A Nectin1 Mutant Mouse Model Is Resistant to Pseudorabies Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analysis of Nectin1 Expression
2.3. Viral Culture and Titration
2.4. Viral Infection in Mice
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
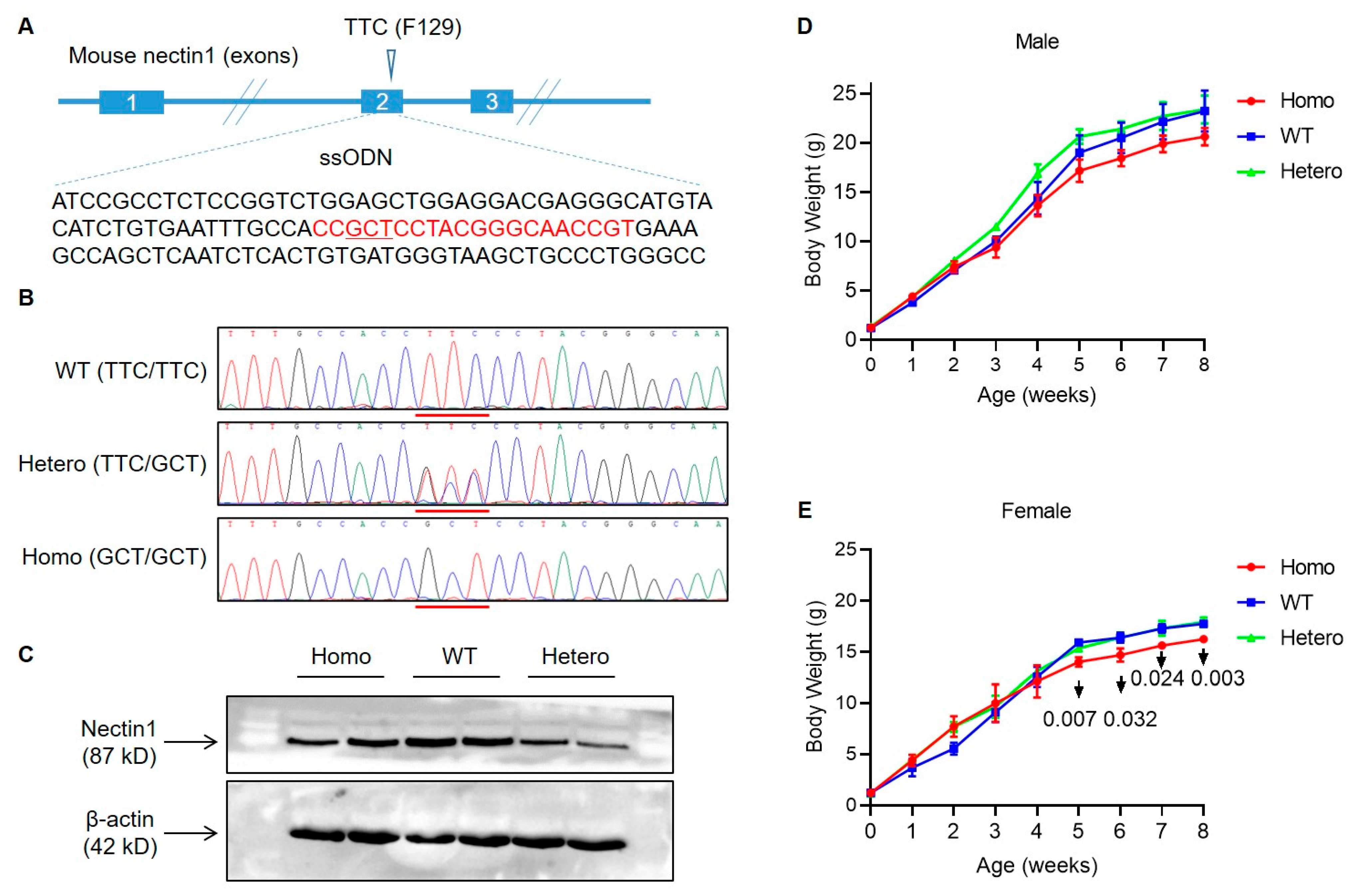
3.1. Generation of Nectin1 (F129A) Mutant Mice
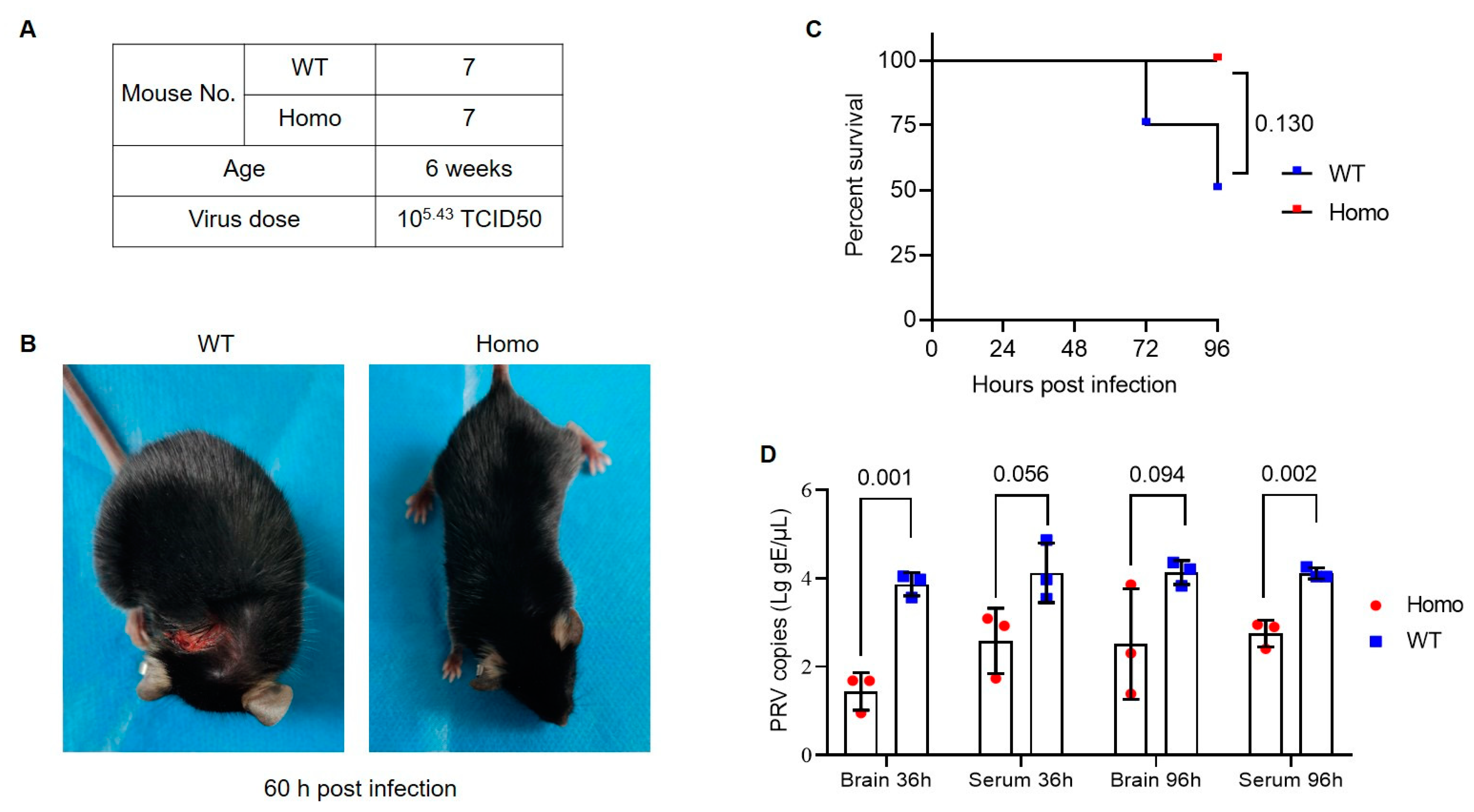
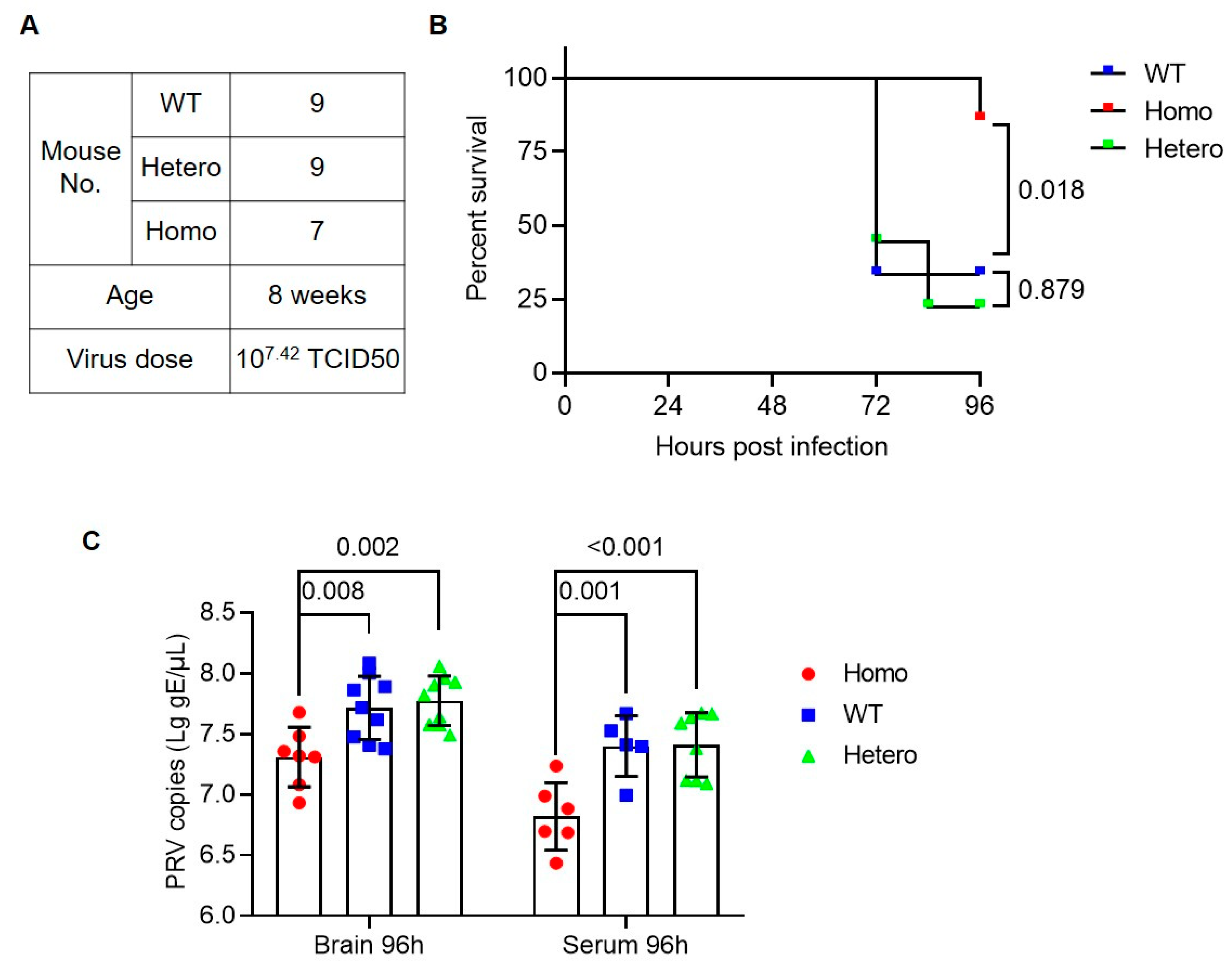
3.2. Anti-PRV Ability of Nectin1 (F129A) Mutant Mice
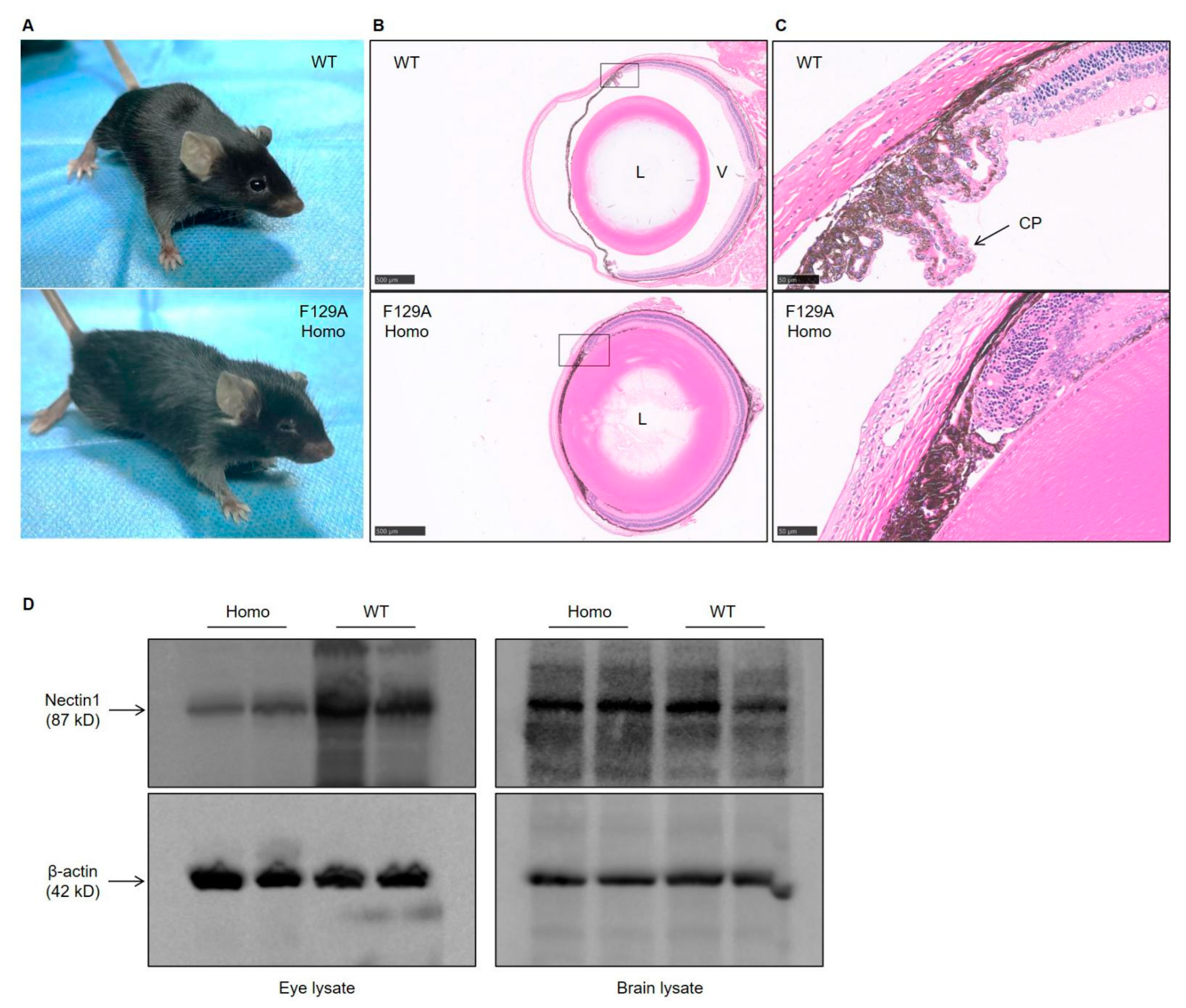
3.3. Defect in Eye Development in Nectin1 (F129A) Mutant Mice
4. Discussion
4.1. Nectin1 Modification Resists PRV Infection in Hosts
4.2. Correlation of PRV Infection in Mice and Pigs
4.3. Nectin1 (F129A) Mutation Impairs Nectin1 Physiologic Function
4.4. Exploration of Ideal Anti-PRV Gene Targets with Breeding Values in Pigs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current status and challenge of pseudorabies virus infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, F.A. Aujeszky’s disease virus: Opportunities and challenges. Vet. Res. 2000, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Aujeszky’s disease (pseudorabies) virus: The virus and molecular pathogenesis—State of the art, June 1999. Vet. Res. 2000, 31, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Pathogenesis of neurotropic herpesviruses: Role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 2003, 92, 197–206. [Google Scholar] [CrossRef]
- Cheng, Z.; Kong, Z.; Liu, P.; Fu, Z.; Zhang, J.; Liu, M.; Shang, Y. Natural infection of a variant pseudorabies virus leads to bovine death in China. Transbound. Emerg. Dis. 2019, 67, 518–522. [Google Scholar] [CrossRef]
- Jin, H.L.; Gao, S.M.; Liu, Y.; Zhang, S.F.; Hu, R.L. Pseudorabies in farmed foxes fed pig offal in Shandong province, China. Arch. Virol. 2016, 161, 445–448. [Google Scholar] [CrossRef]
- Wong, G.; Lu, J.; Zhang, W.; Gao, G.F. Pseudorabies virus: A neglected zoonotic pathogen in humans? Emerg. Microbes Infect. 2019, 8, 150–154. [Google Scholar] [CrossRef]
- Steinrigl, A.; Revilla-Fernandez, S.; Kolodziejek, J.; Wodak, E.; Bago, Z.; Nowotny, N.; Schmoll, F.; Kofer, J. Detection and molecular characterization of Suid herpesvirus type 1 in Austrian wild boar and hunting dogs. Vet. Microbiol. 2012, 157, 276–284. [Google Scholar] [CrossRef]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691. [Google Scholar] [CrossRef]
- Freuling, C.M.; Müller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef]
- Tong, W.; Liu, F.; Zheng, H.; Liang, C.; Zhou, Y.J.; Jiang, Y.F.; Shan, T.L.; Gao, F.; Li, G.X.; Tong, G.Z. Emergence of a Pseudorabies virus variant with increased virulence to piglets. Vet. Microbiol. 2015, 181, 236–240. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, D.J.; Cook, S. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J. Mol. Biol. 1994, 238, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Spear, P.G. Herpes simplex virus: Receptors and ligands for cell entry. Cell Microbiol. 2004, 6, 401–410. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J.; Spear, P.G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 1998, 280, 1618–1620. [Google Scholar] [CrossRef]
- Petermann, P.; Rahn, E.; Their, K.; Hsu, M.J.; Rixon, F.J.; Kopp, S.J.; Knebel-Mörsdorf, D. Role of nectin-1 and herpesvirus entry mediator as cellular receptors for herpes simplex virus 1 on primary murine dermal fibroblasts. J. Virol. 2015, 89, 9407–9416. [Google Scholar] [CrossRef][Green Version]
- Petermann, P.; Their, K.; Rahn, E.; Rixon, F.J.; Bloch, W.; Özcelik, S.; Krummenacher, C.; Barron, M.J.; Dixon, M.J.; Scheu, S.; et al. Entry mechanisms of herpes simplex virus 1 into murine epidermis: Involvement of nectin-1 and herpesvirus entry mediator as cellular receptors. J. Virol. 2015, 89, 262–274. [Google Scholar] [CrossRef]
- Kopp, S.J.; Karaba, A.H.; Cohen, L.K.; Banisadr, G.; Miller, R.J.; Muller, W.J. Pathogenesis of neonatal herpes simplex 2 disease in a mouse model is dependent on entry receptor expression and route of inoculation. J. Virol. 2013, 87, 474–481. [Google Scholar] [CrossRef]
- Karaba, A.H.; Kopp, S.J.; Longnecker, R. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J. Virol. 2011, 85, 10041–10047. [Google Scholar] [CrossRef]
- Taylor, J.M.; Lin, E.; Susmarski, N.; Yoon, M.; Zago, A.; Ware, C.F.; Pfeffer, K.; Miyoshi, J.; Takai, Y.; Spear, P.G. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2007, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Z.; Song, C.; Wu, Z.; Yang, H. Resistance to pseudorabies virus by knockout of nectin1/2 in pig cells. Arch. Virol. 2020, 165, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Struyf, F.; Martinez, W.M.; Spear, P.G. Mutations in the N-terminal domains of nectin-1 and nectin-2 reveal differences in requirements for entry of various alphaherpesviruses and for nectin-nectin interactions. J. Virol. 2002, 76, 12940–12950. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lu, G.; Qi, J.; Wu, L.; Tian, K.; Luo, T.; Shi, Y.; Yan, J.; Gao, G.F. Structural basis of nectin-1 recognition by pseudorabies virus glycoprotein D. PLoS Pathog. 2017, 13, e1006314. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Yue, D.; Chen, Z.; Yang, F.; Ye, F.; Lin, S.; He, B.; Cheng, Y.; Wang, J.; Chen, Z.; Lin, X.; et al. Crystal structure of bovine herpesvirus 1 glycoprotein D bound to nectin-1 reveals the basis for its low-affinity binding to the receptor. Sci. Adv. 2020, 6, eaba5147. [Google Scholar] [CrossRef]
- Brittle, E.E.; Reynolds, A.E.; Enquist, L.W. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 2004, 78, 12951–12963. [Google Scholar] [CrossRef]
- Laval, K.; Vernejoul, J.B.; Van Cleemput, J.; Koyuncu, O.O.; Enquist, L.W. Virulent Pseudorabies Virus Infection Induces a Specific and Lethal Systemic Inflammatory Response in Mice. J. Virol. 2018, 92, e01614-18. [Google Scholar] [CrossRef]
- Laval, K.; Enquist, L.W. The Neuropathic Itch Caused by Pseudorabies Virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef]
- Inagaki, M.; Irie, K.; Ishizaki, H.; Tanaka-Okamoto, M.; Morimoto, K.; Inoue, E.; Ohtsuka, T.; Miyoshi, J.; Takai, Y. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 2005, 132, 1525–1537. [Google Scholar] [CrossRef]
- Yoshida, K.; Tomioka, Y.; Kase, S.; Morimatsu, M.; Shinya, K.; Ohno, S. Transgenic mice generating group, Ono E. Microphthalmia and lack of vitreous body in transgenic mice expressing the first immunoglobulin-like domain of nectin-1. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhou, Y.; Mu, Y.; Liu, Z.; Hou, S.; Xiong, Y.; Fang, L.; Ge, C.; Wei, Y.; Zhang, X.; et al. CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance. eLife 2020, 9, e57132. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Amagai, K.; Taharaguchi, S.; Tomioka, Y.; Yoshino, S.; Watanabe, Y.; Cherel, P.; Houdebine, L.M.; Adam, M.; Eloit, M.; et al. Transgenic mice expressing a soluble form of porcine nectin-1/herpesvirus entry mediator C as a model for pseudorabies-resistant livestock. Proc. Natl. Acad. Sci. USA 2004, 101, 16150–16155. [Google Scholar] [CrossRef]
- Ono, E.; Tomioka, Y.; Taharaguchi, S.; Cherel, P. Comparison of protection levels against pseudorabies virus infection of transgenic mice expressing a soluble form of porcine nectin-1/HveC and vaccinated mice. Vet. Microbiol. 2006, 114, 327–330. [Google Scholar] [CrossRef]
- Baury, B.; Geraghty, R.J.; Masson, D.; Lustenberger, P.; Spear, P.G.; Denis, M.G. Organization of the rat Tage4 gene and herpesvirus entry activity of the encoded protein. Gene 2001, 265, 185–194. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Xiao, T.; Wang, Z.; Nie, X.; Li, X.; Qian, P.; Qin, L.; Han, X.; Zhang, J.; et al. CRISPR screening of porcine sgRNA library identifies host factors associated with Japanese encephalitis virus replication. Nat. Commun. 2020, 11, 5178. [Google Scholar] [CrossRef]
- Yu, C.; Zhong, H.; Yang, X.; Li, G.; Wu, Z.; Yang, H. Establishment of a pig CRISPR/Cas9 knockout library for functional gene screening in pig cells. Biotechnol. J. 2021, in press. [Google Scholar] [CrossRef]
- Hölper, J.E.; Grey, F.; Baillie, J.K.; Regan, T.; Parkinson, N.J.; Höper, D.; Thamamongood, T.; Schwemmle, M.; Pannhorst, K.; Wendt, L.; et al. A genome-wide CRISPR/Cas9 screen reveals the requirement of host sphingomyelin synthase 1 for infection with pseudorabies virus mutant gD(-)Pass. Viruses 2021, 13, 1574. [Google Scholar] [CrossRef]
- Tan, W.S.; Rong, E.; Dry, I.; Lillico, S.G.; Law, A.; Whitelaw, C.B.A.; Dalziel, R.G. Genome-wide CRISPR knockout screen reveals membrane tethering complexes EARP and GARP important for Bovine Herpes Virus Type 1 replication. bioRxiv 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, C.; Zhang, Q.; Hong, L.; Gu, T.; Zheng, E.; Xu, Z.; Li, Z.; Song, C.; Cai, G.; et al. A Nectin1 Mutant Mouse Model Is Resistant to Pseudorabies Virus Infection. Viruses 2022, 14, 874. https://doi.org/10.3390/v14050874
Yang X, Yu C, Zhang Q, Hong L, Gu T, Zheng E, Xu Z, Li Z, Song C, Cai G, et al. A Nectin1 Mutant Mouse Model Is Resistant to Pseudorabies Virus Infection. Viruses. 2022; 14(5):874. https://doi.org/10.3390/v14050874
Chicago/Turabian StyleYang, Xiaohui, Chuanzhao Yu, Qiuyan Zhang, Linjun Hong, Ting Gu, Enqin Zheng, Zheng Xu, Zicong Li, Changxu Song, Gengyuan Cai, and et al. 2022. "A Nectin1 Mutant Mouse Model Is Resistant to Pseudorabies Virus Infection" Viruses 14, no. 5: 874. https://doi.org/10.3390/v14050874
APA StyleYang, X., Yu, C., Zhang, Q., Hong, L., Gu, T., Zheng, E., Xu, Z., Li, Z., Song, C., Cai, G., Wu, Z., & Yang, H. (2022). A Nectin1 Mutant Mouse Model Is Resistant to Pseudorabies Virus Infection. Viruses, 14(5), 874. https://doi.org/10.3390/v14050874







