Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Phage
2.2. Phage Propagation and Titration
2.3. Materials
2.4. Phage Stability in Liquid Polyvinyl Alcohol (PVA)
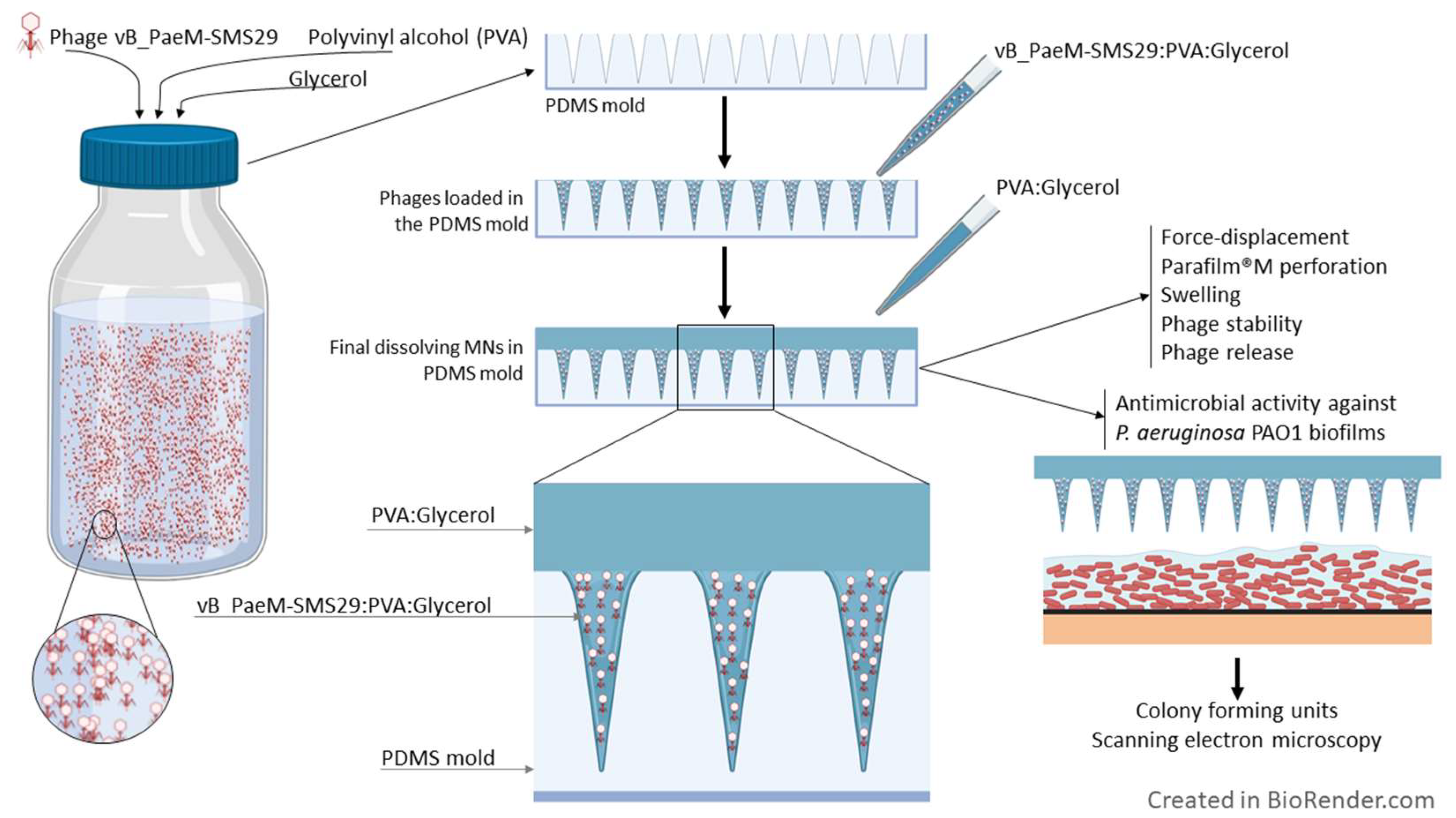
2.5. Microneedle Array Fabrication
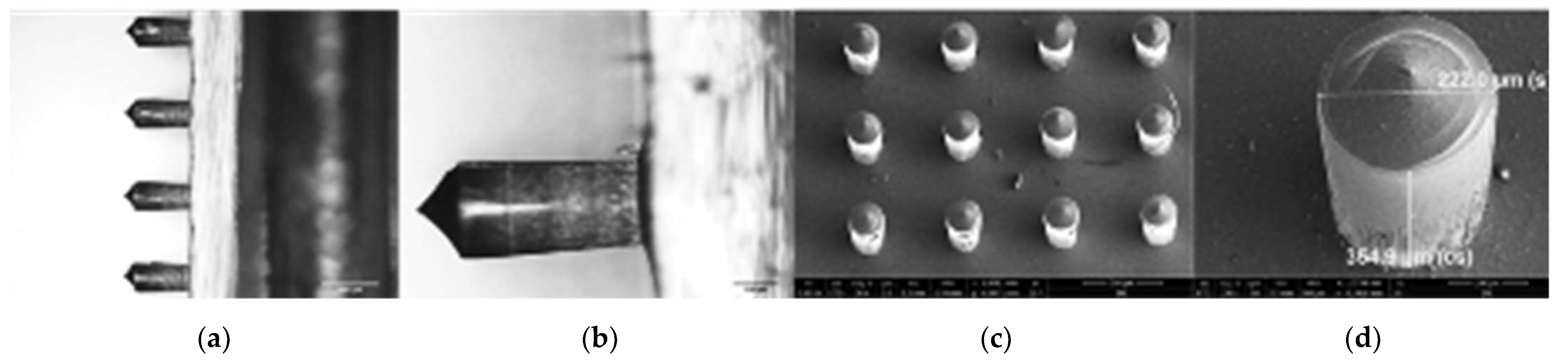
2.6. Polydimethylsiloxane Molds
2.7. Polyvinyl Alcohol Microneedle Fabrication
2.8. Phage Stability and Release from PVAG MNs
2.9. Determination of the Swelling and Solubility Characteristics
2.10. Optical and Scanning Electron Microscopy (SEM) of the MNs
2.11. Transmission Electron Microscopy (TEM) of the Phage
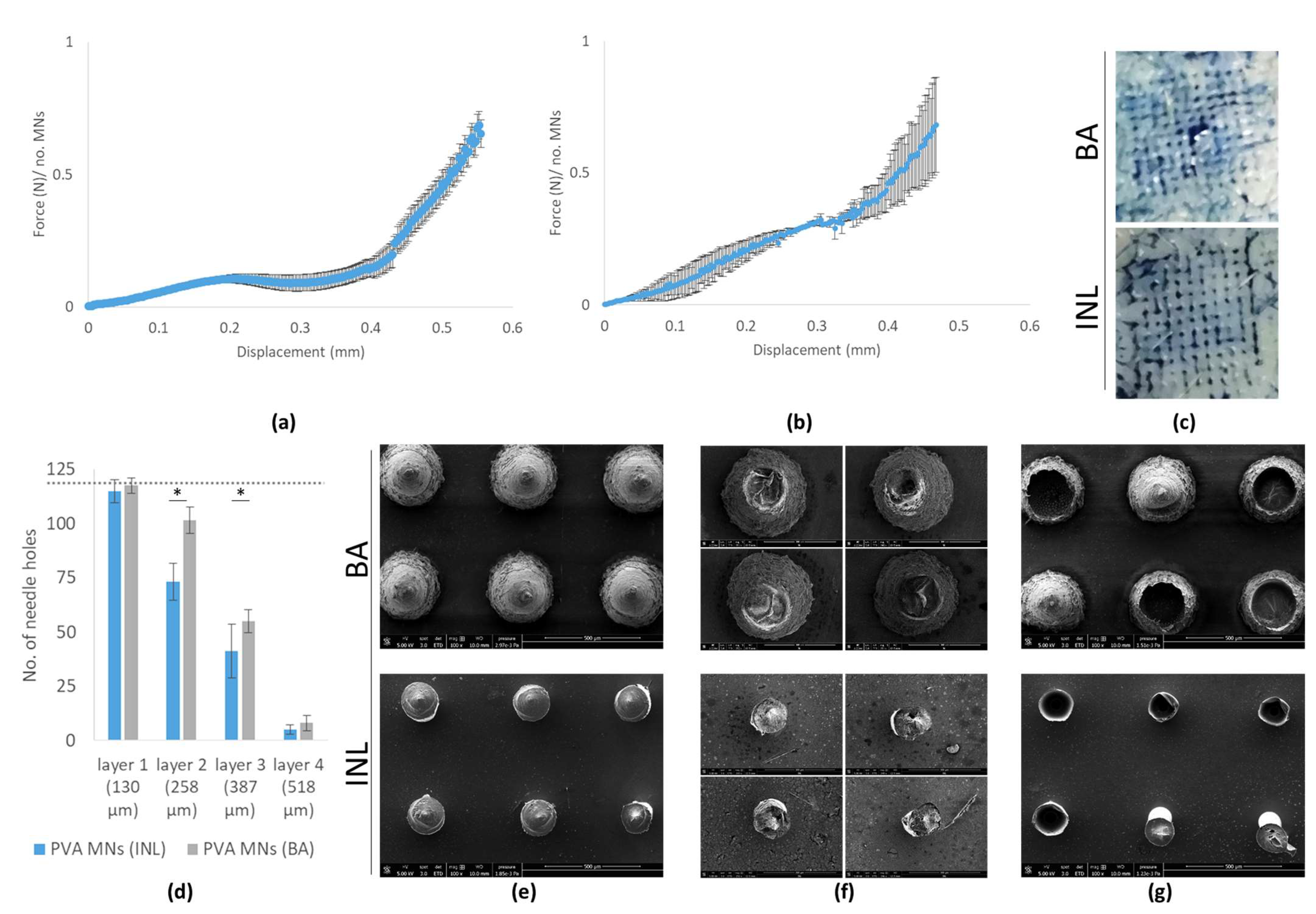
2.12. Force-Displacement
2.13. Perforation Studies
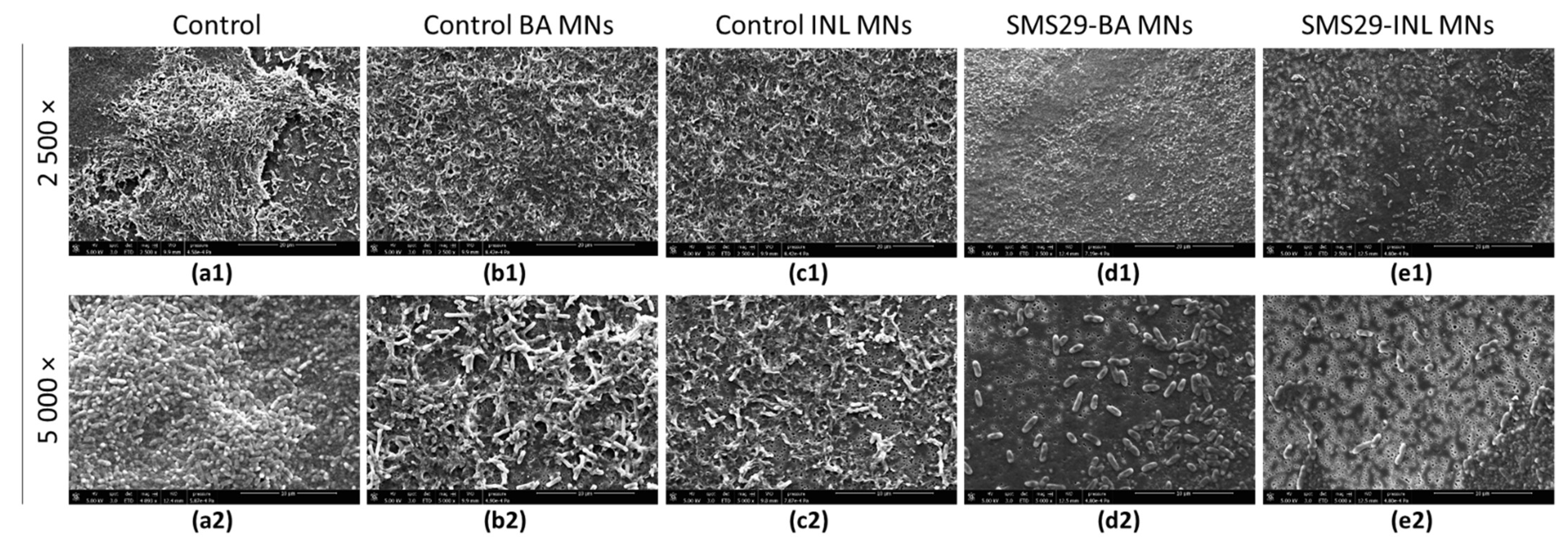
2.14. Antibiofilm Activity
2.15. Statistical Analysis
3. Results and Discussion
3.1. Phage vB_PaeM-SMS29
3.2. Selection of the PVA for MN Fabrication
| Polyvinyl Alcohol (PVA) | Characteristics (MW, Hydrolysis Degree, Viscosity) | After Cooling to 65 °C | Film after 24 h at 40 °C |
|---|---|---|---|
| Mowiol 4-98 | MW 27,000, 98–99 mol% hydrolyzed, 4–5 mPa·s | Liquid | Slightly wrinkled film |
| Mowiol 4-88 | MW 31,000, 86.7–88.7 mol% hydrolyzed; 3.5–4.5 mPa·s | Liquid | Uniform film |
| 31,000–50,000 | MW 31,000–50,000, 5.4–6.5 cP; 99+% hydrolyzed | Liquid | Slightly wrinkled film |
| Mowiol 8-88 | MW 67,000, 86.7–88.7 mol% hydrolyzed; 7–9 mPa·s | Two phases | Not possible to use |
| 85,000–124,000 | MW 85,000–124,000, 28–32 cP; 99+% hydrolyzed | Two phases | Not possible to use |
| Mowiol 18-88 | MW 130,000, 86.7–88.7 mol% hydrolyzed; 16–20 mPa·s | Two phases | Not possible to use |
| 146,000–186,000 | MW 146,000–186,000, 55–65 cP; 99+% hydrolyzed | Two phases | Not possible to use |
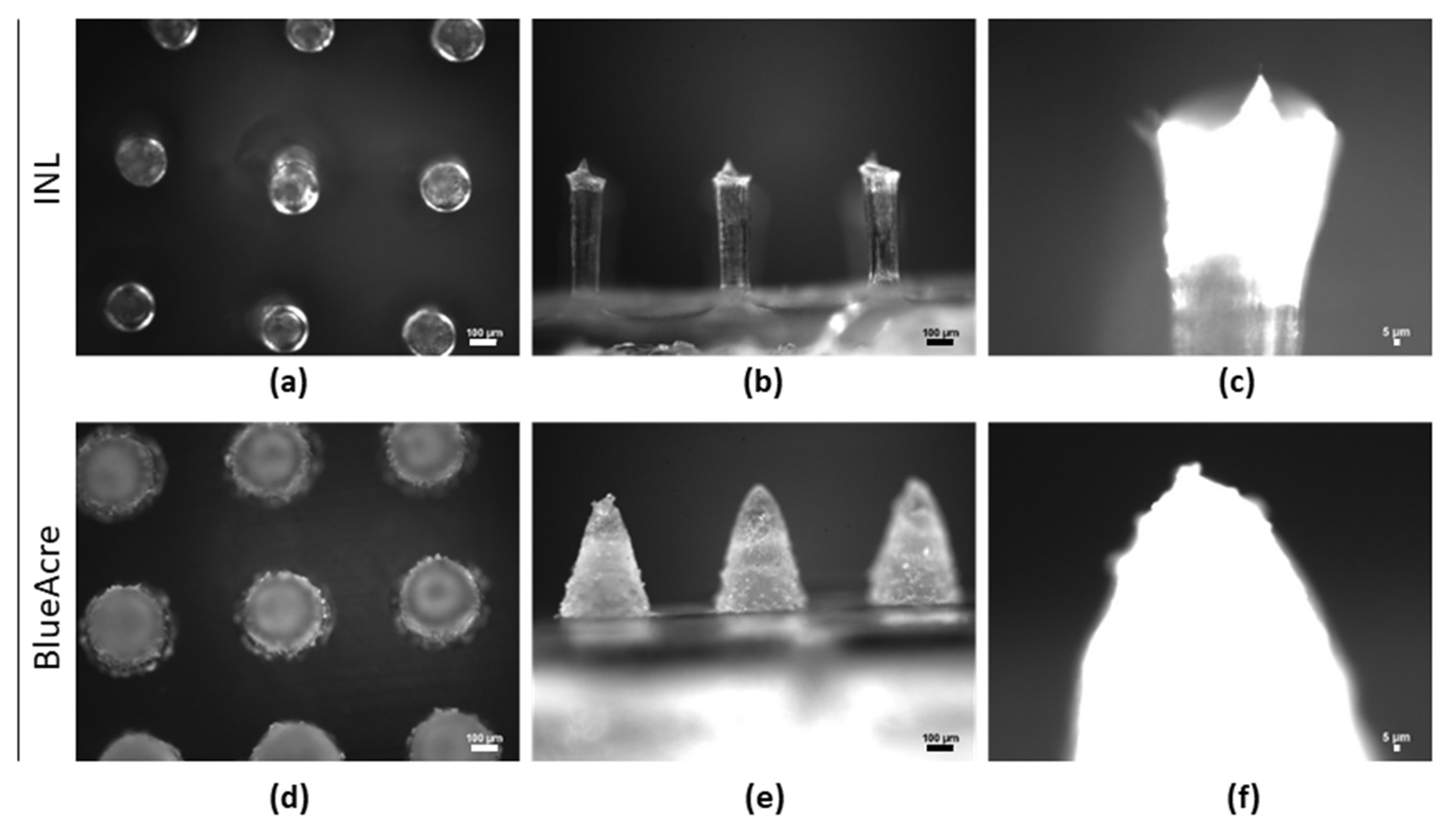
3.3. Microneedle Characterization
3.4. Phage-Loaded MNs
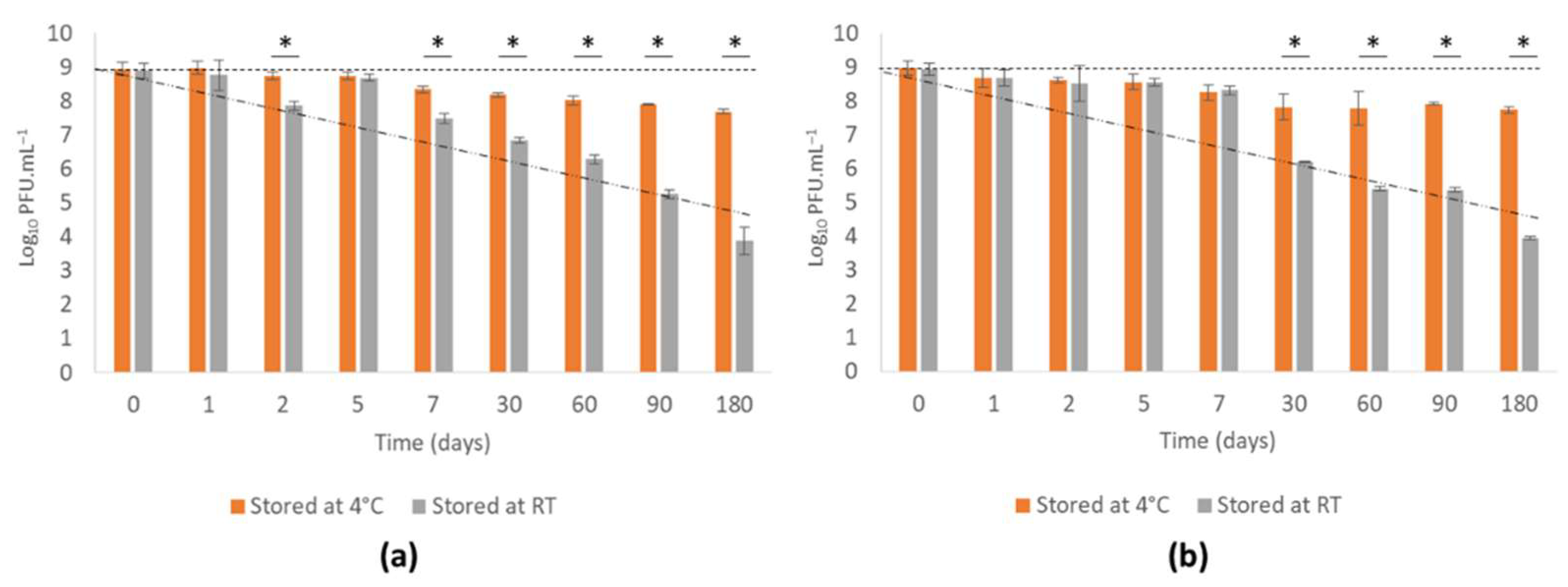
3.4.1. Phage vB_PaeM-SMS29 Stability in MNS during Storage
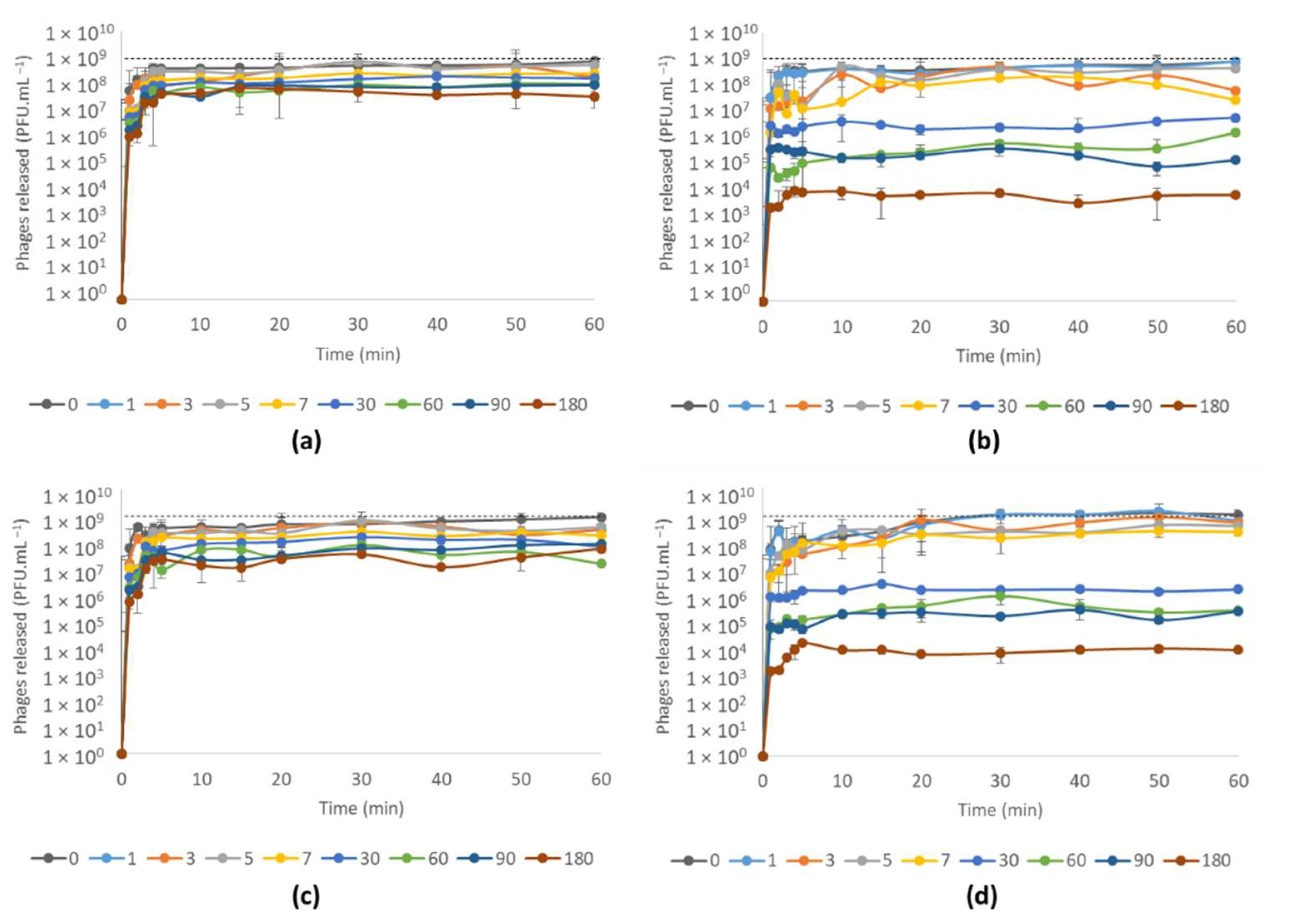
3.4.2. Phage vB_PaeM-SMS29 Release from MNs after Storage at Different Temperatures for Six Months
3.4.3. Antimicrobial Efficacy of Phage vB_PaeM-SMS29-Loaded MNs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-of-Care Fluorescence Imaging. Diagnostics 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef] [PubMed]
- González, J.F.; Hahn, M.M.; Gunn, J.S. Chronic biofilm-based infections: Skewing of the immune response. Pathog. Dis. 2018, 76, fty023. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef]
- Pinto, A.M.; Silva, M.D.; Pastrana, L.M.; Bañobre-López, M.; Sillankorva, S. The clinical path to deliver encapsulated phages and lysins. FEMS Microbiol. Rev. 2021, 45, fuab019. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, M.H.; Im, S.H.; Park, O.O. Electrically bistable Ag nanocrystal-embedded metal-organic framework microneedles. RSC Adv. 2016, 6, 64885–64889. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, D.D.; Chen, Y.; Guo, X.D. A fabrication method of microneedle molds with controlled microstructures. Mater. Sci. Eng. C 2016, 65, 135–142. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Polymer-ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Yu, H.; Li, C.; Feng, J.; Haq, F.; Khan, A.; Khan, R.U. Preparation, properties and challenges of the microneedles-based insulin delivery system. J. Control. Release 2018, 288, 173–188. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.-N.; Quan, Y.-S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle Patch-Mediated Treatment of Bacterial Biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef]
- Peng, K.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Domínguez-Robles, J.; Ramadon, D.; Chambers, P.; McCarthy, H.O.; Larrañeta, E.; Donnelly, R.F. Dissolving microneedle patches loaded with amphotericin B microparticles for localised and sustained intradermal delivery: Potential for enhanced treatment of cutaneous fungal infections. J. Control. Release 2021, 339, 361–380. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Tekko, I.A.; McCarthy, H.O.; Ahmed, N.; Rehman, A.U.; Donnelly, R.F. Microneedle liquid injection system assisted delivery of infection responsive nanoparticles: A promising approach for enhanced site-specific delivery of carvacrol against polymicrobial biofilms-infected wounds. Int. J. Pharm. 2020, 587, 119643. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C 2021, 120, 111786. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, I.; Nejati, S.; Selvamani, V.; Jiang, H.; Chittiboyina, S.; Grant, J.; Mutlu, Z.; Waimin, J.; Abutaleb, N.S.; Seleem, M.N.; et al. Flexible microneedle array patch for chronic wound oxygenation and biofilm eradication. ACS Appl. Bio Mater. 2021, 4, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.D.R.; Boas, D.V.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Jarow, J.P.; Lurie, P.; Ikenberry, S.C.; Lemery, S. Overview of FDA’s Expanded access program for investigational drugs. Ther. Innov. Regul. Sci. 2017, 51, 177–179. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA-J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Paris, V.; Slawomirski, L.; Colbert, A.; Delaunay, N.; Oderkirk, J. Innovation, access and value in pharmaceuticals. In New Health Technologies—Managing Access, Value and Sustainability; OECD Publishing: Paris, France, 2017; pp. 81–116. [Google Scholar]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and function of phage encoded depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Pinto, G.; Oliveira, F.; Vilas-Boas, D.; Almeida, C.; Sillankorva, S.; Cerca, N.; Azeredo, J. The protective effect of Staphylococcus epidermidis biofilm matrix against phage predation. Viruses 2020, 12, 1076. [Google Scholar] [CrossRef]
- Milho, C.; Andrade, M.; Boas, D.V.; Alves, D.; Sillankorva, S. Antimicrobial assessment of phage therapy using a porcine model of biofilm infection. Int. J. Pharm. 2019, 557, 112–123. [Google Scholar] [CrossRef]
- Pinto, A.M.; Faustino, A.; Pastrana, L.M.; Bañobre-López, M.; Sillankorva, S. Pseudomonas aeruginosa PAO 1 in vitro time-kill kinetics using single phages and phage formulations—Modulating Death, Adaptation, and Resistance. Antibiotics 2021, 10, 877. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd, ed.; Cold Spring Harbor Protocols: New York, NY, USA, 1989. [Google Scholar]
- Adams, M. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959; ISBN 9781588296825. [Google Scholar]
- Hildebrand, A.; Remmert, M.; Biegert, A.; Söding, J. Fast and accurate automatic structure prediction with HHpred. Proteins Struct. Funct. Bioinform. 2009, 77, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Amado, I.R.; Gaspar, J. Dissolving microneedles for the delivery of peptides—Towards tolerance-inducing vaccines. Int. J. Pharm. 2020, 586, 119590. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, L.; Winter, M.A.; Pocock, K.J.; Bremmell, K.E.; Thierry, B. Efficient microfluidic negative enrichment of circulating tumor cells in blood using roughened PDMS. Analyst 2015, 140, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 22, 1B-1. [Google Scholar] [CrossRef]
- Moons, P.; Faster, D.; Aertsen, A. Lysogenic conversion and phage resistance development in phage exposed Escherichia coli biofilms. Viruses 2013, 5, 150–161. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, P.; Lin, Z.; Wang, T. Characterization of two Pseudomonas aeruginosa viruses vB_PaeM_SCUT-S1 and vB_PaeM_SCUT-S2. Viruses 2019, 11, 318. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Bim, F.L.; Monteiro, R.M.; Macedo, A.P.; Santos, E.S.; Silva-Lovato, C.H.; Paranhos, H.F.O.; Melo, L.D.R.; Santos, S.B.; Watanabe, E. Identification and characterization of new bacteriophages to control multidrug-resistant Pseudomonas aeruginosa biofilm on endotracheal tubes. Front. Microbiol. 2020, 11, 580779. [Google Scholar] [CrossRef]
- Pires, D.P.; Dötsch, A.; Anderson, E.M.; Hao, Y.; Khursigara, C.M.; Lam, J.S.; Sillankorva, S.; Azeredo, J. A Genotypic analysis of five P. aeruginosa strains after biofilm infection by phages targeting different cell surface receptors. Front. Microbiol. 2017, 8, 1229. [Google Scholar] [CrossRef]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Rodríguez, A.; Martínez, B.; García, P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE 2018, 13, e0205728. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.B.; Carvalho, C.M.; Sillankorva, S.R.; Nicolau, A.; Ferreira, E.C.; Azeredo, J. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 2009, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Senecal, A.; Nugen, S. Electrospun water-soluble polymer nanofibers for the dehydration and storage of sensitive reagents. Nanotechnology 2014, 25, 225101. [Google Scholar] [CrossRef]
- Merabishvili, M.; Vervaet, C.; Pirnay, J.-P.; De Vos, D.; Verbeken, G.; Mast, J.; Chanishvili, N.; Vaneechoutte, M. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLoS ONE 2013, 8, e68797. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.-E. Effect of glycerol on sustained insulin release from PVA hydrogels and its application in diabetes therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pushp, P.; Bhaskar, R.; Kelkar, S.; Sharma, N.; Pathak, D.; Gupta, M.K. Plasticized poly(vinylalcohol) and poly(vinylpyrrolidone) based patches with tunable mechanical properties for cardiac tissue engineering applications. Biotechnol. Bioeng. 2021, 118, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef]
- Bonfante, G.; Lee, H.; Bao, L.; Park, J.; Takama, N.; Kim, B. Comparison of polymers to enhance mechanical properties of microneedles for bio-medical applications. Micro Nano Syst. Lett. 2020, 8, 13. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Baek, S.-K.; Park, J.-H.; Betz, A.R.; Choi, S.-O. Fabrication of circular obelisk-type multilayer microneedles using micro-milling and spray deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Tekko, I.A.; Jomaa, M.H.; Vora, L.; McAlister, E.; Volpe-Zanutto, F.; Nethery, M.; Baine, P.T.; Mitchell, N.; McNeill, D.W.; et al. Two-photon polymerisation 3D printing of microneedle array templates with versatile designs: Application in the development of polymeric drug delivery systems. Pharm. Res. 2020, 37, 174. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W.; Tremblay, D.; Moineau, S. Long term bacteriophage preservation. WFCC Newsl. 2004, 38, 35–40. [Google Scholar]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Nogueira, F.; Karumidze, N.; Kusradze, I.; Goderdzishvili, M.; Teixeira, P.; Gouveia, I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2475–2484. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef]
- Wroe, J.A.; Johnson, C.T.; García, A.J. Bacteriophage delivering hydrogels reduce biofilm formation in vitro and infection in vivo. J. Biomed. Mater. Res.-Part A 2020, 108, 39–49. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Morales, S.; Britton, W.; Kutter, E.; Chan, H.-K. Microfluidic-assisted bacteriophage encapsulation into liposomes. Int. J. Pharm. 2018, 545, 176–182. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Okamoto, Y.; Morales, S.; Kutter, E.; Chan, H.K. Hydrogel formulations containing non-ionic polymers for topical delivery of bacteriophages. Int. J. Pharm. 2021, 605, 120850. [Google Scholar] [CrossRef]


| Sample | Degree of Swelling (%) | Time until Full Disintegration (min) | Dissolution (%) * |
|---|---|---|---|
| PVA | 62.7 ± 18.7 | 7 ± 2 | 90.9 ± 5.3 |
| PVA: 0.1% glycerol | 61.9 ± 17.2 | 11 ± 2 | 95.9 ± 1.2 |
| PVA:PVP | 67.1 ± 35.7 | 9 ± 2 | 80.8 ± 10.4 |
| PVA:PVP: 0.1% glycerol | 62.0 ± 34.1 | 11 ± 2 | 90.9 ± 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillankorva, S.; Pires, L.; Pastrana, L.M.; Bañobre-López, M. Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles. Viruses 2022, 14, 964. https://doi.org/10.3390/v14050964
Sillankorva S, Pires L, Pastrana LM, Bañobre-López M. Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles. Viruses. 2022; 14(5):964. https://doi.org/10.3390/v14050964
Chicago/Turabian StyleSillankorva, Sanna, Liliana Pires, Lorenzo M. Pastrana, and Manuel Bañobre-López. 2022. "Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles" Viruses 14, no. 5: 964. https://doi.org/10.3390/v14050964
APA StyleSillankorva, S., Pires, L., Pastrana, L. M., & Bañobre-López, M. (2022). Antibiofilm Efficacy of the Pseudomonas aeruginosa Pbunavirus vB_PaeM-SMS29 Loaded onto Dissolving Polyvinyl Alcohol Microneedles. Viruses, 14(5), 964. https://doi.org/10.3390/v14050964









