Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is a human delta retrovirus that causes adult T-cell leukemia/lymphoma (ATL) in 3–5% of the infected population after decades of clinical latency. HTLV-1 Tax is a potent activator of IKK/NF-κB and a clastogen. While NF-κB activities are associated with cell survival and proliferation, constitutive NF-κB activation (NF-κB hyperactivation) by Tax leads to senescence and oncogenesis. Until recently, the mechanisms underlying the DNA damage and senescence induced by Tax and NF-κB were unknown. Current data indicate that NF-κB hyperactivation by Tax causes the accumulation of a nucleic acid structure known as an R-loop. R-loop excision by the transcription-coupled nucleotide excision repair (TC-NER) endonucleases, Xeroderma pigmentosum F (XPF), and XPG, in turn, promotes DNA double-strand breaks (DSBs). NF-κB blockade prevents Tax-induced R-loop accumulation, DNA damage, and senescence. In the same vein, the silencing of XPF and XPG mitigates Tax senescence, while deficiency in either or both frequently occurs in ATL of all types. ATL cells maintain constitutively active NF-κB, accumulate R-loops, and resist Tax-induced senescence. These results suggest that ATL cells must have acquired adaptive changes to prevent senescence and benefit from the survival and proliferation advantages conferred by Tax and NF-κB. In this review, the roles of R-loops in Tax- and NF-κB-induced DNA DSBs, senescence, and ATL development, and the epigenetic and genetic alterations that arise in ATL to reduce R-loop-associated DNA damage and avert senescence will be discussed.
1. Introduction
Human T-cell leukemia virus type-1 (HTLV-1) is a complex human delta retrovirus that causes an aggressive CD4+ T-cell malignancy called Adult T-cell Leukemia/Lymphoma (ATL) [1,2]. HTLV-1 is endemic in southwestern Japan, Africa, South America, the Caribbean Islands, and Central Australia and infects approximately 10–20 million people worldwide [2]. The majority of HTLV-1-infection is asymptomatic. However, 3–5% of infected individuals develop ATL after decades of clinical latency. ATL is classified as the aggressive acute and lymphomatous subtypes and the indolent chronic and smoldering subtypes [3]. HTLV-1 infection also leads to inflammatory and immune-mediated diseases, including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [4,5], uveitis, arthritis, conjunctivitis, dermatitis, and susceptibility to helminthic and bacterial infections. More recently, HTLV-1 has been associated with bronchiectasis in the indigenous people of Central Australia [6,7].
HTLV-1 is transmitted by cell-to-cell contact between infected T-lymphocytes and uninfected target cells. Human-to-human transmission occurs via the transfer of virus-infected cells through breastfeeding, transfusion of cell-containing blood or blood products, organ transplantation, and sexual intercourse. In infected individuals, HTLV-1 proviral DNAs integrate into host chromosomes. Latently infected cells are maintained by mitotic expansion. De novo infection within infected individuals occurs continually [8], likely due to intermittent viral reactivation and spread to uninfected cells.
The HTLV-1 proviral DNA is approximately 9 kb in size and is flanked by 5′- and 3′- long terminal repeats (LTR). The 5′ side of the proviral DNA harbors retroviral genes that encode structural and enzymatic proteins (gag, pol, env). In contrast, the 3′ pX region contains several overlapping open reading frames (ORFs) that encode regulatory proteins (p12, p13, p30, Tax, and Rex) [9]. The anti-sense strand of the HTLV-1 proviral genome encodes another regulatory protein called HBZ, HTLV-1 basic domain leucine zipper protein, whose coding sequence resides primarily between the env and pX regions [10].
Tax and HBZ are regulatory proteins that play indispensable roles in the HTLV-1 viral life cycle and ATL development. Tax is a weak or conditional DNA binding protein. It drives LTR-mediated viral mRNA transcription by forming protein complexes with the basic domain-leucine zipper transcription factors, CREB and ATF-1, to assemble on three composite enhancer DNA elements in the U3 region of the LTR. LTR-bound Tax, in turn, recruits transcriptional co-activators including CBP/p300 to promote robust viral trans-activation [9]. Tax also exerts pleiotropic effects on cell signaling. It potently activates TAK1, IKK-NF-κB, JNK, p38 kinase, and the mTOR pathways to facilitate viral replication and cell survival [11]. Curiously, Tax is also a potent clastogen that induces DNA DSBs and micronuclei formation [12] and inhibits DNA damage repair [13], activities associated with senescence induction [9].
ATL exhibits extensive genomic instability and chromosomal abnormalities compared to other lymphoid malignancies. The whole-genome and exome sequencing of 426 ATL patients had previously identified, on average, 7.9 point mutations/106 bases of ATL DNA and 59.5 structural variations per ATL genome [14]. The genomic instability of ATL is thought to be caused by Tax. Tax expression is lost or silenced in approximately 50% of ATL cells due to nonsense mutations or deletions in the Tax coding sequence or deletions or DNA methylation in the 5′ LTR [15]. In Tax-null ATL cells, gain-of-function somatic mutations in the T/B cell receptor signaling pathways supplant Tax to drive NF-κB activation [14].
HBZ antagonizes many activities of Tax, including LTR and NF-κB activation [16]. It is crucial for establishing and maintaining HTLV-1 latency and the mitotic expansion of latently infected T cells. Notably, HBZ is expressed in all ATL cells and is necessary for ATL proliferation [10,17,18]. Interestingly, recent data have indicated that Tax expression occurred intermittently in ATL cell lines, including MT1, where it induced the expression of anti-apoptotic factors via NF-κB to facilitate cell survival [19]. In agreement with these results, Tax and HTLV-1 plus-strand mRNA transcripts have also been shown to express in intense bursts in lymphocytes of infected individuals cultured ex vivo [20]. Most recently, the DNA damage and senescence response induced by Tax has been associated with a nucleic structure known as an R-loop that accumulates due to NF-κB hyperactivation by Tax [21]. This review aims to integrate these recent findings and discuss the role of Tax-/NF-κB-induced co-transcriptional R-loops in ATL development.
2. R-Loops, DNA Double-Strand Breaks, and Genomic Instability
An R-loop is a three-stranded nucleic acid structure consisting of an RNA-DNA hybrid and a displaced single-stranded DNA loop (Figure 1). R-loops occur naturally in the cell. They regulate immunoglobulin isotype switching, CRISPR-mediated genome editing, transcription-coupled nucleotide excision repair (TC-NER), chromatin structure, and transcription [22,23,24]. Of particular relevance here, transcriptional activation/derepression and RNA splicing/elongation/processing/export deficiencies are known to promote R-loop accumulation and genomic instability [25,26,27]. R-loop formation can occur co-transcriptionally (in cis, due to RNA polymerase stalling caused by excess transcriptional activation/derepression or at sites of DNA damage) or post-transcriptionally (in trans, as a result of RNA accumulation due to RNA processing defects or guide RNA-mediated gene targeting).
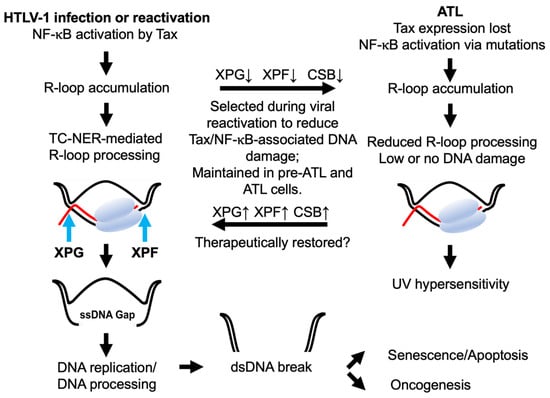
Figure 1.
Tax-/NF-κB-induced co-transcriptional R-loops and DNA damage select for transcription-coupled nucleotide excision repair (TC-NER) deficiencies in ATL. R-loop is a three-stranded nucleic acid structure consisting of an RNA-DNA hybrid and a displaced single-stranded DNA loop. NF-κB hyperactivation by Tax leads to R-loop accumulation. During R-loop processing, XPF and XPG cleave at the 5′ and 3′ ends of the RNA-bound DNA strand to generate a single-stranded DNA (ssDNA) gap (left column). DNA replication or additional ssDNA processing leads to DSBs, resulting in genomic instability, senescence, apoptosis, or oncogenesis (bottom). Co-translational R-loops can also lead to transcription-replication conflicts and collapse of replication forks and DSBs (not depicted). Latently infected T cells deficient in TC-NER survive HTLV-1 reactivation better and evolve into ATL cells (right column). Somatic mutations in TCR signaling develop in ATL to drive Tax-independent NF-κB activation. NF-κB-induced R-loops accumulate in ATL cells without causing senescence due to the down-regulation of TC-NER mediators XPF, XPG, CSB, and other alterations. TC-NER deficiencies in ATL result in hypersensitivity to ultraviolet light (right column). Restoration of TC-NER is expected to increase R-loop excision, leading to DSBs and senescence of ATL cells. DNA double helix, RNA polymerase II, and mRNA are depicted in black, grey, and red.
Co-transcriptionally formed R-loops have recently gained much attention as they regulate gene expression and predispose the genome to DNA damage. The non-template single-stranded DNA of the R-loop is vulnerable to DNA altering agents like activation-induced cytidine deaminase or DNA endonuclease and causes lesions or nicks. R-loops can also interfere with DNA replication to cause replication fork collapse and DNA DSBs [28]. Activation of estrogen-response element E2 by estrogen rapidly increases gene expression, R-loop accumulation, and DNA damage in human breast cancer cell lines [29]. Likewise, TATA-binding protein (TBP) overexpression caused by HRASV12 upregulates genome-wide transcription and R-loop formation in immortalized human fibroblasts [30]. More recently, R-loop excision by the TC-NER endonucleases, Xeroderma pigmentosum F (XPF) and XPG, has been causally linked to the induction of DNA DSBs and genomic instability [23] (Figure 1).
3. Tax Hijacks RNF8 for Canonical NF-κB Activation and DNA Damage Response Perturbation
Tax is a potent activator of canonical and non-canonical IKK/NF-κB signaling, but the underlying mechanisms remained unsolved until recently. Current data indicate that Tax hijacks and aberrantly activates RING finger protein 8 (RNF8), a lysine 63 (K63) ubiquitin E3 ligase critical for signaling DNA DSB repair, and another ubiquitin ligase, linear (M1) ubiquitin assembly complex (LUBAC), to promote the assembly of K63-M1 hybrid polyubiquitin chains in the cytosol [31,32]. This signaling scaffold, in turn, recruits and activates multiple kinases, including TAK1, IKK, mTOR, JNK, p38 kinase, etc., and their downstream effectors, leading to potent activation of canonical NF-κB and other signaling pathways [11]. How Tax activates the non-canonical NF-κB pathway remains unclear to date but likely involves the stabilization and activation of NF-κB-inducing kinase NIK [33].
It should be pointed out that the impact of RNF8 dysregulation by Tax extends beyond the cytosolic activation of the kinase cascades mentioned above. Aberrant RNF8 activation by Tax in the nucleus leads to the assembly of K63-linked polyubiquitin chains that sequester DNA damage response (DDR) factors, including RNF8, BRCA1, DNA-PK, MDC1, etc., into Tax-containing pseudo-DNA damage foci [34] known as Tax speckle structures [35,36,37] that disrupt DDR signaling and interfere with repair of DNA DSBs.
4. NF-κB Hyperactivation by Tax Induces Cellular Senescence
NF-κB impacts immune and inflammatory responses broadly and potently. As such, cellular pathways that lead to NF-κB activation are stringently regulated by negative feedback mechanisms to ensure NF-κB is active only in a short duration [38]. As NF-κB confers survival and proliferative advantages, it is often constitutively activated in T/B cell malignancies, including ATL [14,39,40,41,42,43,44,45].
As HTLV-1 causes ATL in infected individuals and transforms T cells in culture, it was initially thought that HTLV-1 infection induces T cells to proliferate [46]. And since Tax potently activates viral transcription and IKK/NF-κB and other signaling pathways, it has been proposed that via these activities of Tax, especially NF-κB activation and transcriptional activation of interleukins and interleukin receptors, HTLV-1-infected T cells are driven to grow and divide, leading to ATL [47,48,49]. This view, however, is oversimplified and incomplete, especially in light of the discovery of HBZ. Furthermore, persistent stimulation of IKK/NF-κB by Tax comes with a price in the form of the induction of a rapid cellular senescence response (Tax-IRS) mediated by the G1/S cyclin-dependent kinase inhibitors p21Cip1/Waf1 (p21) and p27Kip1 (p27) [50,51,52]. NF-κB blockade by ΔN-I-κBα, a degradation-resistant truncation mutant of I-κBα, the inhibitor of NF-κB, dramatically prevented senescence [53].
Of note, the transcriptional activity of NF-κB is critical for Tax-induced senescence [54]. RNA silencing of p65/RelA and its upstream kinases, especially NIK and IKKα, which drive RelA activation via Ser-536 phosphorylation, mitigated senescence [53,54]. Importantly, senescence induction is not merely a result of Tax over-expression. Naive cells infected by HTLV-1 in culture arrest in senescence [55,56,57], and only infected cells expressing Tax at low levels or not at all escape senescence [56]. As expected, NF-κB inhibition allows infected cells to clonally expand [57].
Tax-induced senescence occurs after cellular passage through an aberrant cell cycle during which the S and G2 phases stall and mitosis is disrupted or impaired [50,58]. As discussed below, the senescence response induced by Tax is correlated with NF-κB- and R-loop-associated DNA DSBs. Other effects of Tax, such as cell cycle perturbation, cell cycle arrest, and apoptosis, are likely associated with the DNA DSBs caused by hyperactivated NF-κB. Finally, NF-κB is constitutively active in ATL cells of all types. As expected, expression or re-expression of Tax in ATL cells no longer induces senescence. These results suggest that ATL cells must have acquired genetic/epigenetic changes that can prevent or mitigate Tax-IRS [59]. Importantly, many ATL cells continue to accumulate R-loops in great abundance, raising the possibility that corrections of the said genetic/epigenetic changes may restore Tax-/NF-κB-/R-loop-associated DNA damage and senescence in ATL cells.
5. Transcription-Coupled Nucleotide Excision Repair and Transcription-Replication Conflict May Underlie the R-Loop-Associated DNA Double-Strand Breaks Caused by Tax
Transcription-coupled nucleotide excision repair (TC-NER) pathway is conserved and ubiquitous in organisms ranging from unicellular bacteria and yeast to mammals. It consists of a multiprotein repair system capable of recognizing and processing DNA structural distortions and lesions induced by UV irradiation, reactive oxygen and nitrogen species, and mutagens that introduce bulky chemical adducts in DNA. TC-NER pathway repairs DNA damage by a “cut and patch” mechanism. Initiation of repair occurs upon the physical blockage of RNA polymerase II (RNAPII) at the sites of DNA lesions/distortions during transcription. RNAPII stalling, in turn, gives rise to an R-loop formed by the nascent RNA transcript, the stalled RNAPII, the DNA template containing the lesion, and the single-stranded complementary DNA strand that loops out (see Figure 1). TC-NER endonucleases XPF and XPG are then recruited to the 5′ and 3′ sides of the R-loop to excise the DNA lesion and the short (24–32 nt) RNA-DNA hybrid from the damaged template strand. Gap-filling DNA repair then occurs using the undamaged DNA strand as a template [60]. Autosomal recessive genetic disorders such as Xeroderma Pigmentosum (XP) and Cockayne Syndrome (CS), are caused by mutations in key mediators of the TC-NER pathway, including XPF, XPG, CSB, etc., that disable the repair of UV-induced DNA damage [61], causing extreme sensitivity to sunlight and increased risk of cutaneous neoplasms.
When R-loops accumulate due to Tax-induced NF-κB hyperactivation, they are thought to be excised much like those that form co-transcriptionally at UV-induced DNA lesions, leading to single-stranded DNA gaps. DNA replication or additional DNA incision then gives rise to DNA DSBs. Indeed, NF-κB blockade prevents Tax-induced R-loop accumulation, DNA damage, and senescence [21,53]. The silencing of XPF and XPG also mitigates Tax senescence, while deficiency in either or both frequently occurs in ATL cells of all types, resulting in sensitivity to UV irradiation [21]. Finally, many Tax-expressing cells progress through the S, G2, and M phases of the cell cycle with difficulties. This aberrant cell cycle is accompanied by a dramatic rise in the mRNA and protein levels of p21 and p27, leading to cellular senescence [50]. Notably, the increase in p21 begins in the S phase, persists through G2 and M phases, and ends with p21 and p27 levels reaching their peaks in an irreversible G1/senescence arrest accompanied by chromosomal abnormalities [50,58]. Excess co-transcriptional R-loops can also cause transcription-replication conflicts (TRC) and jamming of replication fork progression to cause replication fork collapse and DNA DSBs [24]. Whether TRC plays a role in Tax-induced DNA DSBs and senescence induction remains to be demonstrated.
6. NF-κB Hyperactivation and Excess R-Loop Accumulation in ATL Cells
Excess R-loop accumulation threatens genomic integrity and contributes to oncogenic, neurodegenerative, and inflammatory disorders [62,63,64,65]. As discussed in a recent review [64], several cellular factors are known to regulate the homeostasis of R-loops. Notable among them are (i) RNaseH1 and RNaseH2, ribonucleases that specifically degrade the RNA moieties in R-loops; (ii) RNA/DNA helicases like Sen1 in yeast, and Senataxin (SETX) and DHX9 in humans; (iii) DNA topoisomerases that relax DNA-negative supercoiling induced by R-loops; (iv) mRNA biogenesis factors that suppress R-loop formation; and (v) chromatin remodeling factors such as the FACT complex; (vi) BRCA1, BRCA2, and members of the Fanconi anemia pathway that directly or indirectly remove or resolve the R-loops that block DNA replication.
The survival and proliferative advantages conferred by NF-κB for ATL cells outweigh the disadvantages associated with R-loops. NF-κB is constitutively active in ATL cells, and, as expected, R-loop levels therein are significantly elevated (2–3 fold of non-ATL control) [21]. These data raise the questions of what adaptive changes have evolved in ATL cells to allow NF-κB and Tax to be exploited for cell survival and proliferation without triggering extensive DSBs and senescence/cell cycle arrest and whether senescence can be reinstated by reversing such changes.
Do ATL cells mitigate the risk of excess R-loops by preventing their formation or resolving them via the cellular factors described above? Transcriptional repression of RNF8, the K63 ubiquitin E3 ligase hijacked by Tax for canonical NF-κB activation, is common in ATL cells of all types [34]. Many ATL cells also frequently down-regulate TC-NER factors such as XPF and XPG [21]. These alterations can reduce NF-κB-associated R-loop accumulation and moderate R-loop excision to lessen DNA DSBs. A deep dive into the transcriptomic and genomic data of ATL may reveal additional clues.
Other changes in ATL cells likely prevent the senescence response mediated by p21 and p27. In this vein, it is interesting to note that Kaposi’s sarcoma-associated herpesvirus (KSHV) viral cyclin (vCyclin) forms a vCyclin-CDK6 complex that resists p21 and p27 inhibition and targets p27 for degradation to drive cell proliferation [66,67]. Indeed, KSHV vCyclin effectively prevents the senescence response/G1 arrest induced by Tax and KSHV vFLIP, the KSHV-encoded activator of IKK/NF-κB. Remarkably, vCyclin and vFLIP are co-expressed in a bi-cistronic KSHV latency-associated mRNA transcript [51], consistent with their functional co-dependency. Thus, it would not be surprising if some of the key adaptive changes in ATL up-regulate G1 and G1/S cyclins expression and CDK4/6 activities to overcome the p21- and p27-mediated cell cycle blockade integral to Tax-/NF-κB-induced senescence/cell cycle arrest.
7. NF-κB-Induced R-Loops and DNA Double-Strand Breaks in ATL
R-loop accumulation is often a result of aberrant mRNA transcription, processing, or export. [23,68]. Excess mRNA transcription that results from potent NF-κB activation by Tax during viral replication is expected to promote R-loop accumulation and DNA DSBs. Of note, many ATL-significant and indel (insertion and deletion)-containing genes, including B2M, FAS, GATA3, HLA-B, IL-10, NFKBIA, TNFAIP3, and TP53, are transcriptionally activated by Tax via NF-κB. These genes encode proteins that are critical for the negative feedback regulation of the IKK/NF-κB pathway (TNFAIP3 and NFKBIA), tumor suppression (FAS and TP53), and host immune responses (GATA3, IL-10, B2M, and HLA-B). It is tempting to speculate that the indels within these genes originated from non-homologous end joining (NHEJ)-mediated repair of R-loop-induced DNA DSB and emerged as a result of recurrent viral reactivation. R-loop-induced DSBs may also accelerate the genetic exchange between mutant and wild-type alleles via homology-directed repair, leading to loss of heterozygosity and inactivation of tumor suppressor genes. Finally, if indeed Tax-/NF-κB-induced R-loops drive the genomic instability of ATL, why do only a select few among the approximately 500 genes under the transcriptional regulation of NF-κB incur indels and become significant for ATL development? Are they positively selected to favor ATL during the long disease course? Are there more indels in NF-κB-regulated genes of ATL than meet the eye?
8. Concluding Remarks and Future Direction
HTLV-1 infection in cell culture causes most cells to become senescent or senescent-like due apparently to the cytopathic effect of Tax-related DNA damage. Only a minor fraction of infected cells that do not express Tax or express it at low levels manage to survive and persist [56]. The fates of primary CD4+ T cells freshly infected by HTLV-1 in vivo remain unknown. It is well established that infected CD4+ T cells in HTLV-1 carriers show no detectable viral mRNA or protein expression, and a robust CTL response against Tax keeps viral replication in check [69]. Infected cells undergo limited oligoclonal expansion, likely due to HBZ action, giving rise to reservoirs of T cells harboring latent proviral DNA genomes that persist in the infected host and serve as the vehicles of HTLV-1 transmission and pathogenesis. A long-term follow-up of HTLV-1 carriers in Japan had previously demonstrated increased proviral DNA loads (PVLs) preceded the onset of ATL. PVL increase was coupled with the expansion of preleukemic clones that appeared as early as eight years before disease diagnosis [70]. A longitudinal study of Jamaican children who became HTLV-1-infected perinatally also showed that de novo HTLV-1 infections continued to occur. And clones that appeared soon after the initial infections persisted and, on rare occasions, underwent significant expansion [8].
Recent evidence demonstrates that latent HTLV-1 provirus reactivates sporadically and expresses Tax in a short, intense burst of ~19 h to induce anti-apoptotic factors that enhance cell survival. Tax inhibits DNA damage response [13,34], inactivates p53 functionally [71,72], and promotes R-loop-associated DNA DSBs and genomic instability [21]. It is conceivable that intermittent Tax expression during viral reactivation drives R-loop-induced DSBs and genomic instability, giving rise to indels and other structural alterations that confer survival and proliferation advantages and are perpetuated under the influence of HBZ (Figure 2). With frequent recurrence, this process likely leads to ATL-significant mutations that inactivate specific tumor suppressors (FAS and TP53), feedback inhibitors of IKK/NF-κB (TNFAIP3 and NFKBIA), and host immune responses (B2M and HLA-B) under NF-κB control (Figure 2).
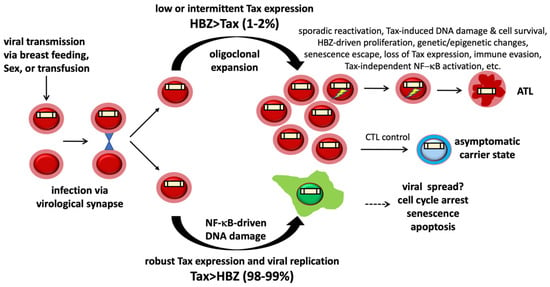
Figure 2.
HTLV-1 and ATL development HTLV-1 infection is cell-mediated and leads to either active viral replication and senescence or latency with low or intermittent Tax expression. HBZ, in turn, stimulates CD4+ T cells latently infected by HTLV-1 (LICs) to expand mitotically. A robust cytotoxic T lymphocyte (CTL) response against Tax keeps HTLV-1 replication in check in most virus carriers. LICs constitute the cell reservoir from which ATL emerges. Intermittent HTLV-1 reactivation (denoted by flash signs), Tax expression, NF-κB activation, and R-loop accumulation drive DNA damage and genomic instability in LICs. Recurrent viral reactivation also selects for epigenetic and genetic changes that mitigate/prevent the DNA damage/senescence response induced by Tax and NF-κB and facilitate the proliferation of ATL cells.
Repeated cycles of viral reactivation likely also select for subclones harboring genetic or epigenetic alterations (such as down-regulation of RNF8, XPF, XPG; and up-regulation of the functional equivalents of KSHV vCyclin, etc.) that mitigate R-loop-associated DNA DSBs and prevent senescence induction by Tax and NF-κB during reactivation, setting the stage for the acquisition of gain-of-function mutations that promote Tax-independent NF-κB activation. Identifying and targeting these alterations can reinstate R-loop-induced DNA damage and senescence (or apoptosis) in NF-κB-addicted ATL cells.
Author Contributions
Conceptualization, C.-Z.G., N.P.; funding acquisition, C.-Z.G.; writing—original draft preparation, C.-Z.G., N.P.; writing—review and editing, C.-Z.G., N.P.; figure preparation, C.-Z.G.; resources, C.-Z.G.; supervision, C.-Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (R21CA216660) and Uniformed Services University (HU0001-14-1-0061).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
As presented.
Conflicts of Interest
The authors declare no competing interest. The opinions and assertions expressed herein are those of the authors’ and do not reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Glossary
| ATF-1 | activating transcription factor 1 |
| ATL | adult T cell leukemia |
| B2M | β2 microglobulin |
| BRCA1 | breast cancer gene 1 |
| BRCA2 | breast cancer gene 2 |
| CBP | CREB-binding protein |
| CREB | cAMP response element-binding protein |
| CSB | cockayne syndrome B |
| CTL | cytotoxic T lymphocyte |
| DDR | DNA damage response |
| DHX9 | DEAH-box helicase 9 |
| DNA DSB | DNA double-strand break |
| DNA-PK | DNA-dependent protein kinase |
| FACT complex | facilitates chromatin transactions complex |
| FAS | FS-7-associated surface antigen, a cell surface death receptor |
| GATA3 | GATA binding protein 3 |
| HBZ | HTLV-1 basic domain leucine zipper protein |
| HLA-B | human leukocyte antigen (HLA) complex-B, major histocompatibility complex class I-B |
| HRAS | Harvey rat sarcoma oncogene |
| HTLV-1 | Human T-cell leukemia virus type 1 |
| Indel | Insertion and deletion |
| IKK | Inhibitor of NF-κB kinase |
| JNK | c-Jun N-terminal kinase |
| LUBAC | linear (M1) ubiquitin assembly complex |
| MDC1 | mediator of DNA damage checkpoint 1 |
| mTOR | mammalian target of rapamycin |
| NHEJ | non-homologous end joining |
| NIK | NF-κB-inducing kinase, mitogen-activated protein kinase kinase kinase 14 |
| NF-κB | nuclear factor kappa B |
| NFKBIA | NF-κB Inhibitor α |
| PVL | proviral load |
| RNAPII | RNA polymerase II |
| RNF8 | RING finger protein 8 |
| Sen1 | yeast homolog of senataxin |
| SETX | senataxin, DNA/RNA helicase that resolves R-loops |
| TAK1 | TGFβ-activated kinase 1, mitogen-activated protein kinase kinase kinase 7 |
| Tax | Trans-activator of the X region |
| Tax-IRS | Tax-induced rapid senescence |
| TC-NER | transcription-coupled nucleotide excision repair |
| TNFAIP3 | TNFα-induced protein 3 |
| TP53 | tumor protein p53 (or p53) |
| XPF | xeroderma pigmentosum F |
| XPG | xeroderma pigmentosum G |
References
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W., Jr.; O’Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, prognostic factors, treatment, and response criteria of adult t-cell leukemia-lymphoma: A proposal from an international consensus meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of htlv-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult t- cell leukaemia-lymphoma. A report from the lymphoma study group (1984–87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de The, G. Antibodies to human t-lymphotropic virus type-i in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Matsumoto, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Tara, M.; Igata, A. Chronic progressive myelopathy associated with elevated antibodies to human t-lymphotropic virus type i and adult t-cell leukemialike cells. Ann. Neurol. 1987, 21, 117–122. [Google Scholar] [CrossRef]
- Einsiedel, L.; Pham, H.; Wilson, K.; Walley, R.; Turpin, J.; Bangham, C.; Gessain, A.; Woodman, R.J. Human t-lymphotropic virus type 1c subtype proviral loads, chronic lung disease and survival in a prospective cohort of indigenous australians. PLoS Negl. Trop. Dis. 2018, 12, e0006281. [Google Scholar] [CrossRef]
- Einsiedel, L.; Chiong, F.; Jersmann, H.; Taylor, G.P. Human t-cell leukaemia virus type 1 associated pulmonary disease: Clinical and pathological features of an under-recognised complication of htlv-1 infection. Retrovirology 2021, 18, 1. [Google Scholar] [CrossRef]
- Umeki, K.; Hisada, M.; Maloney, E.M.; Hanchard, B.; Okayama, A. Proviral loads and clonal expansion of htlv-1-infected cells following vertical transmission: A 10-year follow-up of children in jamaica. Intervirology 2009, 52, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Giam, C.Z. Htlv-1 replication and adult t cell leukemia development. Recent Results Cancer Res. 2021, 217, 209–243. [Google Scholar]
- Matsuoka, M.; Mesnard, J.M. Htlv-1 bzip factor: The key viral gene for pathogenesis. Retrovirology 2020, 17, 2. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Giam, C.Z. Nf-kappab signaling mechanisms in htlv-1-induced adult t-cell leukemia/lymphoma. FEBS J. 2018, 285, 3324–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majone, F.; Semmes, O.J.; Jeang, K.T. Induction of micronuclei by htlv-i tax: A cellular assay for function. Virology 1993, 193, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Marriott, S.J.; Semmes, O.J. Impact of htlv-i tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 2005, 24, 5986–5995. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult t cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Maeda, M.; Morikawa, S.; Taniguchi, Y.; Yasunaga, J.; Nosaka, K.; Tanaka, Y.; Matsuoka, M. Genetic and epigenetic inactivation of tax gene in adult t-cell leukemia cells. Int. J. Cancer 2004, 109, 559–567. [Google Scholar] [CrossRef]
- Giam, C.Z.; Semmes, O.J. Htlv-1 infection and adult t-cell leukemia/lymphoma-a tale of two proteins: Tax and hbz. Viruses 2016, 8, 161. [Google Scholar] [CrossRef]
- Matsuoka, M.; Green, P.L. The hbz gene, a key player in htlv-1 pathogenesis. Retrovirology 2009, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Shaffer, A.L., 3rd; Ceribelli, M.; Zhang, M.; Wright, G.; Huang, D.W.; Xiao, W.; Powell, J.; Petrus, M.N.; Yang, Y.; et al. Targeting the htlv-i-regulated batf3/irf4 transcriptional network in adult t cell leukemia/lymphoma. Cancer Cell 2018, 34, 286–297.e10. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, M.; Yasunaga, J.I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of htlv-1 tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Dey, S.; Ramanayake, S.; Singh, A.; Rueda, D.S.; Bangham, C.R.M. Kinetics of htlv-1 reactivation from latency quantified by single-molecule rna fish and stochastic modelling. PLoS Pathog. 2019, 15, e1008164. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Pasupala, N.; Zhi, H.; Dorjbal, B.; Hussain, I.; Shih, H.M.; Bhattacharyya, S.; Biswas, R.; Miljkovic, M.; Semmes, O.J.; et al. Nf-kappab-induced r-loop accumulation and DNA damage select for nucleotide excision repair deficiencies in adult t cell leukemia. Proc. Natl. Acad. Sci. USA 2021, 118, e2005568118. [Google Scholar] [CrossRef] [PubMed]
- Sollier, J.; Stork, C.T.; Garcia-Rubio, M.L.; Paulsen, R.D.; Aguilera, A.; Cimprich, K.A. Transcription-coupled nucleotide excision repair factors promote r-loop-induced genome instability. Mol. Cell 2014, 56, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollier, J.; Cimprich, K.A. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015, 25, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Muse, T.; Aguilera, A. R loops: From physiological to pathological roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Wahba, L.; Amon, J.D.; Koshland, D.; Vuica-Ross, M. Rnase h and multiple rna biogenesis factors cooperate to prevent rna: DNA hybrids from generating genome instability. Mol. Cell 2011, 44, 978–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amon, J.D.; Koshland, D. Rnase h enables efficient repair of r-loop induced DNA damage. Elife 2016, 5, e20533. [Google Scholar] [CrossRef]
- Li, X.; Manley, J.L. Inactivation of the sr protein splicing factor asf/sf2 results in genomic instability. Cell 2005, 122, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Gan, W.; Guan, Z.; Liu, J.; Gui, T.; Shen, K.; Manley, J.L.; Li, X. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011, 25, 2041–2056. [Google Scholar] [CrossRef] [Green Version]
- Stork, C.T.; Bocek, M.; Crossley, M.P.; Sollier, J.; Sanz, L.A.; Chedin, F.; Swigut, T.; Cimprich, K.A. Co-transcriptional r-loops are the main cause of estrogen-induced DNA damage. Elife 2016, 5, e17548. [Google Scholar] [CrossRef]
- Kotsantis, P.; Silva, L.M.; Irmscher, S.; Jones, R.M.; Folkes, L.; Gromak, N.; Petermann, E. Increased global transcription activity as a mechanism of replication stress in cancer. Nat. Commun. 2016, 7, 13087. [Google Scholar] [CrossRef]
- Ho, Y.K.; Zhi, H.; Bowlin, T.; Dorjbal, B.; Philip, S.; Zahoor, M.A.; Shih, H.M.; Semmes, O.J.; Schaefer, B.; Glover, J.N.; et al. Htlv-1 tax stimulates ubiquitin e3 ligase, ring finger protein 8, to assemble lysine 63-linked polyubiquitin chains for tak1 and ikk activation. PLoS Pathog. 2015, 11, e1005102. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Tokunaga, F.; Goto, E.; Komatsu, G.; Gohda, J.; Saeki, Y.; Tanaka, K.; Takahashi, H.; Sawasaki, T.; Inoue, S.; et al. Htlv-1 tax induces formation of the active macromolecular ikk complex by generating lys63- and met1-linked hybrid polyubiquitin chains. PLoS Pathog. 2017, 13, e1006162. [Google Scholar] [CrossRef] [PubMed]
- Uhlik, M.; Good, L.; Xiao, G.; Harhaj, E.W.; Zandi, E.; Karin, M.; Sun, S.C. Nf-kappab-inducing kinase and ikappab kinase participate in human t-cell leukemia virus i tax-mediated nf-kappab activation. J. Biol. Chem. 1998, 273, 21132–21136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, H.; Guo, X.; Ho, Y.K.; Pasupala, N.; Engstrom, H.A.A.; Semmes, O.J.; Giam, C.Z. Rnf8 dysregulation and down-regulation during htlv-1 infection promote genomic instability in adult t-cell leukemia. PLoS Pathog. 2020, 16, e1008618. [Google Scholar] [CrossRef]
- Semmes, O.J.; Jeang, K.T. Localization of human t-cell leukemia virus type 1 tax to subnuclear compartments that overlap with interchromatin speckles. J. Virol. 1996, 70, 6347–6357. [Google Scholar] [CrossRef] [Green Version]
- Durkin, S.S.; Guo, X.; Fryrear, K.A.; Mihaylova, V.T.; Gupta, S.K.; Belgnaoui, S.M.; Haoudi, A.; Kupfer, G.M.; Semmes, O.J. Htlv-1 tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 2008, 283, 36311–36320. [Google Scholar] [CrossRef] [Green Version]
- Belgnaoui, S.M.; Fryrear, K.A.; Nyalwidhe, J.O.; Guo, X.; Semmes, O.J. The viral oncoprotein tax sequesters DNA damage response factors by tethering mdc1 to chromatin. J. Biol. Chem. 2010, 285, 32897–32905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 years of nf-kappab: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and prioritization of somatic mutations in diffuse large b-cell lymphoma (dlbcl) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884. [Google Scholar] [CrossRef] [Green Version]
- Morin, R.D.; Mungall, K.; Pleasance, E.; Mungall, A.J.; Goya, R.; Huff, R.D.; Scott, D.W.; Ding, J.; Roth, A.; Chiu, R.; et al. Mutational and structural analysis of diffuse large b-cell lymphoma using whole-genome sequencing. Blood 2013, 122, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active myd88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasqualucci, L.; Trifonov, V.; Fabbri, G.; Ma, J.; Rossi, D.; Chiarenza, A.; Wells, V.A.; Grunn, A.; Messina, M.; Elliot, O.; et al. Analysis of the coding genome of diffuse large b-cell lymphoma. Nat. Genet. 2011, 43, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, G.; Dalla-Favera, R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat. Rev. Cancer 2016, 16, 145–162. [Google Scholar] [CrossRef]
- Matsuoka, M.; Jeang, K.T. Human t-cell leukaemia virus type 1 (htlv-1) infectivity and cellular transformation. Nat. Rev. Cancer 2007, 7, 270–280. [Google Scholar] [CrossRef]
- McGuire, K.L.; Curtiss, V.E.; Larson, E.L.; Haseltine, W.A. Influence of human t-cell leukemia virus type i tax and rex on interleukin-2 gene expression. J. Virol. 1993, 67, 1590–1599. [Google Scholar] [CrossRef] [Green Version]
- Siekevitz, M.; Feinberg, M.B.; Holbrook, N.; Wong Staal, F.; Greene, W.C. Activation of interleukin 2 and interleukin 2 receptor (tac) promoter expression by the trans-activator (tat) gene product of human t-cell leukemia virus, type i. Proc. Natl. Acad. Sci. USA 1987, 84, 5389–5393. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, M.; Shibuya, H.; Harada, H.; Hatakeyama, M.; Seiki, M.; Fujita, T.; Inoue, J.; Yoshida, M.; Taniguchi, T. Evidence for aberrant activation of the interleukin-2 autocrine loop by htlv-1-encoded p40x and t3/ti complex triggering. Cell 1987, 48, 343–350. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Giam, C.Z. Activation of the anaphase promoting complex by htlv-1 tax leads to senescence. EMBO J. 2006, 25, 1741–1752. [Google Scholar] [CrossRef] [Green Version]
- Zhi, H.; Zahoor, M.A.; Shudofsky, A.M.; Giam, C.Z. Kshv vcyclin counters the senescence/g1 arrest response triggered by nf-kappab hyperactivation. Oncogene 2014, 34, 496–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhi, H.; Liu, M.; Kuo, Y.L.; Giam, C.Z. Induction of p21(cip1/waf1) expression by human t-lymphotropic virus type 1 tax requires transcriptional activation and mrna stabilization. Retrovirology 2009, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, H.; Yang, L.; Kuo, Y.L.; Ho, Y.K.; Shih, H.M.; Giam, C.Z. Nf-kappab hyper-activation by htlv-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein hbz. PLoS Pathog. 2011, 7, e1002025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.K.; Zhi, H.; Debiaso, D.; Philip, S.; Shih, H.M.; Giam, C.Z. Htlv-1 tax-induced rapid senescence is driven by the transcriptional activity of nf-kappab and depends on chronically activated ikkalpha and p65/rela. J. Virol. 2012, 86, 9474–9483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Yang, L.; Zhang, L.; Liu, B.; Merling, R.; Xia, Z.; Giam, C.Z. Human t-cell leukemia virus type 1 infection leads to arrest in the g1 phase of the cell cycle. J. Virol. 2008, 82, 8442–8455. [Google Scholar] [CrossRef] [Green Version]
- Philip, S.; Zahoor, M.A.; Zhi, H.; Ho, Y.K.; Giam, C.Z. Regulation of human t-lymphotropic virus type i latency and reactivation by hbz and rex. PLoS Pathog. 2014, 10, e1004040. [Google Scholar] [CrossRef]
- Zahoor, M.A.; Philip, S.; Zhi, H.; Giam, C.Z. Nf-kappab inhibition facilitates the establishment of cell lines that chronically produce human t-lymphotropic virus type 1 viral particles. J. Virol. 2014, 88, 3496–3504. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Kotomura, N.; Ho, Y.K.; Zhi, H.; Bixler, S.; Schell, M.J.; Giam, C.Z. Complex cell cycle abnormalities caused by human t-lymphotropic virus type 1 tax. J. Virol. 2011, 85, 3001–3009. [Google Scholar] [CrossRef] [Green Version]
- Shudofsky, A.M.D.; Giam, C.Z. Cells of adult t-cell leukemia evade htlv-1 tax/nf-kappab hyperactivation-induced senescence. Blood Adv. 2019, 3, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- de Boer, J.; Hoeijmakers, J.H. Nucleotide excision repair and human syndromes. Carcinogenesis 2000, 21, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantino, L.; Koshland, D. The yin and yang of r-loop biology. Curr. Opin. Cell Biol. 2015, 34, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skourti-Stathaki, K.; Proudfoot, N.J. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014, 28, 1384–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Pereira, J.M.; Aguilera, A. R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef]
- Richard, P.; Manley, J.L. R loops and links to human disease. J. Mol. Biol. 2017, 429, 3168–3180. [Google Scholar] [CrossRef] [Green Version]
- Laman, H.; Coverley, D.; Krude, T.; Laskey, R.; Jones, N. Viral cyclin-cyclin-dependent kinase 6 complexes initiate nuclear DNA replication. Mol. Cell Biol. 2001, 21, 624–635. [Google Scholar] [CrossRef] [Green Version]
- Verschuren, E.W.; Jones, N.; Evan, G.I. The cell cycle and how it is steered by kaposi’s sarcoma-associated herpesvirus cyclin. J. Gen. Virol. 2004, 85, 1347–1361. [Google Scholar] [CrossRef]
- Crossley, M.P.; Bocek, M.; Cimprich, K.A. R-loops as cellular regulators and genomic threats. Mol. Cell 2019, 73, 398–411. [Google Scholar] [CrossRef] [Green Version]
- Asquith, B.; Hanon, E.; Taylor, G.P.; Bangham, C.R.M. Is human t-cell lymphotropic virus type i really silent? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1013–1019. [Google Scholar] [CrossRef]
- Okayama, A.; Stuver, S.; Matsuoka, M.; Ishizaki, J.; Tanaka, G.; Kubuki, Y.; Mueller, N.; Hsieh, C.C.; Tachibana, N.; Tsubouchi, H. Role of htlv-1 proviral DNA load and clonality in the development of adult t-cell leukemia/lymphoma in asymptomatic carriers. Int. J. Cancer 2004, 110, 621–625. [Google Scholar] [CrossRef]
- Cereseto, A.; Diella, F.; Mulloy, J.C.; Cara, A.; Michieli, P.; Grassmann, R.; Franchini, G.; Klotman, M.E. P53 functional impairment and high p21waf1/cip1 expression in human t-cell lymphotropic/leukemia virus type i-transformed t cells. Blood 1996, 88, 1551–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pise-Masison, C.A.; Mahieux, R.; Jiang, H.; Ashcroft, M.; Radonovich, M.; Duvall, J.; Guillerm, C.; Brady, J.N. Inactivation of p53 by human t-cell lymphotropic virus type 1 tax requires activation of the nf-kappab pathway and is dependent on p53 phosphorylation. Mol. Cell Biol. 2000, 20, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).