Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: HIV-1 Transgenic Rats
2.1.1. Neuroanatomical Assessments
Animals
Immunofluorescence Staining
Neuronal Labeling
2.1.2. Neurocognitive Assessments
Animals
Apparatus
Procedure
2.2. Experiment 2: Post-Mortem HIV-1 Seropositive Individuals with HAND
2.2.1. Neuroanatomical Assessments
Immunofluorescence Staining
Thioflavin-S Staining
2.3. Statistical Analysis
3. Results
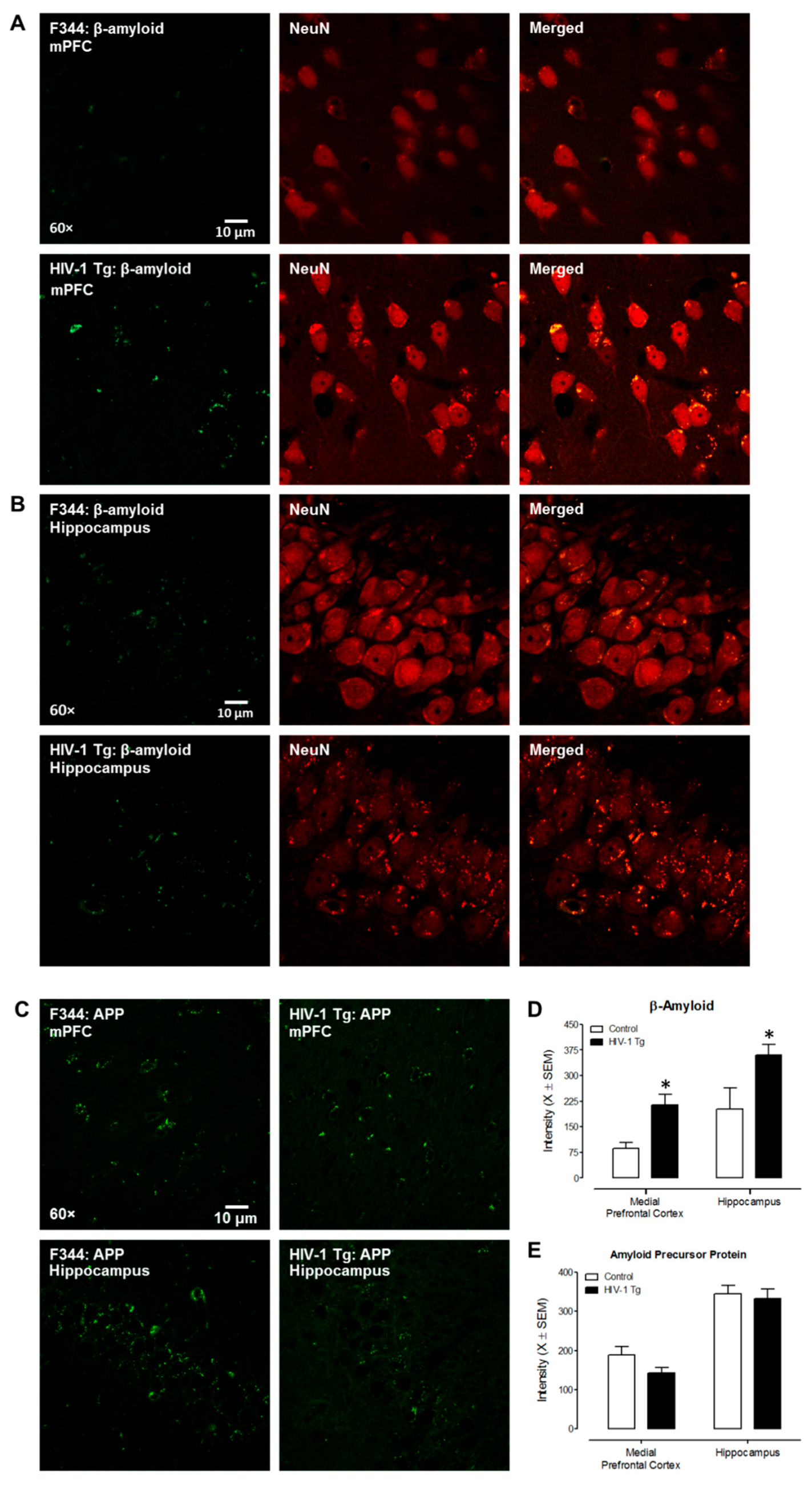
3.1. Experiment 1: HIV-1 Transgenic Rats
3.1.1. Neuroanatomical Assessments
3.1.2. Neurocognitive Assessments
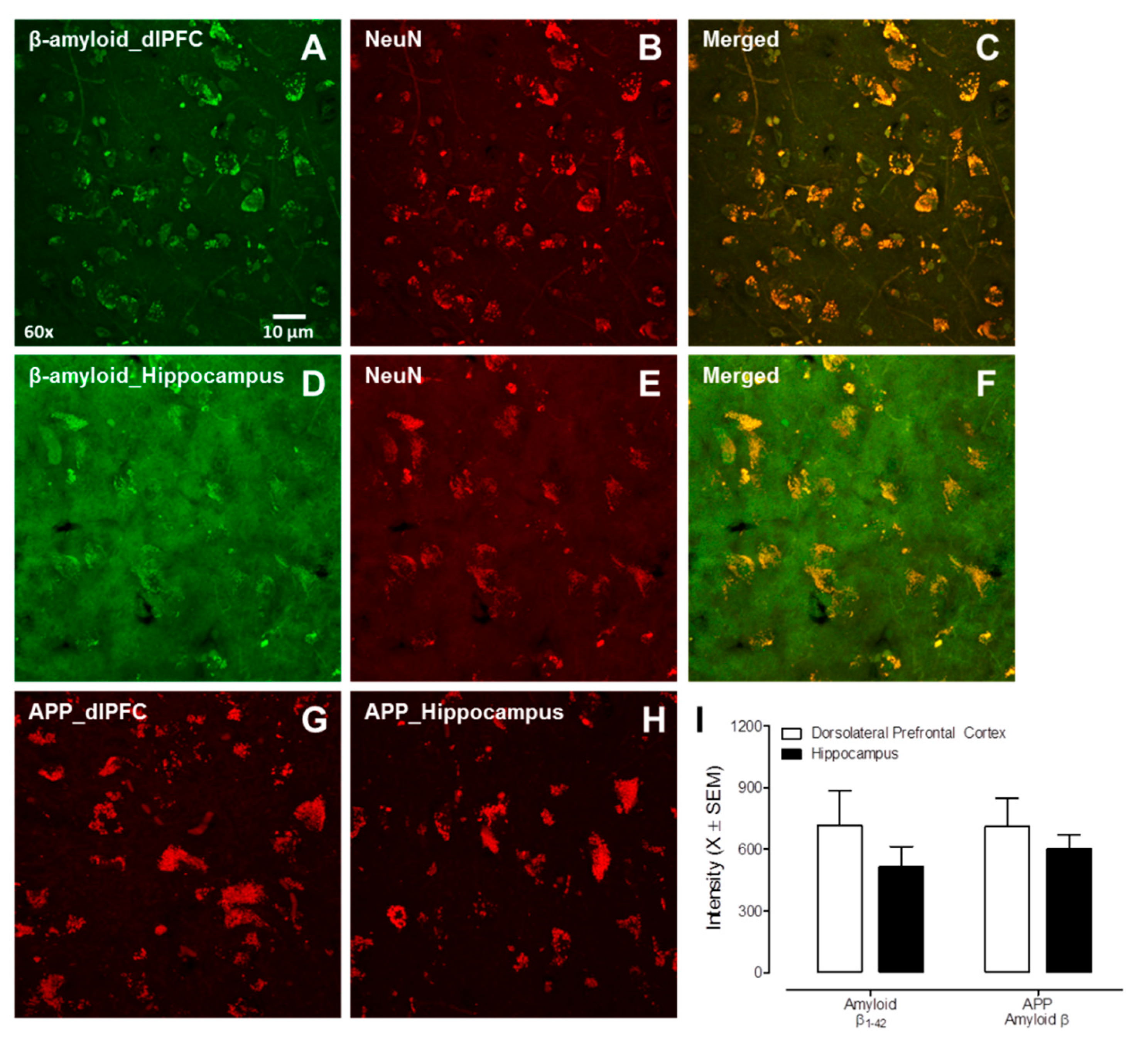
3.2. Experiment 2: Post-Mortem HIV-1 Seropositive Individuals with HAND
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Romley, J.A.; Juday, T. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Aff. 2014, 33, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Teeraananchai, S.; Chaivooth, S. Life expectancy after initiation of combination antiretroviral therapy in Thailand. Antivir. Ther. 2017, 22, 393–402. [Google Scholar] [CrossRef]
- HIV and Aging. Available online: https://www.unaids.org/en/resources/documents/2013/20131101_JC2563_hiv-and-aging (accessed on 14 December 2021).
- Smit, M.; Brinkman, K. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis. 2015, 15, 810–818. [Google Scholar] [CrossRef]
- Valcour, V.; Shikuma, C. Higher frequency of dementia in older HIV-1 individuals: The Hawaii Aging with HIV-1 Cohort. Neurology 2004, 63, 822–827. [Google Scholar] [CrossRef]
- Becker, J.T.; Lopez, O.L. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 2004, 18, S11–S18. [Google Scholar] [CrossRef]
- Vance, D.E.; Wadley, V.G. Cognitive and Everyday Functioning in Older and Younger Adults with and without HIV. Clin. Gerontol. 2011, 34, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Masliah, E. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006, 20, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Gelman, B.B.; Nguyen, T.P. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: Proteomic analysis of human synaptosomes. J. Neuroimmune Pharmacol. 2010, 5, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Dumaop, W. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 2013, 80, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.J.; Calvi, R. Preliminary in vivo evidence of reduced synaptic density in human immunodeficiency virus (HIV) despite antiretroviral therapy. Clin. Infect Dis. 2021, 73, 1404–1411. [Google Scholar] [CrossRef]
- Avdoshina, V.; Mahoney, M. HIV influences microtubule associated protein-2: Potential marker of HIV-associated neurocognitive disorders. AIDS 2020, 34, 979–988. [Google Scholar] [CrossRef]
- Fitting, S.; Xu, R. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: Chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am. J. Pathol. 2010, 177, 1397–1410. [Google Scholar] [CrossRef]
- Fitting, S.; Ignatowska-Jankowska, B.M. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol. Psychiatry 2013, 73, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Digicaylioglu, M. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann. Neurol. 2010, 68, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Speidell, A.; Asuni, G.P. Up-regulation of the p75 neurotrophin receptor is an essential mechanism for HIV-gp120 mediated synaptic loss in the striatum. Brain Behav. Immun. 2020, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, R.F., Jr.; Mactutus, C.F. HIV-1 Transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J. Neuroimmune Pharmacol. 2014, 9, 642–653. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Cook, A.K. Synaptic Connectivity in Medium Spiny Neurons of the Nucleus Accumbens: A Sex-Dependent Mechanism Underlying Apathy in the HIV-1 Transgenic Rat. Front. Behav. Neurosci. 2018, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Li, H. Disruption of timing: NeuroHIV progression in the post-cART era. Sci. Rep. 2019, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Festa, L.K.; Irollo, E. CXCL12-induced rescue of cortical dendritic spines and cognitive flexibility. Elife 2020, 9, e49717. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A. Microglial HIV-1 expression: Role in HIV-1 associated neurocognitive disorders. Viruses 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.Y.; Aksenova, M.V. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci. Lett. 2010, 475, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Hategan, A.; Masliah, E. HIV and Alzheimer’s disease: Complex interactions of HIV-Tat with amyloid beta peptide and Tau protein. J. Neurovirol. 2019, 25, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Bloodgood, B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007, 27, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Tackenberg, C.; Brandt, R. Divergent pathways mediate spine alterations and cell death induced by amyloid-β, wild-type tau, and R406W tau. J. Neurosci. 2009, 29, 14439–14450. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Nagashima, Y. Gamma-secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J. Neurosci. 2009, 29, 13042–13052. [Google Scholar] [CrossRef]
- Olsson, F.; Schmidt, S. Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions. J. Biol. Chem. 2014, 289, 1540–1550. [Google Scholar] [CrossRef]
- Mori, H.; Takio, K. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J. Biol. Chem. 1992, 267, 17082–17086. [Google Scholar] [CrossRef]
- Jarrett, J.T.; Berger, E.P. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef]
- Roher, A.E.; Lowenson, J.D. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 10836–10840. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.; Sadowska, M. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 9271–9276. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A.; Mactutus, C.F.; Booze, R.M. Ballistic Labeling of Pyramidal Neurons in Brain Slices and in Primary Cell Culture. J. Vis. Exp. 2020, 158, 60989. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Booze, R.M.; Mactutus, C.F. Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci. Rep. 2016, 6, 32831. [Google Scholar] [CrossRef]
- Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde in Inren Prinzipien Dargestellt auf Grund des Zellenbaues; Leipzig: Barth, Germany, 1909. [Google Scholar]
- Green, D.A.; Masliah, E. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005, 19, 407–411. [Google Scholar] [CrossRef]
- Achim, C.L.; Adame, A.; Dumaop, W.; Everall, I.P.; Masliah, E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J. Neuroimmune Pharmacol. 2009, 4, 190–199. [Google Scholar] [CrossRef]
- Rempel, H.C.; Pulliam, L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 2005, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Eum, S.Y. HIV-1-induced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol. Cell Neurosci. 2010, 43, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Daily, A.; Nath, A. Tat petides inhibit neprilysin. J. Neurovirol. 2006, 12, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, Y. Rho-kinase inhibitor hydroxyfasudil protects against HIV-1 Tat-induced dysfunction of tight junction and neprilysin/Aβ transfer receptor expression in mouse brain microvessels. Mol. Cell Biochem. 2021, 476, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, H. Estrogen regulates neprilysin activity in rat brain. Neuroscience Lett. 2004, 367, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Atluri, V.S.R.; Hidalgo, M. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: An update. Front Cell Neurosci. 2015, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Leda, A. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol. Cell Neurosci. 2017, 79, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.S.; Searle, J.L. Acoustic variables in the modification of startle reaction in the rat. J. Comp. Physiol. Psychol. 1965, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ison, J.R.; Hammond, G.R. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J. Comp. Physiol. Psychol. 1971, 75, 435–452. [Google Scholar] [CrossRef]
- Ison, J.R.; Agrawal, P. Changes in temporal acuity with age and with hearing impairment in the mouse: A study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J. Acoust. Soc. Am. 1998, 104, 1696–1704. [Google Scholar] [CrossRef]
- Hoffman, H.S.; Ison, J.R. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol. Rev. 1980, 87, 175–189. [Google Scholar] [CrossRef]
- Minassian, A.; Henry, B.L. Prepulse inhibition in HIV-associated Neurocognitive disorders. J. Int. Neuropsychol. Soc. 2013, 7, 255–263. [Google Scholar] [CrossRef]
- Koch, M. The neurobiology of startle. Prog. Neurobiol. 1999, 59, 107–128. [Google Scholar] [CrossRef]
- Groenewegen, H.J.; Vermeulen-Van der Zee, E. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 1987, 23, 103–120. [Google Scholar] [CrossRef]
- Berendse, H.W.; Galis-de Graaf, Y. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992, 316, 314–347. [Google Scholar] [CrossRef] [PubMed]
- Brog, J.S.; Salyapongse, A. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993, 338, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.J.; Caine, S.B. The ventral subiculum modulation of prepulse inhibition is not mediated via dopamine D2 or nucleus accumbens non-NMDA glutamate receptor activity. Eur. J. Phamarcol. 1996, 314, 9–18. [Google Scholar] [CrossRef]
- Zhang, W.N.; Bast, T. Prepulse inhibition in rats with temporary inhibition/inactivation of ventral or dorsal hippocampus. Pharmacol. Biochem. Behav. 2002, 73, 929–940. [Google Scholar] [CrossRef]
- Bubser, M.; Koch, M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology 1994, 113, 487–492. [Google Scholar] [CrossRef]
- Fitting, S.; Booze, R.M. Neonatal intrahippocampal glycoprotein 120 injection: The role of dopaminergic alterations in prepulse inhibition in adult rats. J. Pharmacol. Exp. Ther. 2006, 318, 1352–1358. [Google Scholar] [CrossRef]
- Fagiani, F.; Lanni, C. (Dys)regulation of synaptic activity and neurotransmitter release by β-amyloid: A look beyond Alzheimer’s disease pathogenesis. Front. Mol. Neurosci. 2021, 14, 635880. [Google Scholar] [CrossRef]
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrine. Z. Psychiatr. Physchish-Gerichtl. Med. 1907, 64, 146–148. [Google Scholar]
- Tomlinson, B.E.; Blessed, G. Observations on the brains of demented old people. J. Neurol. Sci. 1970, 11, 205–242. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Esiri, M.M.; Biddolph, S.C. Prevalence of Alzheimer plaques in AIDS. J. Neurol. Neurosurg. Psychiatry 1998, 65, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesen, G.W.; Hyman, B.T. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus 1991, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Mactutus, C.F. An empirical mediation analysis of mechanisms underlying HIV-1-Associated neurocognitive disorders. Brain Res. 2019, 1724, 146436. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.M.; Booze, R.M. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J. Neuroimmune Pharmacol. 2013, 8, 988–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McLaurin, K.A.; Booze, R.M. Temporal processing demands in the HIV-1 transgenic rat: Amodal gating and implications for diagnostis. Int. J. Dev. Neurosci. 2017, 57, 12–20. [Google Scholar] [CrossRef]
- Fitting, S.; Booze, R.M. Neonatal hippocampal Tat injections: Developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int. J. Dev. Neurosci. 2006, 24, 275–283. [Google Scholar] [CrossRef]
- Fitting, S.; Booze, R.M. Intrahippocampal injections of Tat: Effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol. Biochem. Behav. 2006, 84, 189–196. [Google Scholar] [CrossRef]
- Fitting, S.; Booze, R.M. Neonatal intrahippocampal gp120 injection: An examination early in development. Neurotoxicology 2007, 28, 101–107. [Google Scholar] [CrossRef]
- Henry, B.; Geyer, M.A. Prepulse inhibition in HIV-1 gp120 transgenic mice after withdrawal from chronic methamphetamine. Behav. Pharmacol. 2014, 25, 12–22. [Google Scholar] [CrossRef]
- Bachis, A.; Forcelli, P. Expression of gp120 in mice evokes anxiety behavior: Co-occurrence with increased dendritic spines and brain-derived neurotrophic factor in the amygdala. Brain Behav. Immun. 2016, 54, 170–177. [Google Scholar] [CrossRef]
- Paris, J.J.; Singh, H.D. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav. Brain Res. 2015, 291, 209–218. [Google Scholar] [CrossRef]
- McLaurin, K.A.; Booze, R.M. Evolution of the HIV-1 transgenic rat: Utility in assessing the progression of HIV-1-associated neurocognitive disorders. J. Neurovirol. 2018, 24, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Hejl, A.M.; Glenthoj, B. Prepulse inhibition in patents with Alzheimer’s disease. Neurobiol. Aging 2004, 25, 1045–1050. [Google Scholar] [CrossRef]
- Milanini, B.; Valcour, V. Differentiating HIV-associated neurocognitive disorders from Alzheimer’s disease: An emerging issue in geriatric neuroHIV. Curr. HIV/AIDS Rep. 2017, 14, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Milanini, B. Discriminant analysis of neuropsychological testing differentiates HIV-associated neurocognitive disorder from mild cognitive impairment due to Alzheimer’s disease. In Proceedings of the International Society of NeuroVirology, Toronto, Canada, 25–28 October 2016. [Google Scholar]
- Peng, J.; Vigorito, M. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J. Neuroimmunol. 2010, 218, 94–101. [Google Scholar] [CrossRef]
- Moran, L.M.; Booze, R.M. Modeling deficits in attention, inhibition, and flexibility in HAND. J. Neuroimmune Pharmacol. 2014, 9, 508–521. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Booze, R.M. Sex matters: Robust sex differences in signal detection in the HIV-1 transgenic rat. Front. Behav. Neurosci. 2017, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Vigorito, M.; LaShomb, A.L. Spatial learning and memory in HIV-1 transgenic rats. J. Neuroimmune Pharmacol. 2007, 2, 319–328. [Google Scholar] [CrossRef]
- Lashomb, A.L.; Vigorito, M. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J. Neurovirol. 2009, 15, 14–24. [Google Scholar] [CrossRef]
- Repunte-Canonigo, V.; Lafebvre, C. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol. Neurodegener. 2014, 9, 26. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Cysique, L.A.; Maruff, P. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J. Neurovirol. 2004, 10, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Neigh, G.N.; Sundermann, E.E.; Xu, Y.; Scully, E.P.; Maki, P.M. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.A.; Bertrand, S.J. S-Equol mitigates motivational deficits and dysregulation associated with HIV-1. Sci. Rep. 2021, 11, 11870. [Google Scholar] [CrossRef]
- McLaurin, K.A.; Li, H. Neurodevelopmental processes in the prefrontal cortex derailed by chronic HIV-1 viral protein exposure. Cells 2021, 10, 3037. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; McLaurin, K.A.; Mactutus, C.F.; Likins, B.; Huang, W.; Chang, S.L.; Booze, R.M. Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain. Viruses 2022, 14, 1268. https://doi.org/10.3390/v14061268
Li H, McLaurin KA, Mactutus CF, Likins B, Huang W, Chang SL, Booze RM. Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain. Viruses. 2022; 14(6):1268. https://doi.org/10.3390/v14061268
Chicago/Turabian StyleLi, Hailong, Kristen A. McLaurin, Charles F. Mactutus, Benjamin Likins, Wenfei Huang, Sulie L. Chang, and Rosemarie M. Booze. 2022. "Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain" Viruses 14, no. 6: 1268. https://doi.org/10.3390/v14061268
APA StyleLi, H., McLaurin, K. A., Mactutus, C. F., Likins, B., Huang, W., Chang, S. L., & Booze, R. M. (2022). Intraneuronal β-Amyloid Accumulation: Aging HIV-1 Human and HIV-1 Transgenic Rat Brain. Viruses, 14(6), 1268. https://doi.org/10.3390/v14061268






