Understanding the Mechanisms That Drive Phage Resistance in Staphylococci to Prevent Phage Therapy Failure
Abstract
:1. Introduction
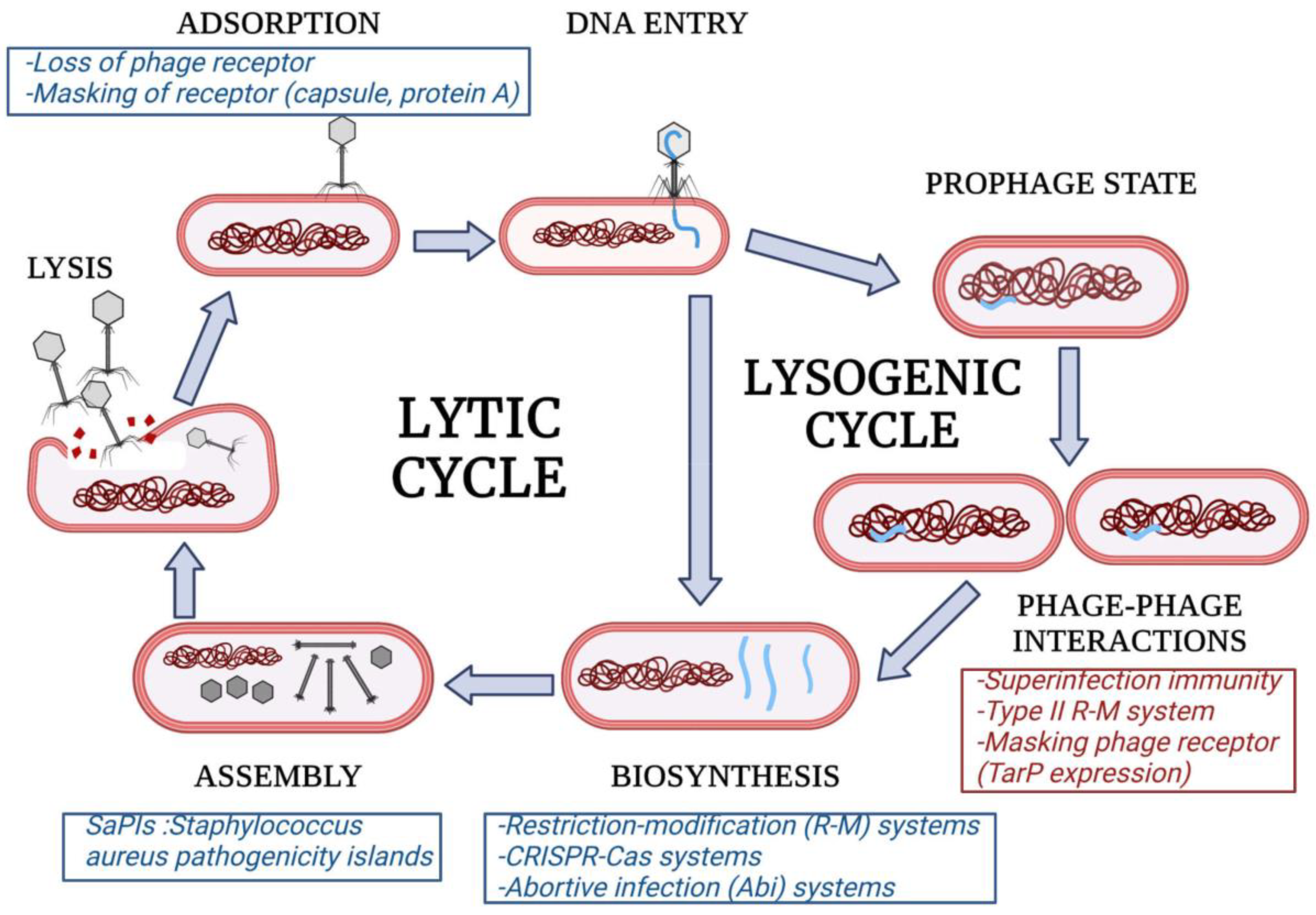
2. Phage Resistance Determinants
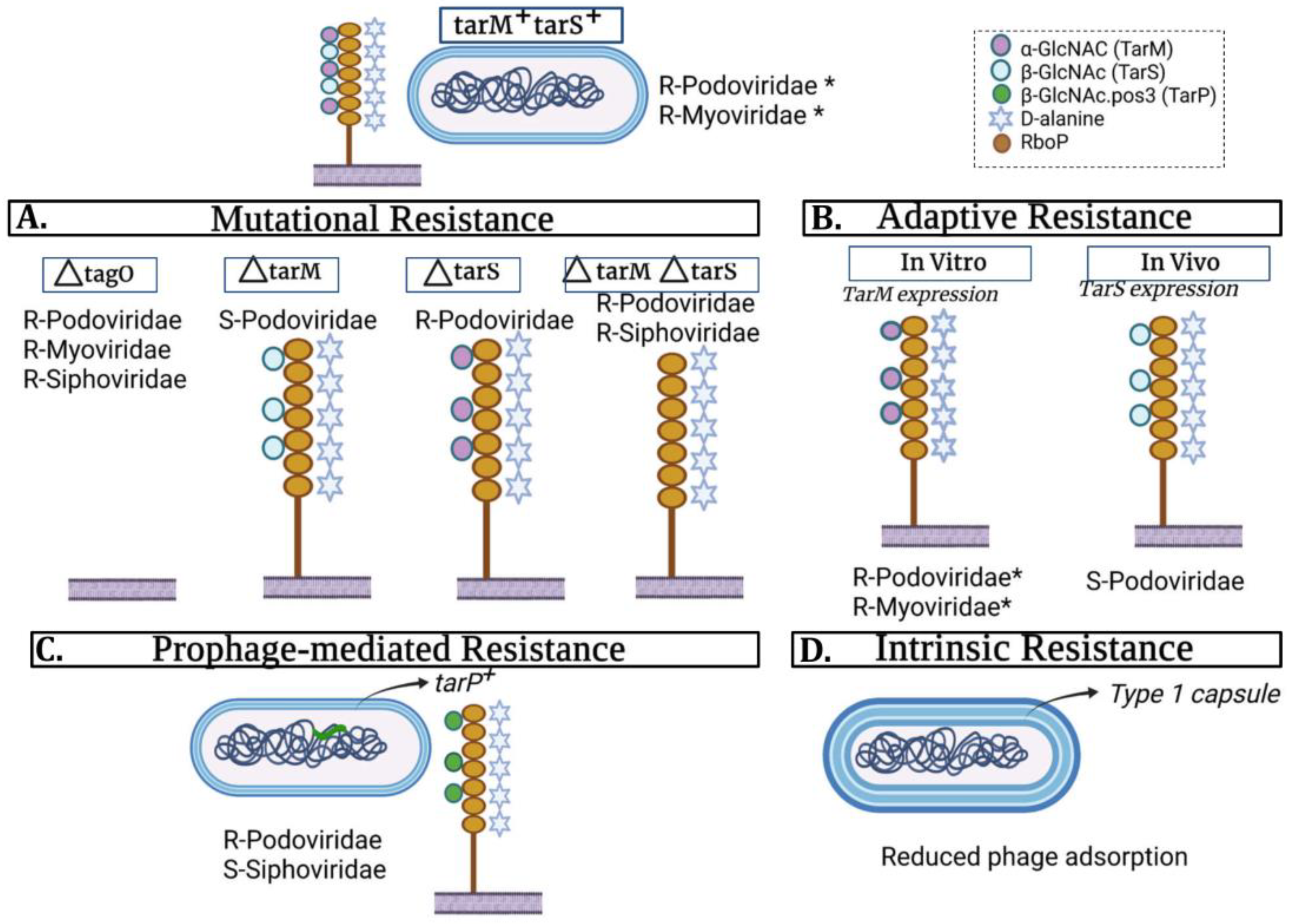
2.1. Inhibition of Phage Adsorption
2.2. Interference with Phage Biosynthesis
2.2.1. Restriction–Modification (R-M) Systems
2.2.2. CRISPR-Cas Systems
2.2.3. Abortive Infection
2.3. Assembly Interference
3. Environment-Driven Adaptations That Enhance Phage Resistance
4. Phage–Phage Interactions
5. Overcoming Phage Resistance in Therapeutic and Biocontrol Applications against S. aureus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017.
- Nóbrega, F.; Costa, A.R.; Kluskens, L.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.K.; Koeris, M.S. The next generation of bacteriophage therapy. Curr. Opin. Microbiol. 2011, 14, 524–531. [Google Scholar] [CrossRef]
- Chang, Y.; Bai, J.; Lee, J.-H.; Ryu, S. Mutation of a Staphylococcus aureus temperate bacteriophage to a virulent one and evaluation of its application. Food Microbiol. 2019, 82, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.; Narita-Yamada, S.; Wakabayashi, Y.; Kamio, Y. Identification of ORF636 in Phage φSLT Carrying Panton-Valentine Leukocidin Genes, Acting as an Adhesion Protein for a Poly(Glycerophosphate) Chain of Lipoteichoic Acid on the Cell Surface of Staphylococcus aureus. J. Bacteriol. 2009, 191, 4674–4680. [Google Scholar] [CrossRef] [Green Version]
- Winstel, V.; Sanchez-Carballo, P.; Holst, O.; Xia, G.; Peschel, A. Biosynthesis of the Unique Wall Teichoic Acid of Staphylococcus aureus Lineage ST395. mBio 2014, 5, e00869-14. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Corrigan, R.M.; Winstel, V.; Goerke, C.; Gründling, A.; Peschel, A. Wall Teichoic Acid-Dependent Adsorption of Staphylococcal Siphovirus and Myovirus. J. Bacteriol. 2011, 193, 4006–4009. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, I.; Osada, K.; Azam, A.H.; Asakawa, H.; Miyanaga, K.; Tanji, Y. The Presence of Two Receptor-Binding Proteins Contributes to the Wide Host Range of Staphylococcal Twort-Like Phages. Appl. Environ. Microbiol. 2016, 82, 5763–5774. [Google Scholar] [CrossRef] [Green Version]
- Hrichi, S.; Chaabane-Banaoues, R.; Bayar, S.; Flamini, G.; Oulad El Majdoub, Y.; Mangraviti, D.; Mondello, L.; El Mzoughi, R.; Babba, H.; Mighri, Z.; et al. Botanical and Genetic Identification Followed by Investigation of Chemical Composition and Biological Activities on the Scabiosa atropurpurea L. Stem from Tunisian Flora. Molecules 2020, 25, 5032. [Google Scholar] [CrossRef]
- Fernández, L.; Duarte, A.C.; Martínez, B.; Rodríguez, A.; García, P. Draft Genome Sequences of the Bap-Producing Strain Staphylococcus aureus V329 and Its Derived Phage-Resistant Mutant BIM-1. Microbiol. Resour. Announc. 2021, 10, e0050021. [Google Scholar] [CrossRef]
- Azam, A.H.; Kadoi, K.; Miyanaga, K.; Usui, M.; Tamura, Y.; Cui, L.; Tanji, Y. Analysis host-recognition mechanism of staphylococcal kayvirus ΦSA039 reveals a novel strategy that protects Staphylococcus aureus against infection by Staphylococcus pseudintermedius Siphoviridae phages. Appl. Microbiol. Biotechnol. 2019, 103, 6809–6823. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gerlach, D.; Du, X.; Larsen, J.; Stegger, M.; Kühner, P.; Peschel, A.; Xia, G.; Winstel, V. An accessory wall teichoic acid glycosyltransferase protects Staphylococcus aureus from the lytic activity of Podoviridae. Sci. Rep. 2015, 5, 17219. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Takemura-Uchiyama, I.; Kato, S.; Sato, M.; Ujihara, T.; Matsui, H.; Hanaki, H.; Daibata, M.; Matsuzaki, S. In silico analysis of AHJD -like viruses, Staphylococcus aureus phages S24-1 and S13′, and study of phage S24-1 adsorption. MicrobiologyOpen 2014, 3, 257–270. [Google Scholar] [CrossRef]
- Nordström, K.; Forsgren, A. Effect of Protein A on Adsorption of Bacteriophages to Staphylococcus aureus. J. Virol. 1974, 14, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, B.J.; Holmes, K.M. Staphylococcus aureus cell surface: Capsule as a barrier to bacteriophage adsorption. Infect. Immun. 1979, 23, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE—A Database for DNA Restriction and Modification: Enzymes, Genes and Genomes. Nucleic Acids Res. 2015, 43, D298–D299. [Google Scholar] [CrossRef] [PubMed]
- Bickle, T.A.; Krueger, D.H. Biology of DNA restriction. Microbiol. Rev. 1993, 57, 434–450. [Google Scholar] [CrossRef]
- Sadykov, M.R. Restriction-Modification Systems as a Barrier for Genetic Manipulation of Staphylococcus aureus. In The Genetic Manipulation of Staphylococci; Bose, J.L., Ed.; Springer: New York, NY, USA, 2014; pp. 9–23. [Google Scholar]
- Waldron, D.E.; Lindsay, J.A. Sau1: A Novel Lineage-Specific Type I Restriction-Modification System That Blocks Horizontal Gene Transfer into Staphylococcus aureus and between S. aureus Isolates of Different Lineages. J. Bacteriol. 2006, 188, 5578–5585. [Google Scholar] [CrossRef] [Green Version]
- Roberts, G.A.; Houston, P.J.; White, J.H.; Chen, K.; Stephanou, A.S.; Cooper, L.P.; Dryden, D.T.; Lindsay, J.A. Impact of target site distribution for Type I restriction enzymes on the evolution of methicillin-resistant Staphylococcus aureus (MRSA) populations. Nucleic Acids Res. 2013, 41, 7472–7484. [Google Scholar] [CrossRef] [Green Version]
- Cooper, L.P.; Roberts, G.A.; White, J.H.; Luyten, Y.A.; Bower, E.K.; Morgan, R.D.; Roberts, R.J.; Lindsay, J.; Dryden, D.T. DNA target recognition domains in the Type I restriction and modification systems of Staphylococcus aureus. Nucleic Acids Res. 2017, 45, 3395–3406. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.-Y.; Corvaglia, A.R.; Chan, S.-H.; Zheng, Y.; Linder, P. A type IV modification-dependent restriction enzyme SauUSI from Staphylococcus aureus subsp. aureus USA300. Nucleic Acids Res. 2011, 39, 5597–5610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flaherty, S.; Coffey, A.; Edwards, R.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. Genome of Staphylococcal Phage K: A New Lineage of Myoviridae Infecting Gram-Positive Bacteria with a Low G+C Content. J. Bacteriol. 2004, 186, 2862–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, S.; Seegers, J.F.M.L.; Fitzgerald, G.F.; van Sinderen, D. Molecular Characterization of a Phage-Encoded Resistance System in Lactococcus lactis. Appl. Environ. Microbiol. 1999, 65, 1891–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.N.; Steens, J.A.; Staals, R.H.; Westra, E.R.; van Houte, S. Coevolution between bacterial CRISPR-Cas systems and their bacteriophages. Cell Host Microbe 2021, 29, 715–725. [Google Scholar] [CrossRef]
- Cruz-López, E.A.; Rivera, G.; Cruz-Hernández, M.A.; Martínez-Vázquez, A.V.; Castro-Escarpulli, G.; Flores-Magallón, R.; Vázquez-Cisneros, K.; Cruz-Pulido, W.L.; Bocanegra-García, V. Identification and Characterization of the CRISPR/Cas System in Staphylococcus aureus Strains from Diverse Sources. Front. Microbiol. 2021, 12, 656996. [Google Scholar] [CrossRef]
- Cao, L.; Gao, C.-H.; Zhu, J.; Zhao, L.; Wu, Q.; Li, M.; Sun, B. Identification and functional study of type III-A CRISPR-Cas systems in clinical isolates of Staphylococcus aureus. Int. J. Med Microbiol. 2016, 306, 686–696. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, T.; Li, Y.; Xiao, W.; Liang, X.; Duan, G.; Yang, H. Characterization of 67 Confirmed Clustered Regularly Interspaced Short Palindromic Repeats Loci in 52 Strains of Staphylococci. Front. Microbiol. 2021, 12, 736565. [Google Scholar] [CrossRef]
- Li, Q.; Xie, X.; Yin, K.; Tang, Y.; Zhou, X.; Chen, Y.; Xia, J.; Hu, Y.; Ingmer, H.; Li, Y.; et al. Characterization of CRISPR-Cas system in clinical Staphylococcus epidermidis strains revealed its potential association with bacterial infection sites. Microbiol. Res. 2016, 193, 103–110. [Google Scholar] [CrossRef]
- Rossi, C.C.; Andrade-Oliveira, A.L.; Giambiagi-Demarval, M. CRISPR tracking reveals global spreading of antimicrobial resistance genes by Staphylococcus of canine origin. Veter. Microbiol. 2019, 232, 65–69. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Lu, J.-J.; Lin, L.-C.; Lin, H.-C.; Chang, S.-C. Phylogenetic Distribution of CRISPR-Cas Systems in Staphylococcus lugdunensis. Microbiol. Spectr. 2021, 9, e0124721. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.N.J.; Staals, R.H.J.; Fineran, P.C.; Bondy-Denomy, J.; Gilmore, M.S. CRISPR-Cas-Mediated Phage Resistance Enhances Horizontal Gene Transfer by Transduction. mBio 2018, 9, e02406-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, C.Y.; Mathai, J.; Rostøl, J.T.; Varble, A.; Banh, D.V.; Marraffini, L.A. Type III-A CRISPR immunity promotes mutagenesis of staphylococci. Nature 2021, 592, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Hynes, A.P.; Rousseau, G.M.; Agudelo, D.; Goulet, A.; Amigues, B.; Loehr, J.; Romero, D.A.; Fremaux, C.; Horvath, P.; Doyon, Y.; et al. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Watters, K.E.; Shivram, H.; Fellmann, C.; Lew, R.J.; McMahon, B.; Doudna, J.A. Potent CRISPR-Cas9 inhibitors from Staphylococcus genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 6531–6539. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.L. Phages Fight Back: Inactivation of the CRISPR-Cas Bacterial Immune System by Anti-CRISPR Proteins. PLoS Pathog. 2016, 12, e1005282. [Google Scholar] [CrossRef] [Green Version]
- Pawluk, A.; Staals, R.H.; Taylor, C.; Watson, B.N.; Saha, S.; Fineran, P.; Maxwell, K.L.; Davidson, A.R. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat. Microbiol. 2016, 1, 16085. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive Infection: Bacterial Suicide as an Antiviral Immune Strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Depardieu, F.; Didier, J.-P.; Bernheim, A.; Sherlock, A.; Molina, H.; Duclos, B.; Bikard, D. A Eukaryotic-like Serine/Threonine Kinase Protects Staphylococci against Phages. Cell Host Microbe 2016, 20, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Moller, A.G.; Petit, R.A.; Read, T.D. Species-Scale Genomic Analysis of Staphylococcus aureus Genes Influencing Phage Host Range and Their Relationships to Virulence and Antibiotic Resistance Genes. mSystems 2022, 7, e0108321. [Google Scholar] [CrossRef]
- Novick, R. Pathogenicity Islands and Their Role in Staphylococcal Biology. Microbiol. Spectr. 2019, 7, GPP3-0062-2019. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Podkowik, M.; Ibarra-Chávez, R.; del Sol, F.G.; Ram, G.; Chen, J.; Marina, A.; Novick, R.P.; Penadés, J.R. A regulatory cascade controls Staphylococcus aureus pathogenicity island activation. Nat. Microbiol. 2021, 6, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Alamar, M.C.; Guzmán-Markevitch, K.; Žiemytė, M.; Ortí, L.; Bernabé-Quispe, P.; Pineda-Lucena, A.; Pemán, J.; Tormo-Mas, M. Mobilisation Mechanism of Pathogenicity Islands by Endogenous Phages in Staphylococcus aureus clinical strains. Sci. Rep. 2018, 8, 16742. [Google Scholar] [CrossRef]
- Ram, G.; Chen, J.; Kumar, K.; Ross, H.F.; Ubeda, C.; Damle, P.K.; Lane, K.D.; Penadés, J.R.; Christie, G.E.; Novick, R.P. Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc. Natl. Acad. Sci. USA 2012, 109, 16300–16305. [Google Scholar] [CrossRef] [Green Version]
- Frígols, B.; Quiles-Puchalt, N.; Mir-Sanchis, I.; Donderis, J.; Elena, S.F.; Buckling, A.; Novick, R.; Marina, A.; Penadés, J.R. Virus Satellites Drive Viral Evolution and Ecology. PLoS Genet. 2015, 11, e1005609. [Google Scholar] [CrossRef]
- Patterson, A.G.; Jackson, S.; Taylor, C.; Evans, G.B.; Salmond, G.P.; Przybilski, R.; Staals, R.; Fineran, P.C. Quorum Sensing Controls Adaptive Immunity through the Regulation of Multiple CRISPR-Cas Systems. Mol. Cell 2016, 64, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Høyland-Kroghsbo, N.M.; Paczkowski, J.; Mukherjee, S.; Broniewski, J.; Westra, E.; Bondy-Denomy, J.; Bassler, B.L. Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system. Proc. Natl. Acad. Sci. USA 2017, 114, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.; Svenningsen, S.L.; Middelboe, M. Quorum Sensing Determines the Choice of Antiphage Defense Strategy in Vibrio anguillarum. mBio 2015, 6, e00627-15. [Google Scholar] [CrossRef] [Green Version]
- Hoque, M.M.; Bin Naser, I.; Bari, S.M.N.; Zhu, J.; Mekalanos, J.J.; Faruque, S.M. Quorum Regulated Resistance of Vibrio cholerae against Environmental Bacteriophages. Sci. Rep. 2016, 6, 37956. [Google Scholar] [CrossRef]
- Mistretta, N.; Brossaud, M.; Telles, F.; Sanchez, V.; Talaga, P.; Rokbi, B. Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Sci. Rep. 2019, 9, 3212. [Google Scholar] [CrossRef] [Green Version]
- Moller, A.G.; Winston, K.; Ji, S.; Wang, J.; Davis, M.N.H.; Solís-Lemus, C.R.; Read, T.D. Genes Influencing Phage Host Range in Staphylococcus aureus on a Species-Wide Scale. mSphere 2021, 6, e01263-20. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.L.; Li, F.; Koo, B.-M.; El-Halfawy, O.; French, S.; Gross, C.A.; Strynadka, N.C.; Brown, E.D. Identification of Two Phosphate Starvation-induced Wall Teichoic Acid Hydrolases Provides First Insights into the Degradative Pathway of a Key Bacterial Cell Wall Component. J. Biol. Chem. 2016, 291, 26066–26082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, A.M.; Schneider, J.; Unsleber, S.; Xia, G.; Mayer, C.; Peschel, A. Staphylococcus aureus counters phosphate limitation by scavenging wall teichoic acids from other staphylococci via the teichoicase GlpQ. J. Biol. Chem. 2018, 293, 14916–14924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Eder, S.; Hulett, F.M. Analysis of Bacillus subtilis tagAB and tagDEF Expression during Phosphate Starvation Identifies a Repressor Role for PhoP-P. J. Bacteriol. 1998, 180, 753–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, L.; González, S.; Campelo, A.B.; Martínez, B.; Rodríguez, A.; García, P. Low-level predation by lytic phage phiIPLA-RODI promotes biofilm formation and triggers the stringent response in Staphylococcus aureus. Sci. Rep. 2017, 7, 40965. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; García, P.; Rodríguez, A. Environmental pH is a key modulator of Staphylococcus aureus biofilm development under predation by the virulent phage phiIPLA-RODI. ISME J. 2021, 15, 245–259. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- González, S.; Fernández, L.; Gutiérrez, D.; Campelo, A.B.; Rodríguez, A.; García, P. Analysis of Different Parameters Affecting Diffusion, Propagation and Survival of Staphylophages in Bacterial Biofilms. Front. Microbiol. 2018, 9, 2348. [Google Scholar] [CrossRef]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Esteban, P.P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T.A. Combined Use of Bacteriophage K and a Novel Bacteriophage To Reduce Staphylococcus aureus Biofilm Formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef] [Green Version]
- Curtin, J.J.; Donlan, R.M. Using Bacteriophages to Reduce Formation of Catheter-Associated Biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2006, 50, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Vandenheuvel, D.; Martínez, B.; Rodriguez, A.; Lavigne, R.; García, P. Two Phages, phiIPLA-RODI and phiIPLA-C1C, Lyse Mono- and Dual-Species Staphylococcal Biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.; Fernández, L.; Campelo, A.B.; Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. The Behavior of Staphylococcus aureus Dual-Species Biofilms Treated with Bacteriophage phiIPLA-RODI Depends on the Accompanying Microorganism. Appl. Environ. Microbiol. 2017, 83, e02821-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerca, N.; Oliveira, R.; Azeredo, J. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of staphylococcus bacteriophage K. Lett. Appl. Microbiol. 2007, 45, 313–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, D.; Briers, Y.; Rodriguez-Rubio, L.; Martínez, B.; Rodriguez, A.; Lavigne, R.; García, P. Role of the Pre-neck Appendage Protein (Dpo7) from Phage vB_SepiS-phiIPLA7 as an Anti-biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Stewart, G.C. Regulatory Elements of the Staphylococcus aureus Protein A (Spa) Promoter. J. Bacteriol. 2004, 186, 3738–3748. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef]
- Garvey, P.; Hill, C.; Fitzgerald, G.F. The Lactococcal Plasmid pNP40 Encodes a Third Bacteriophage Resistance Mechanism, One Which Affects Phage DNA Penetration. Appl. Environ. Microbiol. 1996, 62, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, R.M.; Carroll, D.; Kong, H.; Higgins, L.; Keane, C.T.; Coleman, D.C. Sau42I, a BcgI-like restriction–modification system encoded by the Staphylococcus aureus quadruple-converting phage π42. Microbiology 2005, 151 Pt 4, 1301–1311. [Google Scholar] [CrossRef] [Green Version]
- Gerlach, D.; Guo, Y.; De Castro, C.; Kim, S.-H.; Schlatterer, K.; Xu, F.-F.; Pereira, C.; Seeberger, P.H.; Ali, S.; Codée, J.; et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 2018, 563, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Azam, A.H.; Tanji, Y. Peculiarities of Staphylococcus aureus phages and their possible application in phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 4279–4289. [Google Scholar] [CrossRef]
- Lehman, S.M.; Mearns, G.; Rankin, D.; Cole, R.A.; Smrekar, F.; Branston, S.D.; Morales, S. Design and Preclinical Development of a Phage Product for the Treatment of Antibiotic-Resistant Staphylococcus aureus Infections. Viruses 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kifelew, L.G.; Warner, M.S.; Morales, S.; Vaughan, L.; Woodman, R.; Fitridge, R.; Mitchell, J.G.; Speck, P. Efficacy of phage cocktail AB-SA01 therapy in diabetic mouse wound infections caused by multidrug-resistant Staphylococcus aureus. BMC Microbiol. 2020, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, M.; Kuptsov, N.; Gorodnichev, R.; Bespiatykh, D.; Guliaev, A.; Letarova, M.; Kulikov, E.; Veselovsky, V.; Malakhova, M.; Letarov, A.; et al. Contribution of Podoviridae and Myoviridae bacteriophages to the effectiveness of anti-staphylococcal therapeutic cocktails. Sci. Rep. 2020, 10, 18612. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Practical Method for Isolation of Phage Deletion Mutants. Methods Protoc. 2018, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Borin, J.M.; Avrani, S.; Barrick, J.E.; Petrie, K.L.; Meyer, J.R. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc. Natl. Acad. Sci. USA 2021, 18, e2104592118. [Google Scholar] [CrossRef]
- Castledine, M.; Sierocinski, P.; Inglis, M.; Kay, S.; Hayward, A.; Buckling, A.; Padfield, D. Greater Phage Genotypic Diversity Constrains Arms-Race Coevolution. Front. Cell. Infect. Microbiol. 2022, 12, 834406. [Google Scholar] [CrossRef]
- Moreno, D.S.; Visram, Z.; Mutti, M.; Restrepo-Córdoba, M.; Hartmann, S.; Kremers, A.; Tišáková, L.; Schertler, S.; Wittmann, J.; Kalali, B.; et al. ε2-Phages Are Naturally Bred and Have a Vastly Improved Host Range in Staphylococcus aureus over Wild Type Phages. Pharmaceuticals 2021, 14, 325. [Google Scholar] [CrossRef]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef] [Green Version]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Cass, J.; Barnard, A.; Fairhead, H. Engineered Bacteriophage as a Delivery Vehicle for Antibacterial Protein, SASP. Pharmaceuticals 2021, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.-B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.V.; Abdelbary, H. Does Treatment Order Matter? Investigating the Ability of Bacteriophage to Augment Antibiotic Activity against Staphylococcus aureus Biofilms. Front. Microbiol. 2018, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Family | Tail Morphology | Genome Size (kb) | Life Cycle | Receptor Molecule 1 |
|---|---|---|---|---|
| Myoviridae | Long, non-flexible, contractile | 120–140 | Virulent | WTA backbone and sometimes α-O-GlcNAc or β-O-GlcNAc |
| Siphoviridae | Long, flexible, non-contractile | 39–43 | Temperate | α-O-GlcNAc and/or β-O-GlcNAc |
| Podoviridae | Short, non-contractile | 120–140 | Virulent | β-O-GlcNAc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado, A.; Fernández, L.; Rodríguez, A.; García, P. Understanding the Mechanisms That Drive Phage Resistance in Staphylococci to Prevent Phage Therapy Failure. Viruses 2022, 14, 1061. https://doi.org/10.3390/v14051061
Jurado A, Fernández L, Rodríguez A, García P. Understanding the Mechanisms That Drive Phage Resistance in Staphylococci to Prevent Phage Therapy Failure. Viruses. 2022; 14(5):1061. https://doi.org/10.3390/v14051061
Chicago/Turabian StyleJurado, Andrea, Lucía Fernández, Ana Rodríguez, and Pilar García. 2022. "Understanding the Mechanisms That Drive Phage Resistance in Staphylococci to Prevent Phage Therapy Failure" Viruses 14, no. 5: 1061. https://doi.org/10.3390/v14051061
APA StyleJurado, A., Fernández, L., Rodríguez, A., & García, P. (2022). Understanding the Mechanisms That Drive Phage Resistance in Staphylococci to Prevent Phage Therapy Failure. Viruses, 14(5), 1061. https://doi.org/10.3390/v14051061







