Characterization of a Human Sapovirus Genotype GII.3 Strain Generated by a Reverse Genetics System: VP2 Is a Minor Structural Protein of the Virion
Abstract
:1. Introduction
2. Materials and Methods
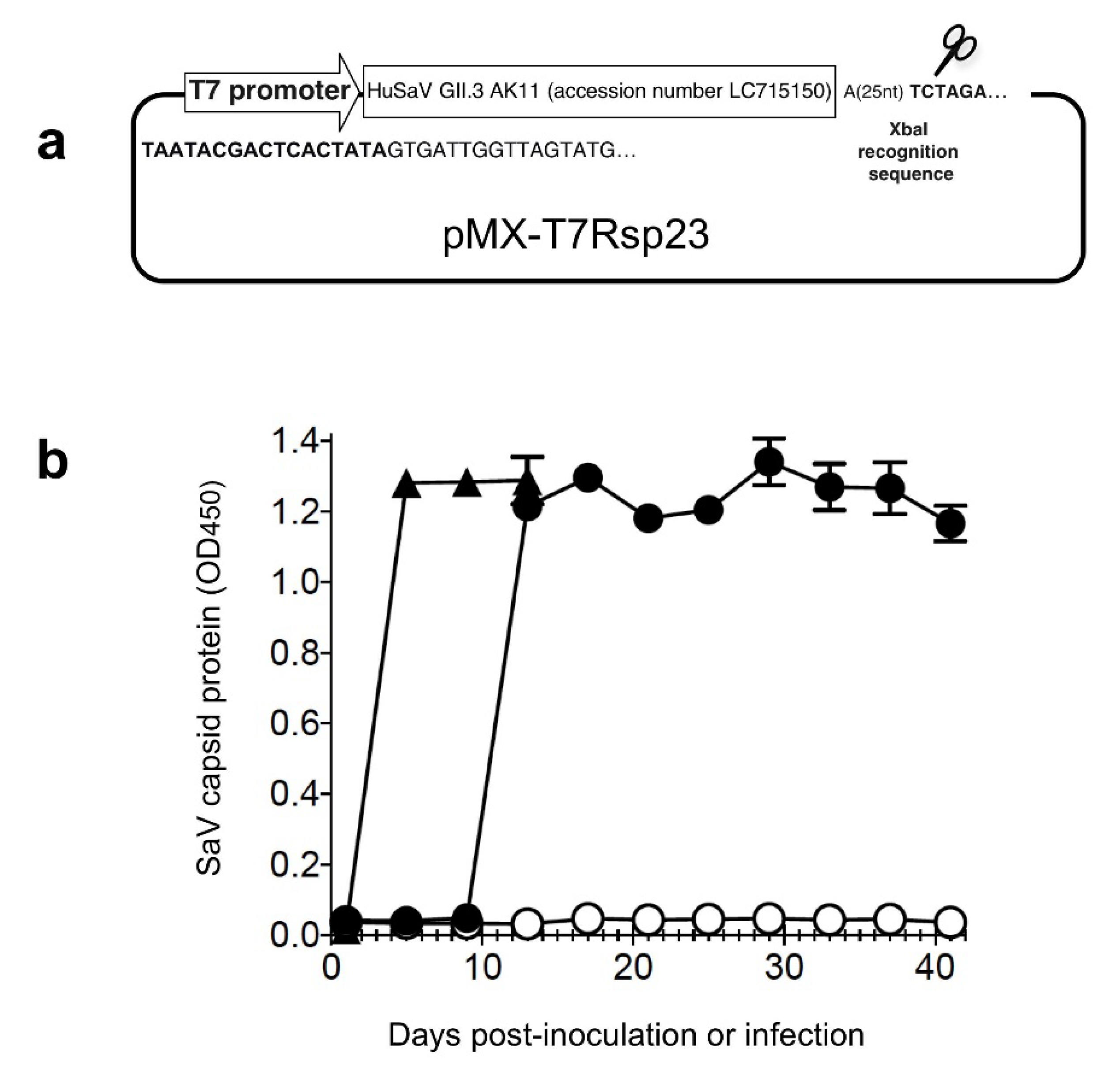
2.1. In Vitro Transcription of HuSaV GII.3 RNA
2.2. Cell Culture, Transfection, and Virus Inoculation
2.3. Antigen Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of HuSaV GII.3 Capsid VP1 Protein
2.4. Real-Time Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) for the Detection of HuSaV GII.3 RNA
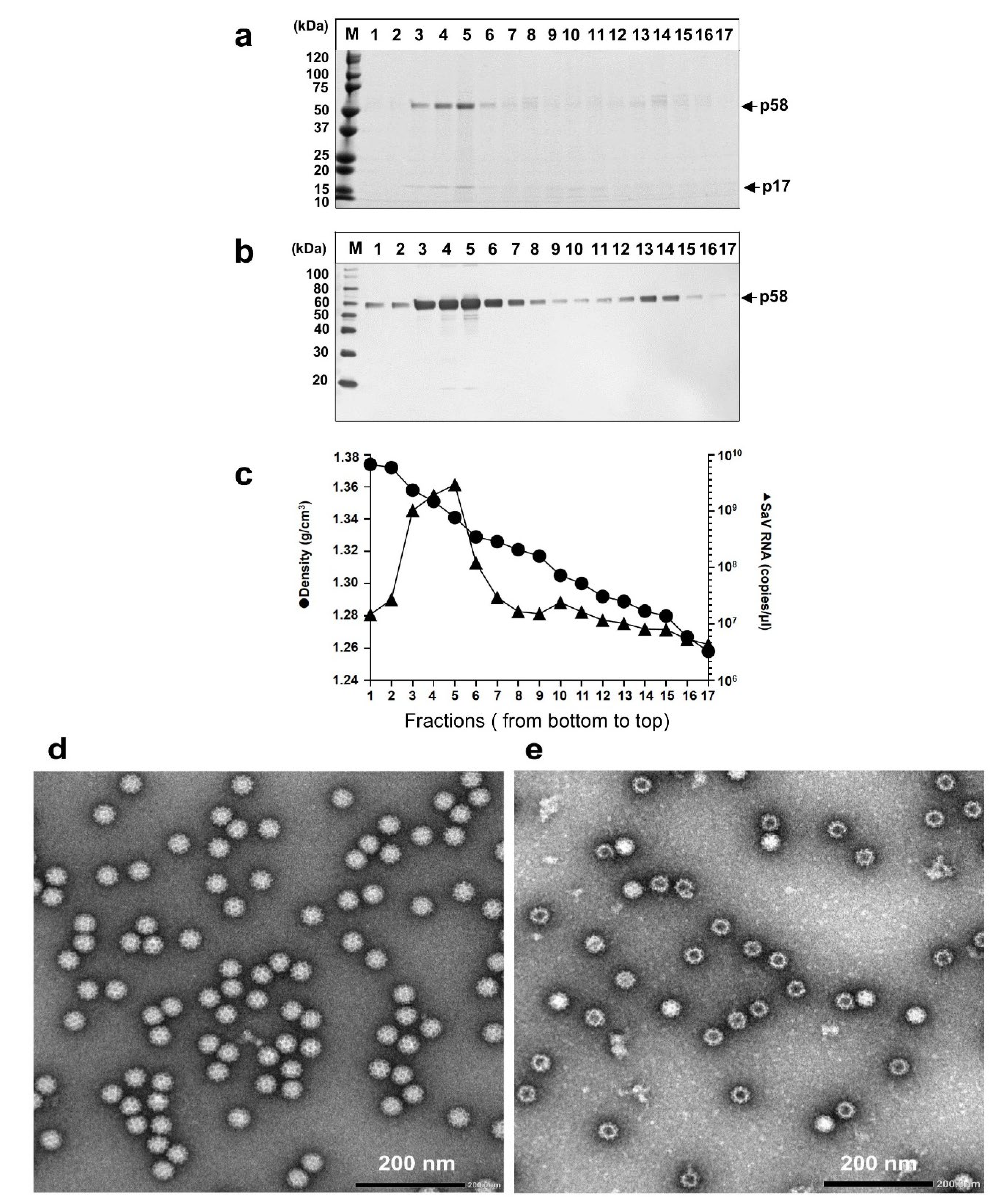
2.5. Purification of HuSaV Particles
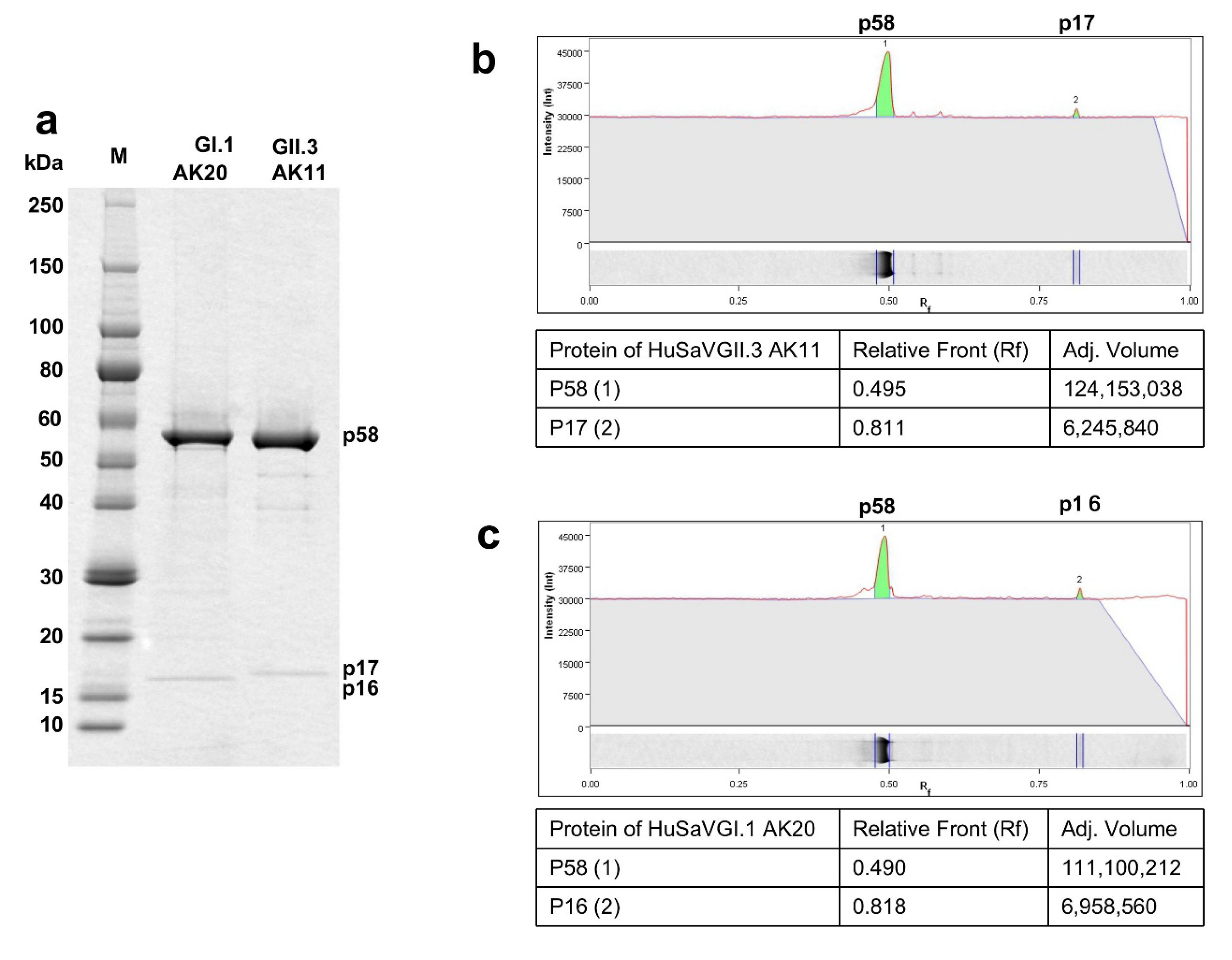
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
2.7. Transmission Electron Microscopy (TEM)
2.8. Viral Genome Sequencing
2.9. Mass Spectrometry Analysis
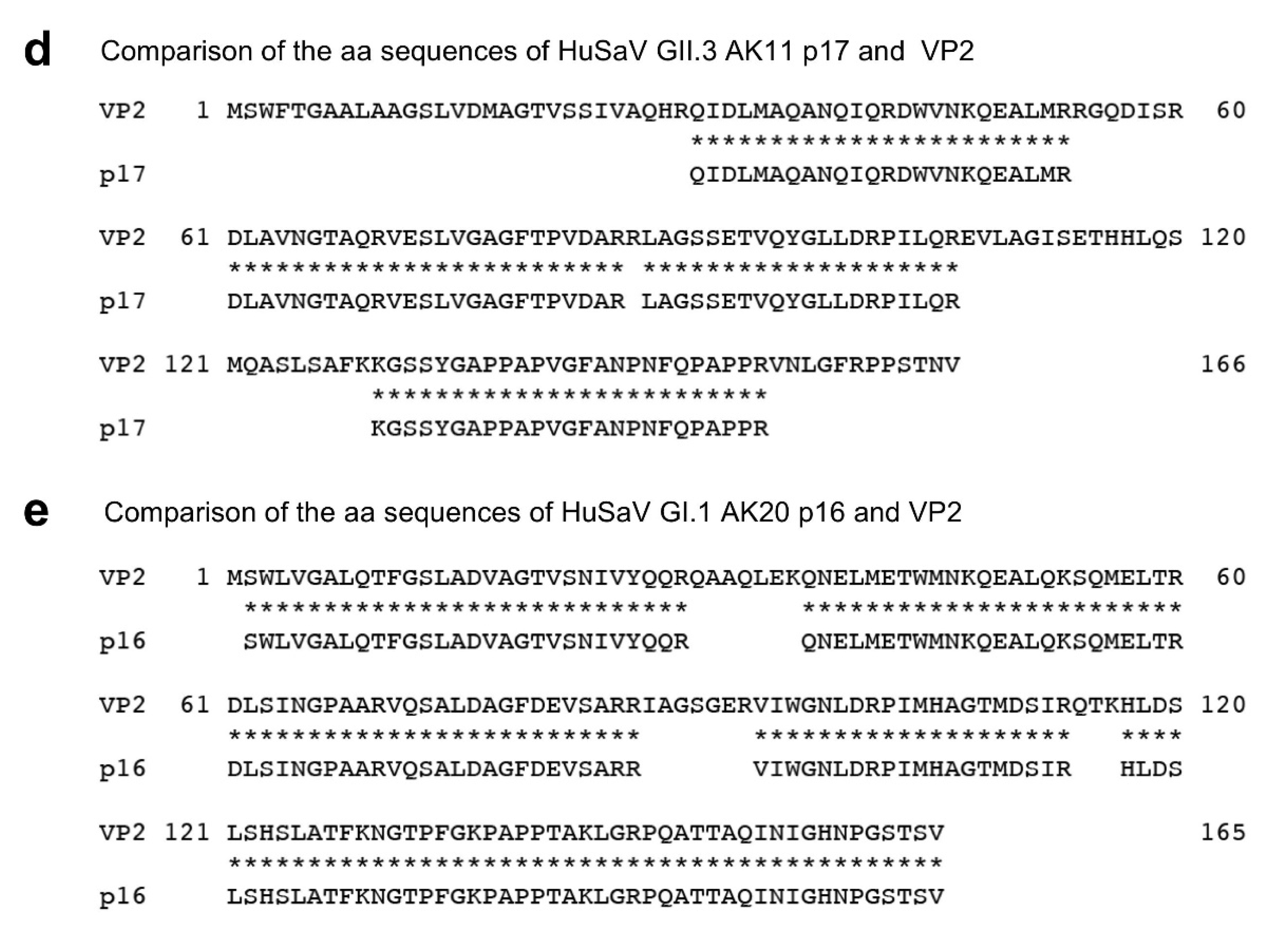
2.10. N-Terminal Amino Acid Sequence Analysis
3. Results
3.1. Generation of the Infectious HuSaV GII.3 AK11 Strain
3.2. Purification and Characterization of the HuSaV GII.3 AK11 Virions
3.3. Analyses of the Capsid Proteins of AK11 and AK20 Virion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oka, T.; Wang, Q.; Katayama, K.; Saif, L.J. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 2015, 28, 32–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, T.; Lu, Z.; Phan, T.; Delwart, E.L.; Saif, L.J.; Wang, Q. Genetic Characterization and Classification of Human and Animal Sapoviruses. PLoS ONE 2016, 11, e0156373. [Google Scholar] [CrossRef]
- Yinda, C.K.; Conceição-Neto, N.; Zeller, M.; Heylen, E.; Maes, P.; Ghogomu, S.M.; Van Ranst, M.; Matthijnssens, J. Novel highly divergent sapoviruses detected by metagenomics analysis in straw-colored fruit bats in Cameroon. Emerg. Microbes Infect. 2017, 6, e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, W.; Cui, L.; Shen, Q.; Hua, X. Metagenomic identification, genetic characterization and genotyping of porcine sapoviruses. Infect. Genet. Evol. 2018, 62, 244–252. [Google Scholar] [CrossRef]
- Hansman, G.S.; Oka, T.; Sakon, N.; Takeda, N. Antigenic diversity of human sapoviruses. Emerg. Infect. Dis. 2007, 13, 1519–1525. [Google Scholar] [CrossRef]
- Kitamoto, N.; Oka, T.; Katayama, K.; Li, T.-C.; Takeda, N.; Kato, Y.; Miyoshi, T.; Tanaka, T. Novel monoclonal antibodies broadly reactive to human recombinant sapovirus-like particles. Microbiol. Immunol. 2012, 56, 760–770. [Google Scholar] [CrossRef]
- Oka, T.; Miyashita, K.; Katayama, K.; Wakita, T.; Takeda, N. Distinct genotype and antigenicity among genogroup II sapoviruses. Microbiol. Immunol. 2009, 53, 417–420. [Google Scholar] [CrossRef]
- Wu, F.T.; Oka, T.; Kuo, T.Y.; Doan, Y.H.; Tzu-Chi Liu, L. Sapoviruses detected from acute gastroenteritis outbreaks and hospitalized children in Taiwan. J. Formos. Med. Assoc. 2021, 120, 1591–1601. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Kong, X.; Li, H.; Ba, Q.Z.; Jin, M.; Wang, Y.; Duan, Z. Two gastroenteritis outbreaks caused by sapovirus in Shenzhen, China. J. Med. Virol. 2018, 90, 1695–1702. [Google Scholar] [CrossRef]
- Neo, F.J.X.; Loh, J.J.P.; Ting, P.; Yeo, W.X.; Gao, C.Q.H.; Lee, V.J.M.; Tan, B.H.; Ng, C.G. Outbreak of caliciviruses in the Singapore military, 2015. BMC Infect. Dis. 2017, 17, 719. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, R.; Ding, X.; Freedman, S.B.; Lee, B.E.; Ali, S.; Luong, J.; Xie, J.; Chui, L.; Wu, Y.; Pang, X. Molecular Epidemiology of Human Sapovirus among Children with Acute Gastroenteritis in Western Canada. J. Clin. Microbiol. 2021, 59, e0098621. [Google Scholar] [CrossRef]
- Cilli, A.; Luchs, A.; Morillo, S.G.; Carmona, R.D.C.C.; dos Santos, F.C.; Maeda, A.Y.; Primo, D.; Pacheco, G.T.; Souza, E.V.; Medeiros, R.S.; et al. Surveillance and molecular characterization of human sapovirus in patients with acute gastroenteritis in Brazil, 2010 to 2017. J. Clin. Virol. 2021, 140, 104844. [Google Scholar] [CrossRef]
- Song, K.; Lin, X.; Liu, Y.; Ji, F.; Zhang, L.; Chen, P.; Zhao, C.; Song, Y.; Tao, Z.; Xu, A. Detection of Human Sapoviruses in Sewage in China by Next Generation Sequencing. Food Environ. Virol. 2021, 13, 270–280. [Google Scholar] [CrossRef]
- Oka, T.; Yamamoto, M.; Katayama, K.; Hansman, G.; Ogawa, S.; Miyamura, T.; Takeda, N. Identification of the cleavage sites of sapovirus open reading frame 1 polyprotein. J. Gen. Virol. 2006, 87 Pt 11, 3329–3338. [Google Scholar] [CrossRef]
- Oka, T.; Yokoyama, M.; Katayama, K.; Tsunemitsu, H.; Yamamoto, M.; Miyashita, K.; Ogawa, S.; Motomura, K.; Mori, H.; Nakamura, H.; et al. Structural and biological constraints on diversity of regions immediately upstream of cleavage sites in calicivirus precursor proteins. Virology 2009, 394, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Kim, Y.; Saif, L.J.; Green, K.Y. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 2004, 101, 8733–8738. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, P.; Karakasiliotis, I.; Bailey, D.; Chaudhry, Y.; Evans, D.J.; Goodfellow, I.G. Bioinformatic and functional analysis of RNA secondary structure elements among different genera of human and animal caliciviruses. Nucleic Acids Res. 2008, 36, 2530–2546. [Google Scholar] [CrossRef] [Green Version]
- Desselberger, U. Caliciviridae Other Than Noroviruses. Viruses 2019, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Parwani, A.V.; Saif, L.J.; Kang, S.Y. Biochemical characterization of porcine enteric calicivirus: Analysis of structural and nonstructural viral proteins. Arch. Virol. 1990, 112, 41–53. [Google Scholar] [CrossRef]
- Numata, K.; Hardy, M.E.; Nakata, S.; Chiba, S.; Estes, M.K. Molecular characterization of morphologically typical human calicivirus Sapporo. Arch. Virol. 1997, 142, 1537–1552. [Google Scholar] [CrossRef]
- Oka, T.; Hansman, G.; Katayama, K.; Ogawa, S.; Nagata, N.; Miyamura, T.; Takeda, N. Expression of sapovirus virus-like particles in mammalian cells. Arch. Virol. 2006, 151, 399–404. [Google Scholar] [CrossRef]
- Hansman, G.S.; Oka, T.; Katayama, K.; Takeda, N. Enhancement of sapovirus recombinant capsid protein expression in insect cells. FEBS Lett. 2006, 580, 4047–4050. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Wang, Q.; Saif, L.J.; Green, K.Y. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J. Virol. 2005, 79, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Stoltzfus, G.T.; Zhu, C.; Jung, K.; Wang, Q.; Saif, L.J. Attempts to grow human noroviruses, a sapovirus, and a bovine norovirus in vitro. PLoS ONE 2018, 13, e0178157. [Google Scholar] [CrossRef]
- Takagi, H.; Oka, T.; Shimoike, T.; Saito, H.; Kobayashi, T.; Takahashi, T.; Tatsumi, C.; Kataoka, M.; Wang, Q.; Saif, L.J.; et al. Human sapovirus propagation in human cell lines supplemented with bile acids. Proc. Natl. Acad. Sci. USA 2020, 117, 32078–32085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ami, Y.; Suzaki, Y.; Doan, Y.H.; Takeda, N.; Muramatsu, M.; Li, T.-C. Generation of a Bactrian camel hepatitis E virus by a reverse genetics system. J. Gen. Virol. 2021, 102, 001618. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Iritani, N.; Yamamoto, S.P.; Mori, K.; Ogawa, T.; Tatsumi, C.; Shibata, S.; Harada, S.; Wu, F.-T. Broadly reactive real-time reverse transcription-polymerase chain reaction assay for the detection of human sapovirus genotypes. J. Med. Virol. 2019, 91, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yang, T.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Ishii, K.; Haga, K.; Nakamura, T.; Ochiai, S.; Takaji, W.; et al. Construction and characterization of an infectious cDNA clone of rat hepatitis E virus. J. Gen. Virol. 2015, 96 Pt 6, 1320–1327. [Google Scholar] [CrossRef]
- Sosnovtsev, S.; Green, K.Y. RNA transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require VpG for infectivity. Virology 1995, 210, 383–390. [Google Scholar] [CrossRef]
- Miyazaki, N.; Song, C.; Oka, T.; Miki, M.; Murakami, K.; Iwasaki, K.; Katayama, K.; Murata, K. Atomic Structure of the Human Sapovirus Capsid Reveals a Unique Capsid Protein Conformation in Caliciviruses. J. Virol. 2022, 96, e0029822. [Google Scholar] [CrossRef]
- Conley, M.J.; McElwee, M.; Azmi, L.; Gabrielsen, M.; Byron, O.; Goodfellow, I.G.; Bhella, D. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature 2019, 565, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Sakuma, Y.; Kogasaka, R.; Akihara, M.; Horino, K.; Nakao, T.; Fukui, S. An outbreak of gastroenteritis associated with calicivirus in an infant home. J. Med. Virol. 1979, 4, 249–254. [Google Scholar] [CrossRef]
- Chiba, S.; Sakuma, Y.; Kogasaka, R.; Akihara, M.; Terashima, H.; Horino, K.; Nakao, T. Fecal shedding of virus in relation to the days of illness in infantile gastroenteritis due to calicivirus. J. Infect. Dis. 1980, 142, 247–249. [Google Scholar] [CrossRef]
- Suzuki, H.; Konno, T.; Kutsuzawa, T.; Imai, A.; Tazawa, F.; Ishida, N.; Katsushima, N.; Sakamoto, M. The occurrence of calicivirus in infants with acute gastroenteritis. J. Med. Virol. 1979, 4, 321–326. [Google Scholar] [CrossRef]
- Terashima, H.; Chiba, S.; Sakuma, Y.; Kogasaka, R.; Nakata, S.; Minami, R.; Horino, K.; Nakao, T. The polypeptide of a human calicivirus. Arch. Virol. 1983, 78, 1–7. [Google Scholar] [CrossRef]
- Nakata, S.; Estes, M.K.; Chiba, S. Detection of human calicivirus antigen and antibody by enzyme-linked immunosorbent assays. J. Clin. Microbiol. 1988, 26, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Yamamoto, M.; Miyashita, K.; Ogawa, S.; Katayama, K.; Wakita, T.; Takeda, N. Self-assembly of sapovirus recombinant virus-like particles from polyprotein in mammalian cells. Microbiol. Immunol. 2009, 53, 49–52. [Google Scholar] [CrossRef]
- Nakata, S.; Honma, S.; Kinoshita-Numata, K.; Kogawa, K.; Ukae, S.; Morita, Y.; Adachi, N.; Chiba, S. Members of the family caliciviridae (Norwalk virus and Sapporo virus) are the most prevalent cause of gastroenteritis outbreaks among infants in Japan. J. Infect. Dis. 2000, 181, 2029–2032. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhong, W.; Kaplan, M.; Pickering, L.K.; Matson, D.O. Expression and characterization of Sapporo-like human calicivirus capsid proteins in baculovirus. J. Virol. Methods 1999, 78, 81–91. [Google Scholar] [CrossRef]
- Hansman, G.S.; Oka, T.; Takeda, N. Sapovirus-like particles derived from polyprotein. Virus Res. 2008, 137, 261–265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.-C.; Kataoka, M.; Doan, Y.H.; Saito, H.; Takagi, H.; Muramatsu, M.; Oka, T. Characterization of a Human Sapovirus Genotype GII.3 Strain Generated by a Reverse Genetics System: VP2 Is a Minor Structural Protein of the Virion. Viruses 2022, 14, 1649. https://doi.org/10.3390/v14081649
Li T-C, Kataoka M, Doan YH, Saito H, Takagi H, Muramatsu M, Oka T. Characterization of a Human Sapovirus Genotype GII.3 Strain Generated by a Reverse Genetics System: VP2 Is a Minor Structural Protein of the Virion. Viruses. 2022; 14(8):1649. https://doi.org/10.3390/v14081649
Chicago/Turabian StyleLi, Tian-Cheng, Michiyo Kataoka, Yen Hai Doan, Hiroyuki Saito, Hirotaka Takagi, Masamichi Muramatsu, and Tomoichiro Oka. 2022. "Characterization of a Human Sapovirus Genotype GII.3 Strain Generated by a Reverse Genetics System: VP2 Is a Minor Structural Protein of the Virion" Viruses 14, no. 8: 1649. https://doi.org/10.3390/v14081649
APA StyleLi, T.-C., Kataoka, M., Doan, Y. H., Saito, H., Takagi, H., Muramatsu, M., & Oka, T. (2022). Characterization of a Human Sapovirus Genotype GII.3 Strain Generated by a Reverse Genetics System: VP2 Is a Minor Structural Protein of the Virion. Viruses, 14(8), 1649. https://doi.org/10.3390/v14081649






