Confirmed |
| HSH239.MF545312 | |||||||
| HIV-1C | |||||||
| 17 | 0.964 | PTB19 | 0.021 | - | - | - | Confirmed |
| HSH880.MF545237 | |||||||
| PSD2681 | |||||||
| 06BRRJ_09.KF255844 | |||||||
| Variable | N | (%) | Clustered N (%) | Not Clustered N (%) | OR (95% CI) | p | aOR (95%CI) ˠ | p |
|---|---|---|---|---|---|---|---|---|
| Total | 19 (28.4) | 48 (71.6) | (18.7–40.6) | |||||
| Gender | ||||||||
| Male | 60 | (89.5) | 19 (31.7) | 41 (68.3) | - | - | - | - |
| Female | 7 | (10.5) | 0 (0.0) | 8 (100.0) | - | - | - | - |
| Age (years) | ||||||||
| 18–34 | 31 | (46.3) | 11 (35.5) | 200 (64.5) | 1.0 | |||
| 35 or more | 36 | (53.7) | 8 (22.2) | 28 (77.8) | 0.52 (0.17–1.52) | 0.23 | - | - |
| Skin colour/ethnicity | ||||||||
| White | 22 | (33.3) | 10 (45.5) | 12 (54.5) | 1.0 | 1.0 | ||
| Non-white | 44 | (66.7) | 9 (20.4) | 35 (79.6) | 0.31 (0.10–0.94) | 0.04 | 0.34 (0.09–1.25) | 0.10 |
| Educational (years) # | ||||||||
| 0 | 30 | (46.8) | 7 (23.3) | 23 (76.7) | - | - | - | - |
| 1–12 | 31 | (48.4) | 11 (35.5) | 20 (64.5) | - | - | - | - |
| >12 | 3 | (4.7) | 0 (0.0) | 3 (100.0) | - | - | - | - |
| Naturality | ||||||||
| MS State | 41 | (61.2) | 13 (31.7) | 28 (68.3) | 1.0 | |||
| Other | 26 | (38.8) | 6 (23.1) | 20 (76.9) | 0.71 (0.24–21.2) | 0.4 | - | - |
| Persons in the same cell | ||||||||
| 1–10 | 27 | (40.9) | 8 (29.6) | 19 (70.4) | 1.0 | |||
| 11–30 | 22 | (33.3) | 6 (27.3) | 16 (72.7) | 0.89 (0.22–2.52) | 0.86 | - | - |
| 31 or more | 17 | (25.7) | 5 (29.4) | 12 (70.6) | 0.98 (0.08–1.60) | 0.98 | - | - |
| Time of incarceration # | ||||||||
| Up to 5 years | 50 | (76.9) | 14 (28.0) | 36 (72.0) | 1.0 | |||
| More than 5 years | 15 | (23.1) | 5 (33.3) | 10 (66.7) | 0.69 (0.37–4.43) | 0.69 | - | - |
| Previous incarceration | ||||||||
| No | 19 | (28.4) | 5 (26.3) | 14 (73.7) | 1.0 | |||
| Yes | 48 | (71.6) | 14 (29.2) | 34 (70.8) | 1.15 (0.35–3.81) | 0.81 | - | - |
| Illicit drug use | ||||||||
| No | 1 | (1.5) | 0 (0.0) | 1 (100.0) | - | - | - | - |
| Yes, no injecting drugs | 63 | (97.0) | 9 (14.3) | 54 (85.7) | - | - | - | - |
| Yes, injecting drugs | 1 | (1.5) | 0 (0.0) | 1 (100.0) | - | - | - | - |
| Cocaine base pasta use # | ||||||||
| No | 55 | (84.6) | 13 (23.6) | 42 (76.4) | 1.0 | |||
| Yes | 10 | (15.4) | 6 (60.0) | 4 (40.0) | 4.84 (1.18–19.8) | 0.02 | 3.57 (0.69–18.4) | 0.12 |
| History of homosexual contact # | ||||||||
| No | 51 | (19.1) | 11 (21.6) | 40 (78.4) | 1.0 | |||
| Yes | 12 | (80.9) | 7 (58.3) | 5 (41.7) | 4.90 (1.43–16.82) | 0.01 | 8.03 (1.30–49.23) | 0.02 |
| Number of sexual partners in the last 12 months | ||||||||
| 0 | 15 | (22.4) | 4 (26.7) | 11 (73.3) | 1.0 | |||
| 1-5 | 43 | (64.2) | 11 (25.6) | 32 (74.4) | 0.94 (0.24–3.59) | 0.93 | - | - |
| ≥6 | 9 | (13.4) | 4 (44.4) | 5 (55.6) | 2.20 (0.38–12.6) | 0.37 | - | - |
| Use of condoms in the last 12 months | ||||||||
| Always | 29 | (43.9) | 9 (31.0) | 20 (69.0) | 1.0 | |||
| Occasionally/Never | 37 | (56.1) | 10 (27.0) | 27 (73.0) | 0.82 (0.29–2.39) | 0.72 | - | - |
| History of STI | ||||||||
| No | 30 | (45.5) | 10 (33.3) | 20 (66.7) | 1.0 | |||
| Yes | 36 | (54.5) | 9 (25.0) | 27 (75.0) | 0.7 (0.23–1.95) | 0.46 | - | - |
| Genital ulcers | ||||||||
| No | 62 | (92.5) | 16 (25.8) | 46 (74.2) | 1.0 | |||
| Yes | 5 | (7.5) | 3 (60.0) | 2 (40.0) | 4.31 (0.66–28.2) | 0.12 | - | - |
| HIV-1 Subtype | ||||||||
| B | 49 | (73.2) | 17 (34.7) | 32 (65.3) | 1.0 | |||
| Non-B | 18 | (26.8) | 2 (11.1) | 33 (88.9) | 0.23 (0.04–1.14) | 0.05 | 0.01 (0.01–1.7) | 0.05 |
| ID | Age | Resistance Mutations | HIV-1 Subtype | Type of Exposure | ||
|---|---|---|---|---|---|---|
| NRTI | NNRTI | PI | ||||
| ART-experienced n = 16 | ||||||
| PSD1270 * | 28 | M184I | B | Sex with HIV-infected partner | ||
| PSD2402 | 30 | K103N | URF_BF | Homemade tattooing, multiple sexual partners | ||
| PSD2529 | 27 | K103NS, V106M | B | Irregular condom use, sharing of sharp objects | ||
| PSD2671 | 41 | K103N | B | Sex with HIV-infected and drug user | ||
| PSD2802 | 37 | V106I | B | Multiple sexual partners | ||
| PSD3034 | 40 | M41L, M184V, T215Y | K103N, P225H | B | Sex with drug user | |
| PTB16 | 27 | K103N | B | None | ||
| PTB70 | 26 | V106I | URF_BF | MSM, Irregular condom use, sharing of sharp objects | ||
| PTB165 | 26 | E44D | C | MSM, Irregular condom use, Sex with drug user, sharing of sharp objects | ||
| PTB177 | 54 | Y181YH | B | Irregular condom use, sharing of sharp objects | ||
| PTB204 | 35 | M41L, L74I, M184V, T215Y | K103N, E138A, P255H | B | MSM and sex with drug user | |
| PTB229 | 44 | A62V, K65R, M184V | L100I, K103N | B | None | |
| RT58 | 27 | D67NK70R, T215Y | K103N, V106I, V179T, Y188F, H221Y | I84V, L90M, Q58E, G73S | B | Irregular condom use, MSM and sex with drug user |
| RT64 | 28 | Y181F | URF_BF | Irregular condom use | ||
| RT71 | 44 | F227FL | B | MSM, sharing of sharp objects | ||
| RT75 | 27 | K103N | URF_BF | MSM, history of STI | ||
| ART-naive n = 2 | ||||||
| PSD2368 | 26 | K103N | B | Sharing of sharp objects | ||
| PSD3117 | 47 | K219Q | K103N | L90M | D | History of STI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.S.O.; Cesar, G.A.; Rezende, G.R.d.; Puga, M.A.M.; Weis-Torres, S.M.d.S.; Bandeira, L.M.; Pompílio, M.A.; Croda, J.; Guimarães, M.L.; Motta-Castro, A.R.C. Molecular Epidemiology of HIV-1 among Prisoners in Central Brazil and Evidence of Transmission Clusters. Viruses 2022, 14, 1660. https://doi.org/10.3390/v14081660
Tanaka TSO, Cesar GA, Rezende GRd, Puga MAM, Weis-Torres SMdS, Bandeira LM, Pompílio MA, Croda J, Guimarães ML, Motta-Castro ARC. Molecular Epidemiology of HIV-1 among Prisoners in Central Brazil and Evidence of Transmission Clusters. Viruses. 2022; 14(8):1660. https://doi.org/10.3390/v14081660
Chicago/Turabian StyleTanaka, Tayana Serpa Ortiz, Gabriela Alves Cesar, Grazielli Rocha de Rezende, Marco Antonio Moreira Puga, Sabrina Moreira dos Santos Weis-Torres, Larissa Melo Bandeira, Maurício Antônio Pompílio, Julio Croda, Monick Lindenmeyer Guimarães, and Ana Rita Coimbra Motta-Castro. 2022. "Molecular Epidemiology of HIV-1 among Prisoners in Central Brazil and Evidence of Transmission Clusters" Viruses 14, no. 8: 1660. https://doi.org/10.3390/v14081660
APA StyleTanaka, T. S. O., Cesar, G. A., Rezende, G. R. d., Puga, M. A. M., Weis-Torres, S. M. d. S., Bandeira, L. M., Pompílio, M. A., Croda, J., Guimarães, M. L., & Motta-Castro, A. R. C. (2022). Molecular Epidemiology of HIV-1 among Prisoners in Central Brazil and Evidence of Transmission Clusters. Viruses, 14(8), 1660. https://doi.org/10.3390/v14081660