Increased Ifng and Il10 Expression Correlate with Disease in Rodent Models Experimentally Infected with Modoc Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Infections
2.2. Viral RNA Extraction and RT-qPCR
2.3. Cellular RNA Extraction and Gene Expression Analysis
| Target | Sequence (5′ to 3′) | bp | R2 | Eff. (%) | Accession | Reference | |
|---|---|---|---|---|---|---|---|
| β-Actin | F R | GCTACAGCTTCACCACCACA TCTCCAGGGAGGAAGAGGAT | 123 | 1.000 | 102.4 | XM_013120404.2 | [37] |
| Oas1b | F R | CAGTATGCCCTGGAGCTGC GTACTTGGTGACCAGTTCC | 111 | 0.999 | 103.5 | XM_013119795.2 | This study |
| Ifng | F R | TGTTGCTCTGCCTCACTCAGG AAGACGAGGTCCCCTCCATTC | 130 | 1.000 | 104.2 | AF034482.1 | [41] |
| Tnf | F R | TGAGCCATCGTGCCAATG AGCCCGTCTGCTGGTATCAC | 79 | 0.998 | 97.7 | XM_005086799.3 | [41] |
| Tgfb | F R | TGTGTGCGGCAGCTGTACA TGGGCTCGTGAATCCACTTC | 63 | 1.000 | 100.7 | XM_013125593.2 | [29] |
| IL6 | F R | CCTGAAAGCACTTGAAGAATTCC GGTATGCTAAGGCACAGCACACT | 78 | 1.000 | 112.4 | XM_005087110.2 | [29] |
| IL10 | F R | GGTTGCCAAACCTTATCAGAAATG TTCACCTGTTCCACAGCCTTG | 194 | 1.000 | 99.5 | XM_021232886.1 | [41] |
| MODV-NS5 | F R | CCAGGACAAGTCATGTGGTAGC TCCCAAAGATGTTCCTCACCTT | 101 | 0.998 | 107.5 | NC_003635.1 | [39] |
2.4. Statistical Analysis
3. Results
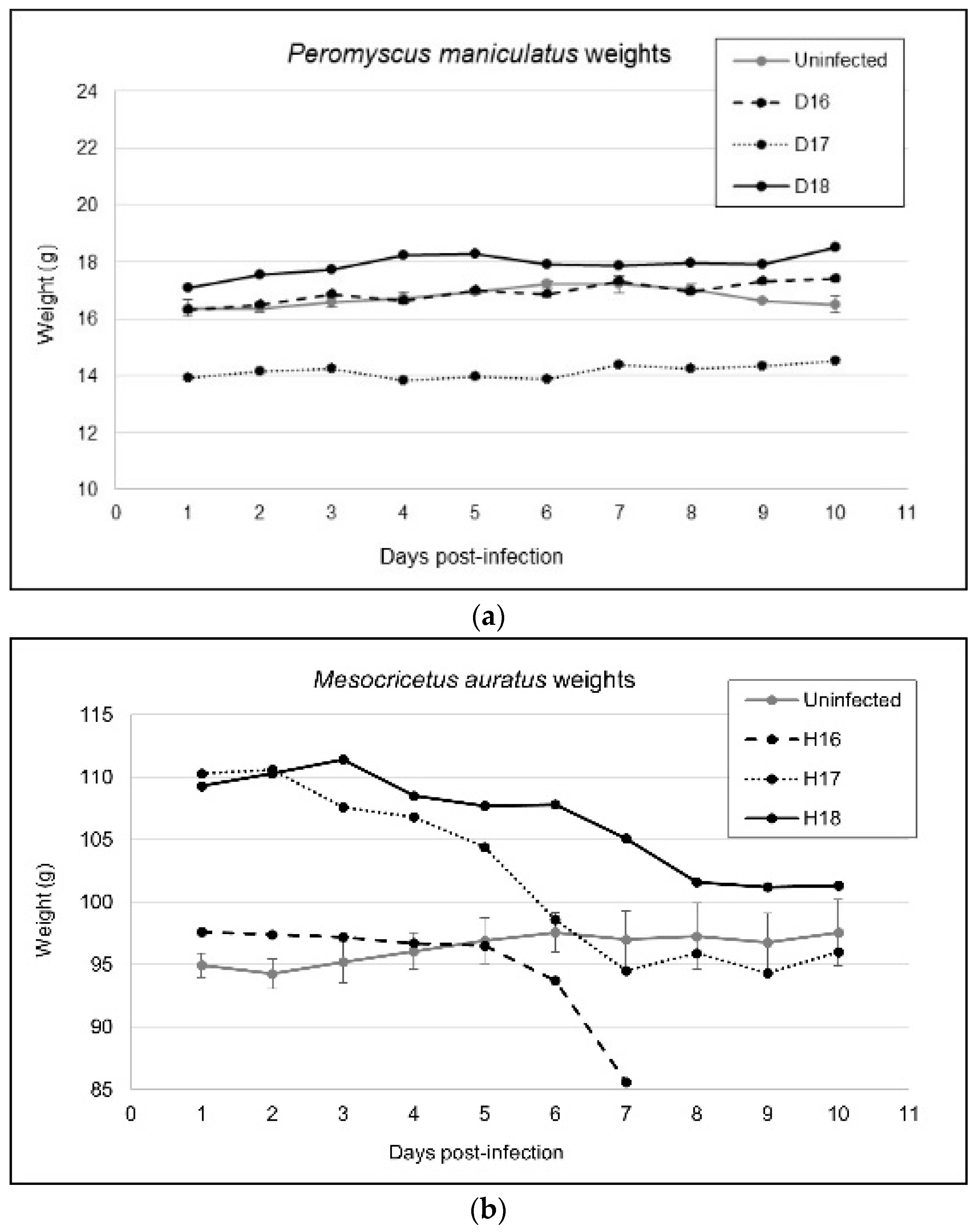
3.1. Variable Clinical Outcomes of MODV Infection
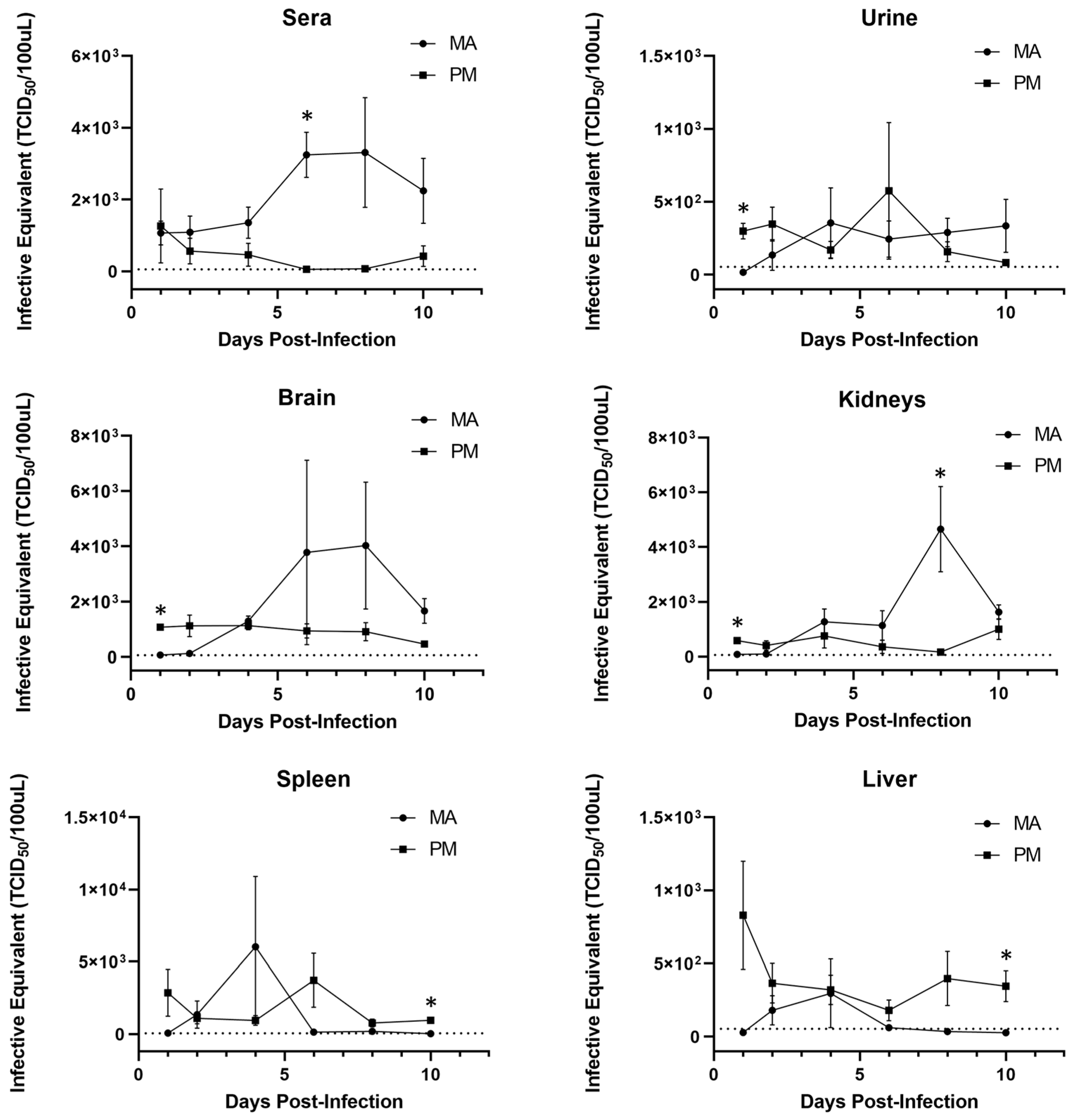
3.2. Viral Replication Kinetics
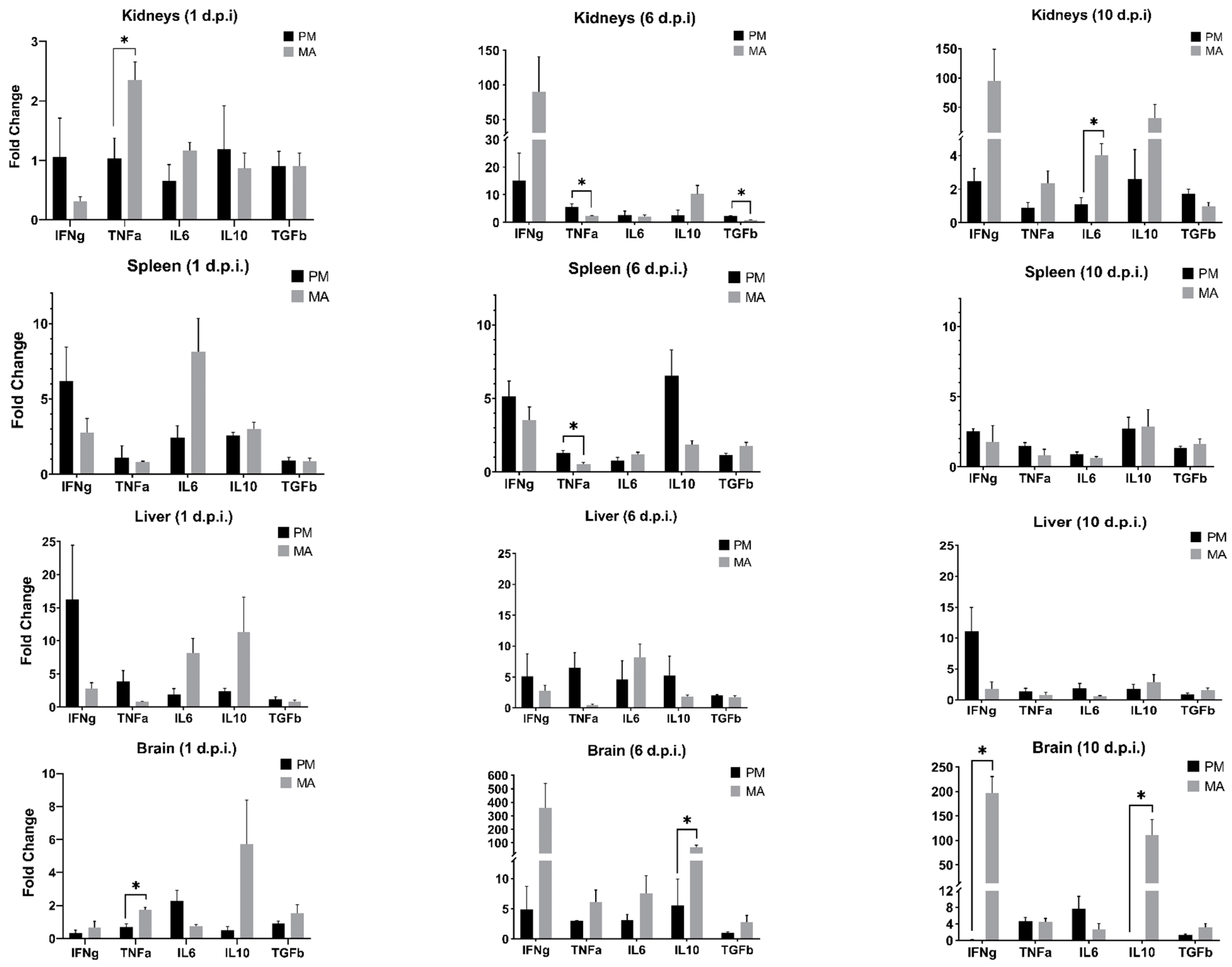
3.3. Differential Transcript Expression
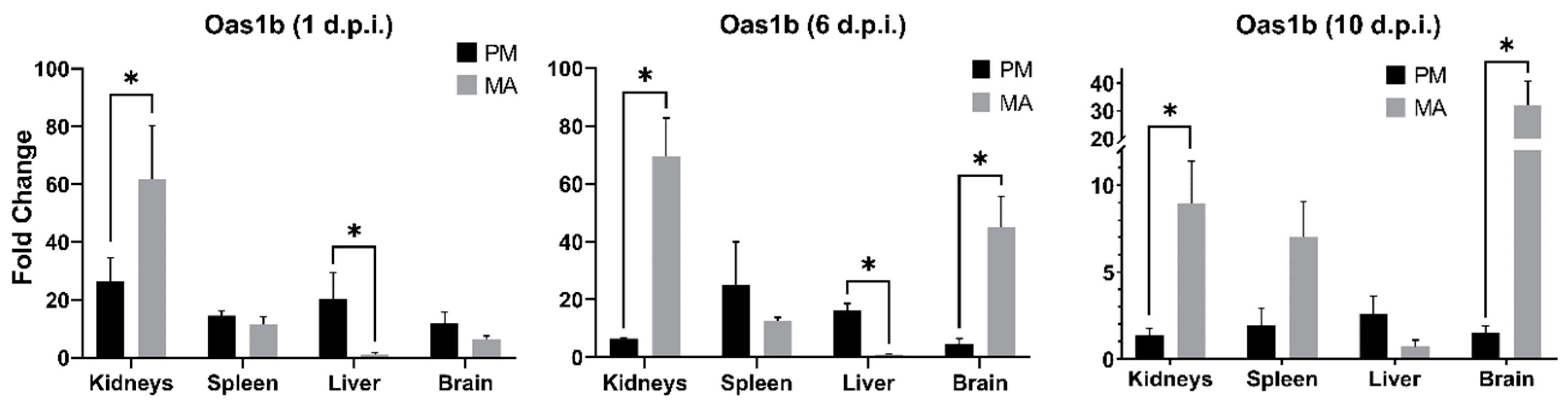
3.4. Oas1b Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Johnson, H.N. Ecological implications of antigenically related mammalian viruses for which arthropod vectors are unknown and avian associated soft tick viruses. Jpn. J. Med. Sci. Biol. 1967, 20, 160–166. [Google Scholar] [PubMed]
- Leyssen, P.; Charlier, N.; Lemey, P.; Billoir, F.; Vandamme, A.M.; de Clercq, E.; de Lamballerie, X.; Neyts, J. Complete genome sequence, taxonomic assignment, and comparative analysis of the untranslated regions of the Modoc virus, a flavivirus with no known vector. Virology 2002, 293, 125–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidet, K.; Garcia-Blanco, M.A. Flaviviral RNAs: Weapons and targets in the war between virus and host. Biochem. J. 2014, 462, 215–230. [Google Scholar] [CrossRef] [PubMed]
- de Borba, L.; Villordo, S.M.; Marsico, F.L.; Carballeda, J.M.; Filomatori, C.V.; Gebhard, L.G.; Pallares, H.M.; Lequime, S.; Lambrechts, L.; Vargas, I.S.; et al. RNA Structure Duplication in the Dengue Virus 3′ UTR: Redundancy or Host Specificity? MBio 2019, 10, e02506-18. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.N. Long-term persistence of Modoc virus in hamster-kidney cells. In vivo and in vitro demonstration. Am. J. Trop. Med. Hyg. 1970, 19, 537–539. [Google Scholar] [CrossRef]
- Saiyasombat, R.; Carrillo-Tripp, J.; Miller, W.A.; Bredenbeek, P.J.; Blitvich, B.J. Substitution of the premembrane and envelope protein genes of Modoc virus with the homologous sequences of West Nile virus generates a chimeric virus that replicates in vertebrate but not mosquito cells. Virol. J. 2014, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Tumban, E.; Maes, N.E.; Schirtzinger, E.E.; Young, K.I.; Hanson, C.T.; Whitehead, S.S.; Hanley, K.A. Replacement of conserved or variable sequences of the mosquito-borne dengue virus 3′ UTR with homologous sequences from Modoc virus does not change infectivity for mosquitoes. J. Gen. Virol. 2013, 94, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.W.; Hardy, J.L. In vitro studies with Modoc virus in Vero cells: Plaque assay and kinetics of growth, neutralization, and thermal inactivation. Appl. Microbiol. 1973, 26, 344–348. [Google Scholar] [CrossRef]
- Sulkin, S.E.; Burns, K.F.; Shelton, D.F.; Wallis, C. Bat salivary gland virus: Infections of man and monkey. Tex. Rep. Biol. Med. 1962, 20, 113–127. [Google Scholar]
- Leyssen, P.; Croes, R.; Rau, P.; Heiland, S.; Verbeken, E.; Sciot, R.; Paeshuyse, J.; Charlier, N.; De Clercq, E.; Meyding-Lamade, U.; et al. Acute encephalitis, a poliomyelitis-like syndrome and neurological sequelae in a hamster model for flavivirus infections. Brain Pathol. 2003, 13, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.P.; Da Rosa, A.P.T.; Nunes, M.R.; Xiao, S.Y.; Tesh, R.B. Pathogenesis of Modoc virus (Flaviviridae; Flavivirus) in persistently infected hamsters. Am. J. Trop. Med. Hyg. 2013, 88, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Brett, D.; Lindenbach, H.-J.T.; Charles, M. Rice: Flaviviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Howley, D.M., Ka, P.M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 2007. [Google Scholar]
- Guzman, A.; Isturiz, R.E. Update on the global spread of dengue. Int. J. Antimicrob. Agents. 2010, 36, S40–S42. [Google Scholar] [CrossRef] [PubMed]
- Vorou, R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: What we know and what we should investigate urgently. Int. J. Infect. Dis. 2016, 48, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.H.; Metz, S.W. Progress and Works in Progress: Update on Flavivirus Vaccine Development. Clin. Ther. 2017, 39, 1519–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. West Nile Virus Disease Cases Reported to CDC by State of Residence, 1999–2017. 2017. Available online: https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-reported-to-CDC-by-state_1999-2017.pdf (accessed on 1 May 2018).
- Centers for Disease Control and Prevention. CDC: Zika Virus Case Count in the US; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018.
- World Health Organization. Japanese Encephalitis; World Health Organization: Geneva, Switzerland, 2015; Volume 2016.
- World Health Organization. Yellow Fever; World Health Organization: Geneva, Switzerland, 2016.
- World Health Organization. Dengue and Severe Dengue; World Health Organization: Geneva, Switzerland, 2016.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Monaghan, A.J.; Sampson, K.M.; Steinhoff, D.F.; Ernst, K.C.; Ebi, K.L.; Jones, B.; Hayden, M.H. The potential impacts of 21st century climatic and population changes on human exposure to the virus vector mosquito Aedes aegypti. Clim. Chang. 2016, 146, 487–500. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Barbour, A.G. Infection resistance and tolerance in Peromyscus spp., natural reservoirs of microbes that are virulent for humans. Semin. Cell Dev. Biol. 2017, 61, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl-Jensen, V.; Bollinger, L.; Safronetz, D.; de Kok-Mercado, F.; Scott, D.P.; Ebihara, H. Use of the Syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers. Viruses 2012, 4, 3754–3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zivcec, M.; Safronetz, D.; Haddock, E.; Feldmann, H.; Ebihara, H. Validation of assays to monitor immune responses in the Syrian golden hamster (Mesocricetus auratus). J. Immunol. Methods 2011, 368, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W.; Hardy, J.L. Characterization of persistent Modoc viral infections in Syrian hamsters. Infect. Immun. 1974, 10, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.W.; Hardy, J.L.; Reeves, W.C. Modoc viral infections in the deer mouse Peromyscus maniculatus. Infect. Immun. 1974, 10, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, A.; Yuill, T.M. Experimental infection and horizontal transmission of Modoc virus in deer mice (Peromyscus maniculatus). J. Wildl. Dis. 1987, 23, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Leyssen, P.; Paeshuyse, J.; Charlier, N.; Van Lommel, A.; Drosten, C.; De Clercq, E.; Neyts, J. Impact of direct virus-induced neuronal dysfunction and immunological damage on the progression of flavivirus (Modoc) encephalitis in a murine model. J. Neurovirol. 2003, 9, 69–78. [Google Scholar] [CrossRef]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.; Azeredo, E.L.; Nogueira, R.M.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Suen, W.W.; Bosco-Lauth, A.; Hartwig, A.E.; Hall, R.A.; Bowen, R.A.; Bielefeldt-Ohmann, H. Kinetics of the West Nile virus induced transcripts of selected cytokines and Toll-like receptors in equine peripheral blood mononuclear cells. Vet. Res. 2016, 47, 61. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.M.; Dung, N.M.; Loan, H.T.; Kneen, R.; Wills, B.; Thu, L.T.; House, D.; White, N.J.; Farrar, J.J.; Hart, C.A.; et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J. Infect. Dis. 2004, 190, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Mallarino, R.; Linden, T.A.; Linnen, C.R.; Hoekstra, H.E. The role of isoforms in the evolution of cryptic coloration in Peromyscus mice. Mol. Ecol. 2017, 26, 245–258. [Google Scholar] [CrossRef]
- Schountz, T.; Acuna-Retamar, M.; Feinstein, S.; Prescott, J.; Torres-Perez, F.; Podell, B.; Peters, S.; Ye, C.; Black, W.C.t.; Hjelle, B. Kinetics of immune responses in deer mice experimentally infected with Sin Nombre virus. J. Virol. 2012, 86, 10015–10027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyssen, P.; Van Lommel, A.; Drosten, C.; Schmitz, H.; De Clercq, E.; Neyts, J. A novel model for the study of the therapy of flavivirus infections using the Modoc virus. Virology 2001, 279, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Espitia, C.M.; Saldarriaga, O.A.; Travi, B.L.; Osorio, E.Y.; Hernandez, A.; Band, M.; Patel, M.J.; Medina, A.A.; Cappello, M.; Pekosz, A.; et al. Transcriptional profiling of the spleen in progressive visceral leishmaniasis reveals mixed expression of type 1 and type 2 cytokine-responsive genes. BMC Immunol. 2014, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Nagata, N.; Iwata-Yoshikawa, N.; Hayasaka, D.; Sato, Y.; Kojima, A.; Kariwa, H.; Takashima, I.; Takasaki, T.; Kurane, I.; Sata, T.; et al. The pathogenesis of 3 neurotropic flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J. Neuropathol. Exp. Neurol. 2015, 74, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Cruz Hernandez, S.I.; Puerta-Guardo, H.N.; Flores Aguilar, H.; Gonzalez Mateos, S.; Lopez Martinez, I.; Ortiz-Navarrete, V.; Ludert, J.E.; Angel, R.M. Primary dengue virus infections induce differential cytokine production in Mexican patients. Mem. Inst. Oswaldo. Cruz. 2016, 111, 161–167. [Google Scholar] [CrossRef]
- Garcia, M.N.; Hause, A.M.; Walker, C.M.; Orange, J.S.; Hasbun, R.; Murray, K.O. Evaluation of prolonged fatigue post-West Nile virus infection and association of fatigue with elevated antiviral and proinflammatory cytokines. Viral. Immunol. 2014, 27, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Kuczera, D.; Assolini, J.P.; Tomiotto-Pellissier, F.; Pavanelli, W.R.; Silveira, G.F. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J. Interferon Cytokine Res. 2018, 38, 69–80. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Interferon-gamma: A historical perspective. Cytokine Growth Factor Rev. 2009, 20, 97–113. [Google Scholar] [CrossRef]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune. Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Wang, T.; Samuel, M.A.; Whitby, K.; Craft, J.; Fikrig, E.; Diamond, M.S. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J. Virol. 2006, 80, 5338–5348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Nicolas, O.; Lewandowska, M.; Ricklin, M.E.; Summerfield, A. Monocyte-Derived Dendritic Cells as Model to Evaluate Species Tropism of Mosquito-Borne Flaviviruses. Front. Cell Infect. Microbiol. 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Tsai, T.T.; Chuang, Y.J.; Lin, Y.S.; Wan, S.W.; Chen, C.L.; Lin, C.F. An emerging role for the anti-inflammatory cytokine interleukin-10 in dengue virus infection. J. Biomed. Sci. 2013, 20, 40. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, R.S.; Kothari, S.T.; Gohil, D.J.; D’Souza, M.; Chowdhary, A.S. Novel evidence of microglial immune response in impairment of Dengue infection of CNS. Immunobiology 2015, 220, 1170–1176. [Google Scholar] [CrossRef]
- Brooks, D.G.; Trifilo, M.J.; Edelmann, K.H.; Teyton, L.; McGavern, D.B.; Oldstone, M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006, 12, 1301–1309. [Google Scholar] [CrossRef]
- Brockman, M.A.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009, 114, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, Y.V.; Brelaz, M.C.; Neves, J.K.; Ferraz, J.C.; Pereira, V.R. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the Development of Pulmonary Tuberculosis. Pulm. Med. 2012, 2012, 745483. [Google Scholar] [CrossRef]
- Best, S.M. The Many Faces of the Flavivirus NS5 Protein in Antagonism of Type I Interferon Signaling. J. Virol. 2017, 91, e01970-16. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Jordan, J.L.; Fredericksen, B.L. How flaviviruses activate and suppress the interferon response. Viruses 2010, 2, 676–691. [Google Scholar] [CrossRef]
- Brinton, M.A. Genetic resistance to flaviviruses. Monoclon. Antib. Immunodiagn. Immunother. 2014, 33, 183–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.B.; Thomas, S.; Swarts, J.; McMillan, A.A.; Ferris, M.T.; Suthar, M.S.; Treuting, P.M.; Ireton, R.; Michael, G., Jr.; Lund, J.M. Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes. mBio 2015, 6, e00493-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashimo, T.; Lucas, M.; Simon-Chazottes, D.; Frenkiel, M.P.; Montagutelli, X.; Ceccaldi, P.E.; Deubel, V.; Guenet, J.L.; Despres, P. A nonsense mutation in the gene encoding 2’–5’-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 2002, 99, 11311–11316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelygin, A.A.; Scherbik, S.V.; Zhulin, I.B.; Stockman, B.M.; Li, Y.; Brinton, M.A. Positional cloning of the murine flavivirus resistance gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9322–9327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherbik, S.V.; Stockman, B.M.; Brinton, M.A. Differential expression of interferon (IFN) regulatory factors and IFN-stimulated genes at early times after West Nile virus infection of mouse embryo fibroblasts. J. Virol. 2007, 81, 12005–12018. [Google Scholar] [CrossRef] [Green Version]
- Fredericksen, B.L.; Gale, M., Jr. West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J. Virol. 2006, 80, 2913–2923. [Google Scholar] [CrossRef] [Green Version]
| Target | Sequence (5′ to 3′) | bp | R2 | Eff. (%) | Accession | Reference | |
|---|---|---|---|---|---|---|---|
| β-Actin | F R | GCTACAGCTTCACCACCACA TCTCCAGGGAGGAAGAGGAT | 123 | 1.000 | 102.0 | XM_006998174.2 | [37] |
| Oas1b | F R | CAGTATGCCCTGGAGCTGC GTACTTGGTGACCAGTTCC | 111 | 0.998 | 98.3 | XM_006970848.1 | This study |
| Ifng | F R | ACAGCAGTGAGGAGAAACGG GACAGGCGGTACATCACTCC | 115 | 0.970 | 94.7 | AY289494.1 | This study |
| Tnf | F R | GGGCTGTACCTCGTCTACTC ACAGGAGGTTGACTTTGTCC | 121 | 0.999 | 100.4 | XM_006995235.2 | This study |
| Tgfb | F R | CGTGGAACTCTACCAGAAATACAGC TCAAAAGACAACCACTCAGGCG | 96 | 0.999 | 95.6 | XM_006988036.2 | [38] |
| IL6 | F R | CCATCCAACTCATCCTGAAAGC CCACAGATTGGTACACATAGGCAC | 101 | 0.999 | 96.1 | AY256518.1 | [38] |
| IL10 | F R | CAGACCTACACGCTTCGAG CCCAGGTAACCCTTAAAGTCC | 128 | 0.999 | 111.2 | XM_006995328.2 | This study |
| MODV-NS5 | F R | CCAGGACAAGTCATGTGGTAGC TCCCAAAGATGTTCCTCACCTT | 101 | 0.998 | 107.5 | NC_003635.1 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, T.J.; Petty, D.; Schountz, T.; Hodges, N.; Hawkinson, A.C. Increased Ifng and Il10 Expression Correlate with Disease in Rodent Models Experimentally Infected with Modoc Virus. Viruses 2022, 14, 1026. https://doi.org/10.3390/v14051026
Sherman TJ, Petty D, Schountz T, Hodges N, Hawkinson AC. Increased Ifng and Il10 Expression Correlate with Disease in Rodent Models Experimentally Infected with Modoc Virus. Viruses. 2022; 14(5):1026. https://doi.org/10.3390/v14051026
Chicago/Turabian StyleSherman, Tyler J., Douglas Petty, Tony Schountz, Natasha Hodges, and Ann C. Hawkinson. 2022. "Increased Ifng and Il10 Expression Correlate with Disease in Rodent Models Experimentally Infected with Modoc Virus" Viruses 14, no. 5: 1026. https://doi.org/10.3390/v14051026
APA StyleSherman, T. J., Petty, D., Schountz, T., Hodges, N., & Hawkinson, A. C. (2022). Increased Ifng and Il10 Expression Correlate with Disease in Rodent Models Experimentally Infected with Modoc Virus. Viruses, 14(5), 1026. https://doi.org/10.3390/v14051026






