Anthropometric Parameters of Children with Congenital Zika Virus Exposure in the First Three Years of Life
Abstract
:1. Introduction
2. Materials and Methods
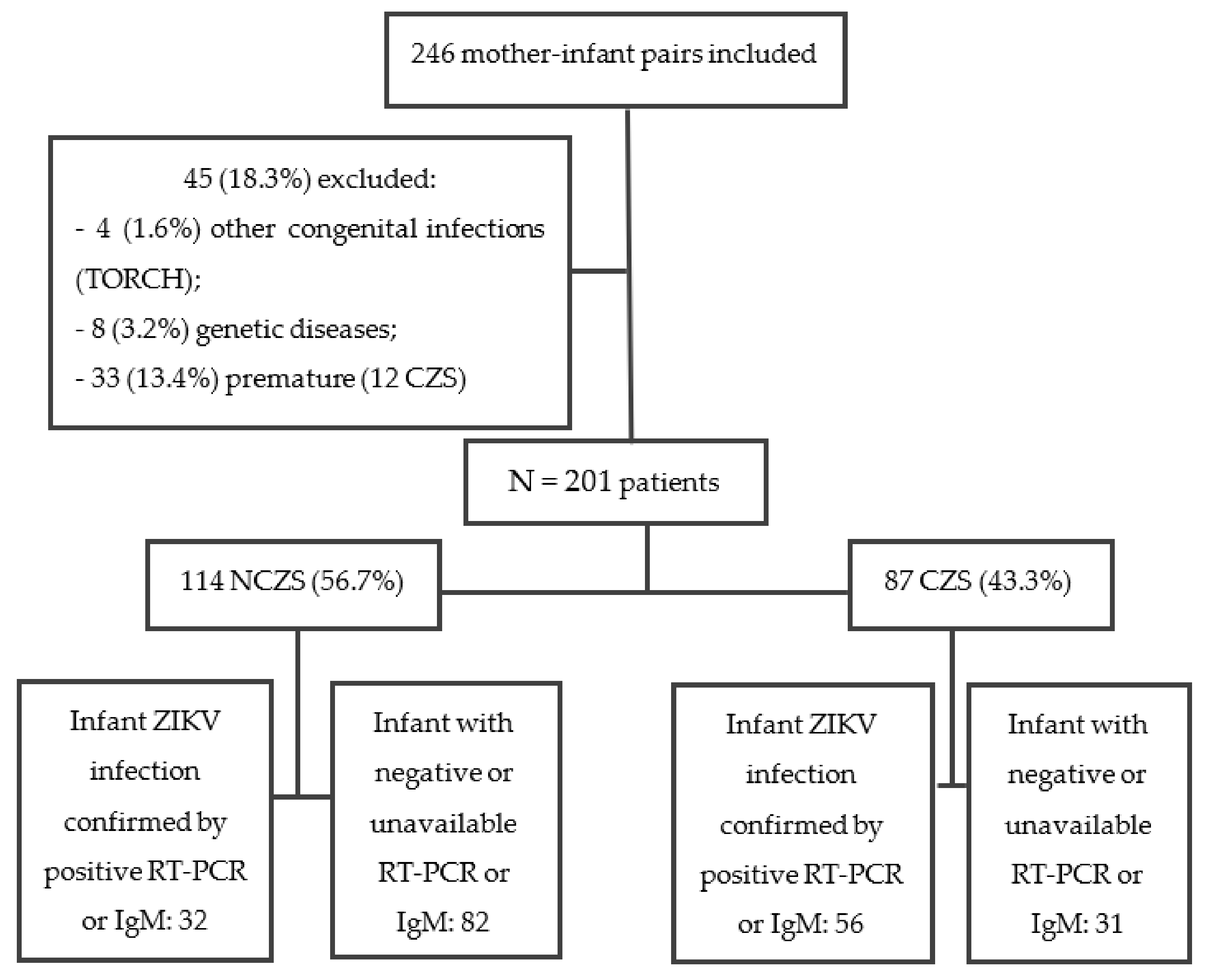
2.1. Inclusion and Exclusion Criteria
2.2. Study Procedures
2.3. Statistical Analysis
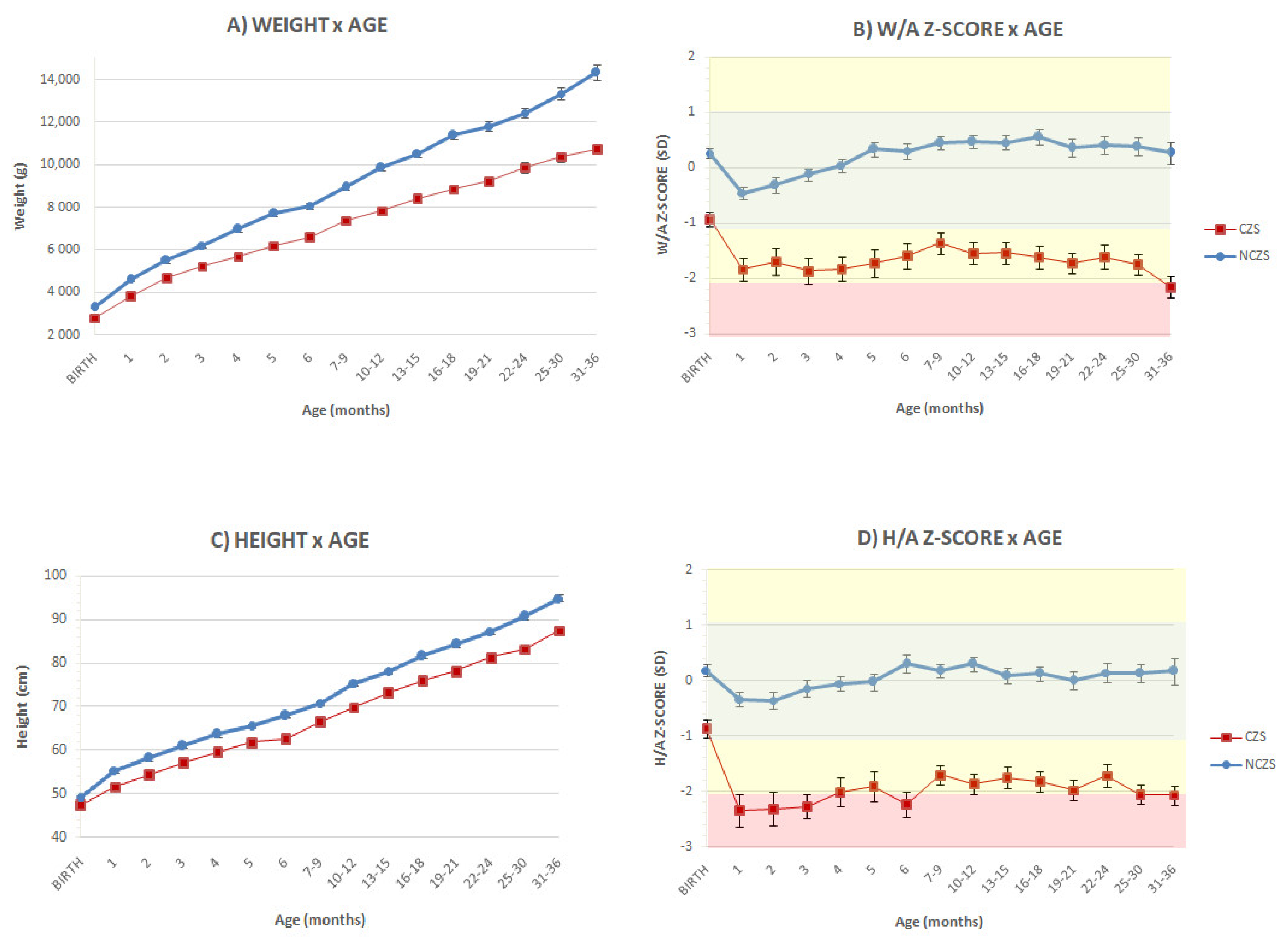
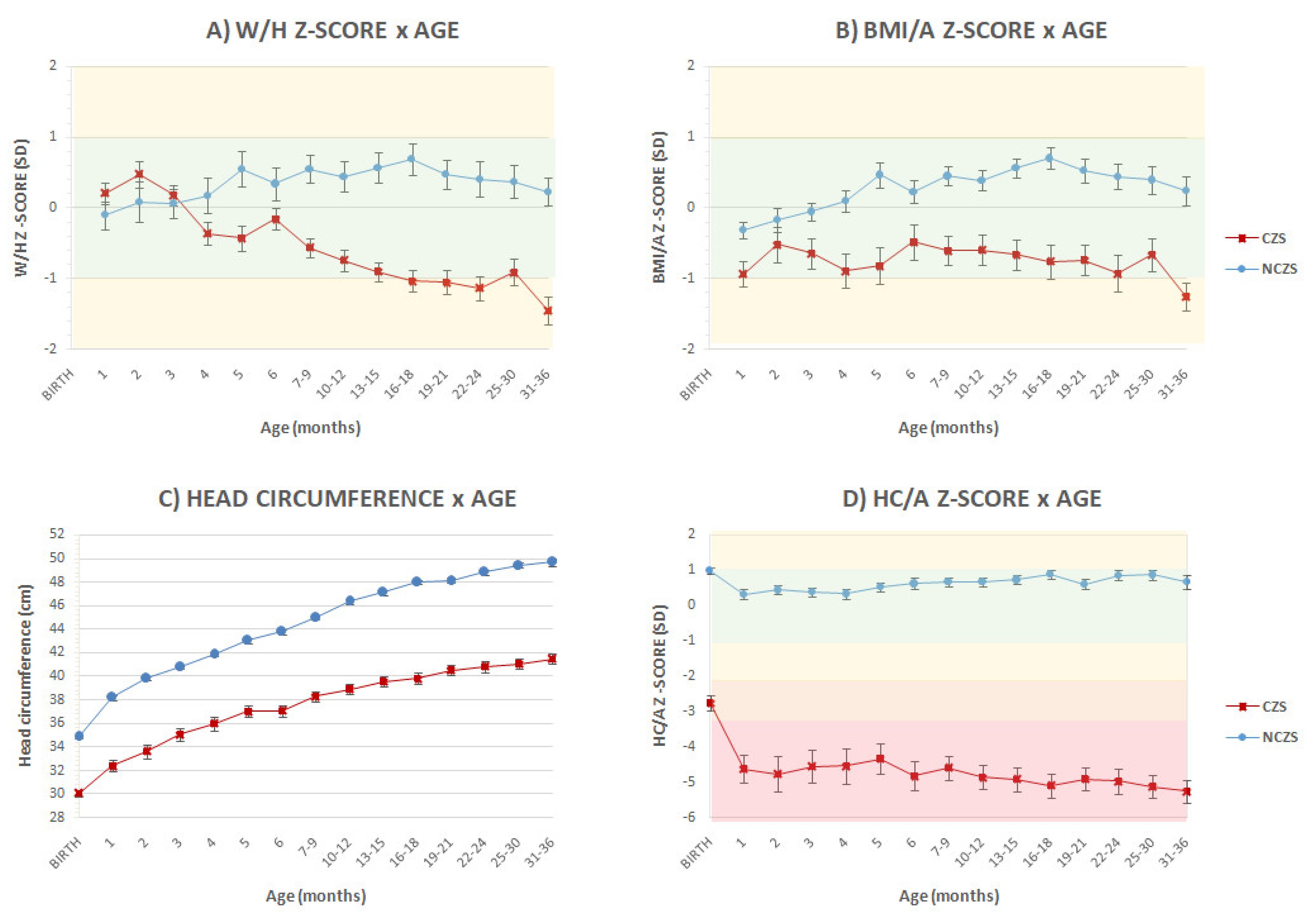
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total | CZS * (n = 87) | p-Value | ||||
|---|---|---|---|---|---|---|
| Negative or Unavailable Mother or Infant RT-PCR or Infant IgM (N = 31) | Positive Mother or Infant RT-PCR or Positive Infant IgM (N = 56) | |||||
| N (%) | N (%) | |||||
| Sex (n = 87) | Fem | 45 | 17 (54.8%) | 28 (50%) | 0.665 | |
| Male | 42 | 14 (45.2%) | 28 (50%) | |||
| Rash by trimester of pregnancy (n = 87) | Absent | 19 | 7 (22.6%) | 12 (21.4%) | 0.934 | |
| 1st | 59 | 20 (64.5%) | 39 (64.3%) | |||
| 2nd | 7 | 3 (9.7%) | 4 (7.1%) | |||
| 3rd | 2 | 1 (3.2%) | 1 (1.8%) | |||
| Microcephaly at birth (n = 87) | Yes | 67 | 23 (74.2%) | 44 (78.6%) | 0.642 | |
| Severe microcephaly | No | 35 | 12 (38.7%) | 23 (41.1%) | 0.83 | |
| Yes | 52 | 19 (61.3%) | 33 (54.9%) | |||
| Abnormal neuroimaging (n = 87) | Yes | 85 | 31 (100%) | 54 (96.4%) | 0.287 | |
| Abnormal ophthalmologic evaluation (n = 87) | Yes | 50 | 17 (19.5%) | 33 (58.9%) | 0.712 | |
| Arthrogryposis (n = 87) | Yes | 15 | 7 (22.6%) | 8 (14.3%) | 0.327 | |
| Small for Gestational Age (n = 87) | Yes | 34 | 9 (29%) | 25 (44.6%) | 0.153 | |
| Neonatal complication (n = 87) | Yes | 31 | 9 (29%) | 22 (39.3%) | 0.721 | |
| Breastfeeding (n = 82) | Yes | 72 | 25 (80.6%) | 47 (83.9%) | 0.744 | |
| Gastrostomy (n = 87) | Yes | 23 | 10 (32.3%) | 13 (23.2%) | 0.36 | |
| Hospitalization | All causes (n = 86) | Yes | 65 | 25 (80.6%) | 40 (71.4%) | 0.135 |
| Missing | 1 | 0 | 1 (1.8%) | |||
| Urinary tract infection (n = 84) | Yes | 19 | 9 (29%) | 10 (17.9%) | 0.386 | |
| Missing | 3 | 1 (3.2%) | 2 (3.6%) | |||
| Respiratory disease (n = 84) | Yes | 42 | 19 (61.3%) | 23 (41.1%) | 0.243 | |
| Missing | 3 | 1 (3.2%) | 2 (3.6%) | |||
| Epilepsy (n = 84) | Yes | 20 | 11 (35.5%) | 9 (16.1%) | 0.107 | |
| Missing | 3 | 1 (3.2%) | 2 (3.6%) | |||
| Surgery (n = 84) | Yes | 32 | 12 (38.7%) | 20 (35.7%) | 0.850 | |
| Missing | 3 | 1 (3.2%) | 2 (3.6%) | |||
| Anticonvulsant drugs (n = 86) | Yes | 72 | 25 (80.6%) | 47 (83.9%) | 0.577 | |
| Missing | 1 | 0 | 1 (1.8%) | |||
| Urinary tract infection (outpatient) (n = 84) | Yes | 27 | 9 (29%) | 18 (32.1%) | 0.180 | |
| Missing | 3 | 1 (3.2%) | 2 (3.6%) | |||
| Death (n = 87) | Yes | 5 | 1 (3.2%) | 4 (7.1%) | 0.452 | |
| Maternal education (n = 84) | 1–4 years | 8 | 6 (19.4%) | 2 (3.6%) | 0.066 | |
| 5–8 years | 18 | 6 (19.4%) | 12 (21.4%) | |||
| High school | 46 | 17 (54.8%) | 29 (51.8%) | |||
| College | 12 | 2 (6.4%) | 10 (17.9%) | |||
| Missing | 3 | 0 | 3 (5.3%) | |||
| Government aid (n = 81) | Yes | 19 | 13 (41.9%) | 6 (10.7%) | 0.005 | |
| Missing | 6 | 1 (3.2%) | 5 (8.9%) | |||
| Family income classification * (n = 84) | A | 1 | 0 | 1 (1.7%) | 0.517 | |
| B | 1 | 0 | 1 (1.7%) | |||
| C | 6 | 3 (9.7%) | 3 (5.4%) | |||
| D | 23 | 6 (19.4%) | 17 (30.4%) | |||
| E | 53 | 22 (70.9%) | 31 (55.4%) | |||
| Missing | 3 | 0 | 3 (5.4%) | |||
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J.; Zikavirus, I. Isolations and serological specificity. Trans. R Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Campos, G.S.; Bande ira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Martines, R.B.; Bhatnagar, J.; Keating, M.K.; Silva-Flannery, L.; Muehlenbachs, A.; Gary, J.; Goldsmith, C.; Hale, G.; Ritter, J.; Rollin, D.; et al. Notes from the Field: Evide nce of Zika Virus Infectionin Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 159–160. [Google Scholar] [CrossRef] [Green Version]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; ResmanRus, K.; VesnaverVipotnik, T.; FabjanVodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovits, D.; Cavalcanti, D.; Pessoa, A.; Doriqui, M.; Neri, J.; Monterode Pina Neto, J.; Wanderley, H.; et al. Possible Association between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- De Oliveira, K.W.; Cortez-Escalante, J.; De Oliveira, W.T.; doCarmo, G.; Henriques, C.; Coelho, G.; de Franca, G. Increase in Reported Prevalence of Microcephalyin Infants Born to Women Living in Areas with Confirmed ZikaVirus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- De Fatima Vasco Aragao, M.; van der Linden, V.; Brainer-Lima, A.M.; Ramon Coeli, R.; Rocha, M.; Sobral da Silva, P.; de Cavalho, M.; van der Linden, A.; de Holanda, A.; Valenca, M. Clinical features and neuroimaging (CTandMRI) findings in presumed Zika virus related congenital infection and microcephaly: Retrospective case series study. BMJ 2016, 353, i1901. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.S.; Aguiar, R.S.; Amorim, M.M.; Arruda, M.B.; Melo, F.O.; Ribeiro, S.T.; Batista, A.G.; Ferreira, T.; DosSantos, M.P.; Sampaio, V.V.; et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016, 73, 1407–1416. [Google Scholar] [CrossRef]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.P.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; de Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 live births with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Ventura, C.V.; Maia, M.; Ventura, B.V.; Van der Linden, V.; Araújo, E.B.; Ramos, R.C.; Rocha, M.A.W.; Carvalho, M.D.C.G.; Belfort, R., Jr.; Ventura, L.O. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq. Bras. Oftalmol. 2016, 79, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Gois, A.L.; Belfort, R. Zika virus in Brazil and macularatrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef] [Green Version]
- De PaulaFreitas, B.; de OliveiraDias, J.R.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R., Jr. Ocular Findings in Infants with Microcephaly Associated with Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linden, V.; Filho, E.L.; Lins, O.G.; van der Linden, A.; de Aragão, M.F.; Brainer-Lima, A.M.; Cruz, D.D.; Rocha, M.A.; Sobral da Silva, P.F.; Carvalho, M.D.; et al. Congenital Zika syndrome with arthrogryposis: Retrospective case series study. BMJ 2016, 354, i3899. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Kliegman, R.; Stanton, B.; Geme, J.S.; Schor, N. Nelson Textbook of Pediatrics, 20th ed.; 2 Volume Set; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- World Health Organization. Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children’s Fundation; World Health Organization: Geneva, Switzerland, 2009.
- De Onis, M. Update on the implementation of the WHO child growth standards. World Rev. Nutr. Diet. 2013, 106, 75–82. [Google Scholar]
- Kuperminc, M.N.; Stevenson, R.D. Growth and nutrition disorders in children with cerebral palsy. Dev. Disabil. Res. Rev. 2008, 14, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.B. Gastrointestinal disorders in children with neurodevelopmental disabilities. Dev. Disabil. Res. Rev. 2008, 14, 128–136. [Google Scholar] [CrossRef]
- Yakut, A.; Dinleyici, E.C.; Idem, S.; Yarar, C.; Dogruel, N.; Colak, O. Serum leptin levels in children with cerebral palsy: Relationship with growth and nutritional status. Neuro. Endocrinol. Lett. 2006, 27, 507–512. [Google Scholar]
- Rempel, G. The importance of good nutrition in children with cerebral palsy. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 39–56. [Google Scholar] [CrossRef]
- Moura da Silva, A.A.; Ganz, J.S.; Sousa, P.D.; Doriqui, M.J.; Ribeiro, M.R.; Branco, M.D.; Queiroz, R.C.; Pacheco, M.J.; Vieira da Costa, F.R.; Silva, F.S. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg. Infect. Dis. 2016, 22, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Prata-Barbosa, A.; Martins, M.M.; Guastavino, A.B.; Cunha, A. Effects of Zika infection on growth. J. Pediatr. 2018, 95, 30–41. [Google Scholar] [CrossRef] [PubMed]
- França, T.L.B.; Mede iros, W.R.; Souza, N.L.; Longo, E.; Pereira, S.A.; França, T.B.O.; Sousa, K.G. Growth and Development of Children with Microcephaly Associated with Congenital Zika Virus Syndrome in Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satterfield-Nash, A.; Kotzky, K.; Allen, J.; Bertolli, J.; Moore, C.A.; Pereira, I.O.; Pessoa, A.; Melo, F.; Santelli, A.C.F.E.S.; Boyle, C.A.; et al. Health and Development at Age 19–24 Months of 19 Children Who Were Born with Microcephaly and Laboratory Evidence of Congenital Zika Virus Infection During the 2015 Zika Virus Outbreak—Brazil, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1347–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valde rramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Leal, M.C.; van der Linden, V.; Bezerra, T.P.; de Valois, L.; Borges, A.C.G.; Antunes, M.M.C.; Brandt, K.G.; Moura, C.X.; Rodrigues, L.C.; Ximenes, C.R. Characteristics of Dysphagiain Infants with Microcephaly Caused by Congenital Zika Virus Infection, Brazil, 2015. Emerg. Infect. Dis. 2017, 23, 1253–1259. [Google Scholar] [CrossRef]
- Van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Júnior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragão, M.F. Description of 13 Infants Born During October 2015–January 2016 with Congenital Zika Virus Infection without Microcephaly at Birth—Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Granger, D.; Hilgart, H.; Misner, L.; Christensen, J.; Bistode au, S.; Palm, J.; Strain, A.K.; Konstantinovski, M.; Liu, D.; Tran, A.; et al. Serologic testing for Zika virus: Comparison of three Zika virus IgM-screening enzyme-linked immunosorbent assays and initial laboratory experiences. J. Clin. Microbiol. 2017, 55, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- INTERGROWTH-21st. Available online: https://intergrowth21.tghn.org/standards-tools/ (accessed on 22 June 2020).
- IBGE. POF—Pesquisade Orçamentos Familiares. Available online: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009/default.shtm (accessed on 2 November 2020).
- WHO. Nutrition Landscape Information System (NLIS) Country Profile Indicators: Interpretation Guide; WHO: Geneva, Switzerland, 2010.
- De Cássia Oliveirade Carvalho-Sauer, R.; da Conceição Nascimento Costa, M.; Paixão, E.S.; de Jesus Silva, N.; Barreto, F.R.; Teixeira, M.G. Cross-sectional study of the anthropometric characteristics of children with congenital Zika syndrome up to 12 months of life. BMC Pediatr. 2020, 20, 479. [Google Scholar]
- Landry, M.L.; StGeorge, K. Laboratory Diagnosis of Zika Virus Infection. Arch. Pathol. Lab. Med. 2017, 141, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.L.; Merriam, A.A.; Ohuma, E.O.; Dighe, M.K.; Gale, M.; Rajagopal, L.; Papageorghiou, A.T.; Gyamfi-Bannerman, C.; Waldorf, K.M.A. Femur-sparing pattern of abnormal fetal grow thin pregnant women from New York City after maternal Zika virus infection. Am. J. Obstet. Gynecol. 2018, 219, 187.e1–187.e20. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernande z, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uraki, R.; Jurado, K.A.; Hwang, J.; Szigeti-Buck, K.; Horvath, T.L.; Iwasaki, A.; Fikrig, E. Fetal Growth Restriction Caused by Sexual Transmission of Zika Virus in Mice. J. Infect. Dis. 2017, 215, 1720–1724. [Google Scholar] [CrossRef] [Green Version]
- Cugola, F.R.; Fernande s, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Sauer, R.; Costa, M.C.N.; Barreto, F.R.; Teixeira, M.G. Congenital Zika Syndrome: Prevalence of low birth weight and associated factors. Bahia, 2015–2017. Int. J. Infect. Dis. 2019, 82, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, C.Y.P.; Caron, D.M.R.; Souza, C.B.; Kozima, P.F.A.; Damasceno, L.; Einspieler, C.; Marschik, P.B.; Brasil, P.; Schmitt, A.C.B.; Nielsen-Saines, K.; et al. Neurodevelopment in the third year of life in children with antenatal ZIKV-exposure. Rev. Saude Publica 2021, 55, 15. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Junior, S.C.G.; Pone, S.M.; Pone, M.V.D.S.; Vasconcelos, Z.; Zin, A.; Vilibor, R.H.H.; Costa, R.P.; Meio, M.D.B.B.; Nielsen-Saines, K.; et al. Neurodevelopment of children exposed intra-uterus by Zika virus: Acaseseries. PLoS ONE 2020, 15, e0229434. [Google Scholar] [CrossRef] [Green Version]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; de Carvalho, L.M.A.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef]
- Paul, A.; Acharya, D.; Neupane, B.; Thompson, E.A.; Gonzalez-Fernande z, G.; Copeland, K.M.; Garrett, M.; Liu, H.; Lopez, M.E.; De Cruz, M.; et al. Congenital Zika Virus Infection in Immunocompetent Mice Causes Postnatal Growth Impediment and Neurobehavioral Deficits. Front. Microbiol. 2018, 9, 2028. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G.C.; Seferovic, M.D.; Fowler, S.W.; Major, A.M.; Gorchakov, R.; Berry, R.; Swennes, A.G.; Murray, K.O.; Suter, M.A.; Aagaard, K.M. Timing of gestational exposure to Zika virus is associated with postnatal growth restriction in a murine mode l. Am. J. Obstet. Gynecol. 2018, 219, 403.e1–403.e9. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.F.M.; Soares, F.V.M.; de Abranches, A.D.; da Costa, A.; Lopes Moreira, M.; de Matos Fonseca, V. Infants with microcephaly due to ZIKA virus exposure: Nutritional status and food practices. Nutr. J. 2019, 18, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.M.D.S.; Miranda-Filho, D.B.; Ximenes, R.A.A.; Montarroyos, U.R.; Martelli, C.M.T.; Brickley, E.B.; Gouveia, M.C.L.; Ramos, R.C.; Rocha, M.Â.W.; Araujo, T.V.B. Comparison of Oropharyngeal Dysphagiain Brazilian Children with Prenatal Exposure to Zika Virus, with and without Microcephaly. Dysphagia 2021, 36, 583–594. [Google Scholar] [CrossRef]
- Gouvea, L.A.; Martins, M.; Vivacqua, D.; Rosseto, J.; Lima, G.; Frota, A.C.; Abreu, T.; Araujo, A.; Hofer, C.B. Complications and Sequelae in Patients with Congenital Microcephaly Associated with Zika Virus Infection: Two-Year Follow-Up. J. Child. Neurol. 2021, 36, 537–544. [Google Scholar] [CrossRef]
- De Fatima Viana Vasco Aragão, M.; van der Linden, V.; Petribu, N.C.; Valenca, M.M.; Parizel, P.M.; de Mello, R.J.V. Congenital Zika Syndrome: The Main Cause of Death and Correspondence between Brain CT and Postmortem Histological Section Findings from the Same Individuals. Top. Magn. Reson. Imaging 2019, 28, 29–33. [Google Scholar] [CrossRef]
- Day, S.M.; Strauss, D.J.; Vachon, P.J.; Rosenbloom, L.; Shavelle, R.M.; Wu, Y.W. Growth patterns in a population of children and adolescents with cerebral palsy. Dev. Med. Child. Neurol. 2007, 49, 167–171. [Google Scholar] [CrossRef]
- Day, S.M. Improving growth charts for children and adolescents with cerebral palsy through evide nce-based clinical practice. Dev. Med. Child. Neurol. 2010, 52, 793. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Conaway, M.; Chumlea, W.C.; Rosenbaum, P.; Fung, E.B.; Hende rson, R.C.; Worley, G.; Liptak, G.; O’Donnell, M.; Samson-Fang, L.; et al. Growth and Health in Children with Moderate-to-Severe Cerebral Palsy. Pediatrics 2006, 118, 1010–1018. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Cruz, G.D.O.; Fontes, J.; de Araujo, G.; Ventura, T.; Monteiro, A.; Moreira, M. Neurogenic bladder in the settings of congenital Zika syndrome: A confirmed and unknown condition for urologists. J. Pediatr. Urol. 2019, 15, 450.e1–450.e7. [Google Scholar] [CrossRef]
- Monteiro, L.M.C.; Cruz, G.N.D.O.; Fontes, J.M.; Salles, T.R.D.S.; Boechat, M.C.B.; Monteiro, A.C.C.; Moreira, M.E.L. Neurogenic bladder findings in patients with Congenital Zika Syndrome: A novel condition. PLoS ONE 2018, 13, e0193514. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; D’Egidio, C.; Mohn, A.; Coppola, G.; Chiarelli, F. Weight gain following treatment with valproic acid: Pathogenetic mechanisms and clinical implications. Obes. Rev. 2011, 12, e32–e43. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Scaparrotta, A.; Agostinelli, S.; DiPillo, S.; Chiarelli, F.; Grosso, S. Topiramate-induced weightloss: A review. Epilepsy Res. 2011, 95, 189–199. [Google Scholar] [CrossRef] [PubMed]
| Total | Participants (N = 201) | p-Value | ||||
|---|---|---|---|---|---|---|
| NCZS (N = 114) | CZS (N = 87) | |||||
| N (%) | N (%) | |||||
| Sex | Fem | 104 (51.7%) | 59 (51.8%) | 45 (51.7%) | 1.00 | |
| Male | 97 (48.3%) | 55 (48.2%) | 42 (48.3%) | |||
| Rash by trimester of pregnancy | Absent | 19 (9.5%) | 0 (0%) | 19 (21.8%) | <0.001 | |
| 1st | 87 (43.3%) | 28 (24.6%) | 59 (67.8%) | |||
| 2nd | 63 (31.3%) | 56 (49.1%) | 7 (8%) | |||
| 3rd | 32 (15.9%) | 30 (26.3%) | 2 (2.3%) | |||
| Head circumference at birth (n = 201) | Normocephaly | 133 (66.2%) | 113 (99.1%) | 20 (23%) | <0.001 | |
| Microcephaly (<−2 SD) | 68 (33.8%) | 1 (0.9%) | 67 (77%) | |||
| Severe microcephaly (<−3 SD) | 52 (25.9%) | 0 (0%) | 52 (59.8%) | |||
| Abnormal neuroimaging (n = 199) | Yes | 87(43.7%) | 3 (2.7%) | 85 (97.7%) | <0.001 | |
| Not performed | 2 | 2 | 0 | |||
| Abnormal ophthalmologic evaluation (n = 199) | Yes | 51 (25.6%) | 1 (0.9%) | 50 (57.5%) | <0.001 | |
| Not performed | 2 | 2 | 0 | |||
| Arthrogryposis (n = 201) | Yes | 15 (7.5%) | 0 (0%) | 15 (17.2%) | <0.001 | |
| Small for Gestational Age (n = 201) | Yes | 41 (20.4%) | 7 (6.1%) | 34 (39.1%) | <0.001 | |
| Neonatal complication (n = 201) | Yes | 47 (23.4%) | 16 (14%) | 31 (35.6%) | <0.001 | |
| Breastfeeding (n = 176) | Yes | 159 (90.3%) | 87 (92.6%) | 72 (87.8%) | 0.287 | |
| Missing | 25 | 20 | 5 | |||
| Gastrostomy (n = 201) | Yes | 23 (11.4%) | 0 (0%) | 23 (26.4%) | <0.001 | |
| Hospitalization (n= 179) | All causes | Yes | 86 (48%) | 21 (22.5%) | 65 (75.5%) | <0.001 |
| Missing | 22 | 21 | 1 | |||
| Urinary tract infection | Yes | 21 (12.3%) | 2 (2.3%) | 19 (22.6%) | 0.002 | |
| Missing | 30 | 27 | 3 | |||
| Respiratory disease | Yes | 46 (26.5%) | 4 (4.6%) | 42 (50%) | <0.001 | |
| Missing | 30 | 27 | 3 | |||
| Epilepsy | Yes | 22 (10.9%) | 2 (2.2%) | 20 (23.8%) | <0.001 | |
| Missing | 30 | 27 | 3 | |||
| Surgery | Yes | 35 (17.4%) | 3 (3.5%) | 32 (38.1%) | <0.001 | |
| Missing | 31 | 28 | 3 | |||
| Anticonvulsant drugs (n= 181) | Yes | 75 (41.4%) | 3 (3.2%) | 72 (83.7%) | <0.001 | |
| Missing | 20 | 19 | 1 | |||
| Urinary tract infection (outpatient) (n = 171) | Yes | 34 (19.9%) | 7 (8%) | 27 (32.2%) | 0.003 | |
| Missing | 30 | 27 | 3 | |||
| Death (n = 201) | Yes | 5 (2.5%) | 0 (0%) | 5 (5.7%) | 0.010 | |
| Maternal education (n = 171) | 1–4 years | 9 (5.3%) | 1 (1.1%) | 8 (9.5%) | <0.001 | |
| 5–8 years | 29 (17%) | 11 (12.6%) | 18 (21.4%) | |||
| High school | 90 (52.6%) | 44 (50.6%) | 46 (54.8%) | |||
| College | 43 (25.1%) | 31 (35.6%) | 12 (14.3%) | |||
| Missing | 30 | 27 | 3 | |||
| Government aid (n= 162) | Yes | 26 (16%) | 7 (8.6%) | 19 (23.5%) | 0.001 | |
| Missing | 39 | 33 | 6 | |||
| Family income classification (n = 179) | A | 4 (2.2%) | 3 (3.1%) | 1 (1.2%) | 0.008 | |
| B | 3 (1.7%) | 2 (2.1%) | 1 (1.2%) | |||
| C | 29 (16.2%) | 23 (24.2%) | 6 (7.1%) | |||
| D | 52 (29.1%) | 29 (30.5%) | 23 (27.4%) | |||
| E | 91 (50.8%) | 38 (40%) | 53 (63.1%) | |||
| Missing | 22 | 19 | 3 | |||
| NCZS (n = 48) | CZS (n = 70) | |
|---|---|---|
| W/A< −3 SD (severely underweight) | 0 (0%) | 22 (31.4%) |
| W/A< −2 AND >−3 SD (underweight) | 0 (0%) | 15 (21.4%) |
| Total W/A < −2 SD | 0 (0%) | 37 (52.8%) |
| H/A < −3 SD (severely stunted) | 1 (2.1%) | 14 (20%) |
| H/A< −2 AND > −3 SD (stunted) | 1 (2.1%) | 26 (37.1%) |
| Total H/A < −2 SD | 2 (4.2%) | 40 (57.1%) |
| W/H< −3 SD (severely wasted) | 0 (0%) | 8 (11.4%) |
| W/H< −2 E > −3 SD (wasted) | 0 (0%) | 18 (25.7%) |
| Total W/H < −2 SD | 0 (0%) | 26 (37.1%) |
| HC/A< −3 SD (severe microcephaly) | 0 (0%) | 57 (81.4%) |
| HC/A < −2 E > −3 SD (microcephaly) | 0 (0%) | 4 (5.7%) |
| Total HC/A < −2 SD | 0 (0%) | 61 (87.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, E.B.d.; Pone, S.M.; Gomes Junior, S.C.d.S.; Soares, F.V.M.; Zin, A.A.; Vasconcelos, Z.F.M.; Ribeiro, C.T.M.; Pereira Junior, J.P.; Moreira, M.E.L.; Nielsen-Saines, K.; et al. Anthropometric Parameters of Children with Congenital Zika Virus Exposure in the First Three Years of Life. Viruses 2022, 14, 876. https://doi.org/10.3390/v14050876
Aguiar EBd, Pone SM, Gomes Junior SCdS, Soares FVM, Zin AA, Vasconcelos ZFM, Ribeiro CTM, Pereira Junior JP, Moreira MEL, Nielsen-Saines K, et al. Anthropometric Parameters of Children with Congenital Zika Virus Exposure in the First Three Years of Life. Viruses. 2022; 14(5):876. https://doi.org/10.3390/v14050876
Chicago/Turabian StyleAguiar, Elisa Barroso de, Sheila Moura Pone, Saint Clair dos Santos Gomes Junior, Fernanda Valente Mendes Soares, Andrea Araujo Zin, Zilton Farias Meira Vasconcelos, Carla Trevisan Martins Ribeiro, José Paulo Pereira Junior, Maria Elisabeth Lopes Moreira, Karin Nielsen-Saines, and et al. 2022. "Anthropometric Parameters of Children with Congenital Zika Virus Exposure in the First Three Years of Life" Viruses 14, no. 5: 876. https://doi.org/10.3390/v14050876
APA StyleAguiar, E. B. d., Pone, S. M., Gomes Junior, S. C. d. S., Soares, F. V. M., Zin, A. A., Vasconcelos, Z. F. M., Ribeiro, C. T. M., Pereira Junior, J. P., Moreira, M. E. L., Nielsen-Saines, K., & Pone, M. V. d. S. (2022). Anthropometric Parameters of Children with Congenital Zika Virus Exposure in the First Three Years of Life. Viruses, 14(5), 876. https://doi.org/10.3390/v14050876






