Identification of Potential mRNA Biomarkers in Milk Small Extracellular Vesicles of Enzootic Bovine Leukosis Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and EBL Diagnosis
2.2. Hematology
2.2.1. Detection of Serum Antibodies against BLV
2.2.2. Detection of BLV Provirus
2.2.3. Measurement of BLV Proviral Load
2.2.4. Measurement of Total Lactate Dehydrogenase (LDH) Activity and Isozymes
2.3. Milk Samples
2.3.1. Milk sEVs Isolation and Characterization
2.3.2. RNA Extraction and cDNA Synthesis
2.3.3. Microarray Analysis
2.3.4. Relative Quantification of mRNA in Milk sEVs by qPCR
2.3.5. Statistical Analysis
3. Results
3.1. BLV Infection and Clinical Status
3.2. Morphology and Nanoparticle Size Analysis of Milk sEVs
3.3. Microarray Analysis
3.4. qPCR for Detection of mRNA Biomarker Candidates
3.5. Validation of the Utility of mRNA Biomarker Candidates
3.6. Correlation between Other Factors and mRNA Biomarker Candidates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, S.M.; Florins, A.; Gillet, N.; de Brogniez, A.; Sánchez-Alcaraz, M.T.; Boxus, M.; Boulanger, F.; Gutiérrez, G.; Trono, K.; Alvarez, I.; et al. Preventive and therapeutic strategies for bovine leukemia virus: Lessons for HTLV. Viruses 2011, 3, 1210–1248. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.; Tsutsui, T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009−2011. J. Vet. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dequiedt, F.; Cantor, G.; Hamilton, V.; Pritchard, S.; Davis, W.; Kerkhofs, P.; Burny, A.; Kettmann, R.; Willems, L. Bovine leukemia virus-induced persistent lymphocytosis in cattle does not correlate with increased ex vivo survival of B lymphocytes. J. Virol. 1999, 73, 1127–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burny, A.; Cleuter, Y.; Kettmann, R.; Mammerickx, M.; Marbaix, G.; Portetelle, D.; Van den Broeke, A.; Willems, L.; Thomas, R. Bovine leukaemia: Facts and hypotheses derived from the study of an infectious cancer. Vet. Microbiol. 1988, 17, 197–218. [Google Scholar] [CrossRef]
- Annual Report on the Outbreak of Livestock Infectious Diseases. Available online: https://www.maff.go.jp/j/syouan/douei/kansi_densen/attach/pdf/kansi_densen-210.pdf (accessed on 8 November 2021).
- Nakanishi, R.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Comparing miRNA in milk small extracellular vesicles among healthy cattle and cattle at high risk for bovine leukemia virus transmission. J. Dairy Sci. 2022; in press. [Google Scholar]
- Ono, S.; Takanashi, M.; Kuroda, M. Development of nucleic acid DDS by exosomes. Drug Deliv. Syst. 2014, 29, 2. [Google Scholar]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.; Mornet, S.; Brisson, A. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Gonzales, P.; Pisitkun, T.; Hoffert, J.; Tchapyjnikov, D.; Star, R.; Kleta, R.; Wang, N.; Knepper, M. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Korvala, J.; Salo, T.; Sormunen, R.; Vered, M. Human saliva-derived exosomes: Comparing methods of isolation. J. Histochem. Cytochem. 2014, 63, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Thaler, J.; Nieuwland, R. Extracellular vesicles in human milk. Pharmaceuticals 2021, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Fahner, C.; Reid, G.; Simpson, R. ExoCarta 2012: Datebase of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, 1241–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaborowski, M.; Balaj, L.; Breakefield, X.; Lai, C. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6977. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.; Wang, M. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.; Silva, J.; Herrera, A.; Pena, C.; Martin, P.; Gil-Calderon, B.; Larriba, M.; Coronado, M.; Soldevilla, B.; Turrion, V.; et al. Exosomes enriched in stemness/metastatic-related mRNAs promote oncogenic potential in breast cancer. Oncotarget 2015, 6, 40575–40587. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Peng, H.; Chen, X.-T.; Yao, Y.-Y.; Chen, L.-P.; Yin, Q.; Shen, W. Chemokine receptor CXCR4 activates the PhoA/ROCK2 pathway in spinal neurons that induces bone cancer pain. Mol. Pain 2020, 16, 1744806920919568. [Google Scholar] [CrossRef]
- The Livestock Mutual Aid Office Handling Guidelines. Available online: https://www.maff.go.jp/j/keiei/nogyohoken/attach/pdf/kokujituuchi-145.pdf (accessed on 8 November 2021).
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.; Rahman, M.; Yamauchi, M.; Takashima, S.; Wakihara, Y.; Kamatari, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. mRNA profile in milk extracellular vesicles from bovine leukemia virus-infected cattle. Viruses 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- National Meat Hygiene Inspection Office Council. Bovine Leukemia. In New Meat Hygiene Inspection Manual; Chuohoki Publishing: Tokyo, Japan, 2011; pp. 171–177. (In Japanese) [Google Scholar]
- Bendixen, H.J. Preventive measures in cattle leukemia: Leukosis enzootica bovis. Ann. N. Y. Acad. Sci. 1963, 108, 1241–1267. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Okada, K.; Ikawa, Y.; Aida, Y. Bovine leukemia virus induces CD5− B cell lymphoma in sheep despite temporarily increasing CD5+ B cells in asymptomatic stage. Virology 1994, 202, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Fechner, H.; Blankenstein, P.; Looman, A.C.; Elwert, J.; Geue, L.; Albrecht, C.; Kurg, A.; Beier, D.; Marquardt, O.; Ebner, D. Provirus variants of the bovine leukemia virus and their relation to the serological status of naturally infected cattle. Virology 1997, 237, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Shimizu, K.; Yamauchi, M.; Takase, H.; Ugawa, S.; Okada, A.; Inoshima, Y. Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS ONE 2019, 14, e0222613. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Takashima, S.; Kamatari, Y.O.; Badr, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Putative internal control genes in bovine milk small extracellular vesicles suitable for nomalization in quantitative real time-plymerase chain reaction. Membranes 2021, 11, 933. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Benmoussa, A.; Ly, S.; Shan, S.T.; Laugier, J.; Boilard, E.; Gilbert, C.; Provost, P. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. J. Extracell. Vesicles 2017, 6, 1401897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, J.; Liao, M.; Dang, W.; Chen, X.; Wu, Y.; Liao, W. Upregulation of RECQL4 expression predicts poor prognosis in hepatocellular carcinoma. Oncol. Lett. 2018, 15, 4248–4254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yuan, K.; Wang, X.; Wang, H.; Wu, Q.; Wu, X.; Peng, J. Overexpression of RECQL4 is associated with poor prognosis in patients with gastric cancer. Oncol. Lett. 2018, 16, 5419–5425. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Glowacka, W.K.; Alberts, P.; Ouchida, R.; Wang, J.Y.; Rotin, D. LAPTM5 protein is a positive regulator of proinflammatory signaling pathways in macrophages. J. Biol. Chem. 2012, 287, 27691–27702. [Google Scholar] [CrossRef] [Green Version]
- Koteluk, O.; Bielicka, A.; Lemanska, Z.; Jozwiak, K.; Klawiter, W.; Mackiewicz, A.; Kazimierczak, U.; Kolenda, T. The landscape of transmembrane protein family members in head and neck cancers: Their biological role and diagnostic utility. Cancers 2021, 13, 4737. [Google Scholar] [CrossRef]
- Li, J.-M.; Tseng, C.-W.; Lin, C.-C.; Law, C.-H.; Chien, Y.-A.; Kuo, W.-H.; Chou, H.-C.; Wang, W.-C.; Chan, H.-L. Upregulation of LGALS1 is associated with oral cancer metastasis. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794622. [Google Scholar] [CrossRef] [Green Version]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Ohata, T.; Yokoo, H.; Kamiyama, T.; Fukai, M.; Aiyama, T.; Hatanaka, Y.; Hatanaka, K.; Wakayama, K.; Orimo, T.; Kakisaka, T.; et al. Fatty acid-binding protein 5 function in hepatocellular carcinoma through induction of epithelial-mesenchymal transition. Cancer Med. 2017, 6, 1049–1061. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Yang, Y.; Zhu, L.; Li, X.; Zhao, W. SRGN promotes colorectal cancer metastasis as a critical downstream target of HIF-1α. Cell Physiol. Biochem. 2018, 48, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, D.; Zhang, Z.; Wang, Y.; Yu, W.; Sun, K.; Maimela, N.R.; Sun, Z.; Liu, J.; Yuan, W.; et al. DEFB4A is a potential prognostic biomarker for colorectal cancer. Oncol. Lett. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 2017, 74, 103–141. [Google Scholar]
- Dai, J.; Su, Y.; Zhong, A.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Mammerickx, M.; Lorenz, R.J.; Straub, O.C.; Donnelly, W.J.C.; Flensburg, J.C.; Gentile, G.; Markson, L.M.; Taylor, S.M.; Ressang, A.A. Bovine hematology. III. Comparative breed studies on the leukocyte parameters of several European cattle breeds as determined in the common reference laboratory. Zbl. Vet. Med. B 1978, 25, 257–267. [Google Scholar]
- Kobayashi, T.; Inaggaki, Y.; Ohnuki, N.; Sato, R.; Murakami, S.; Imakawa, K. Increasing bovine leukemia virus (BLV) proviral load is a risk factor for progression of enzootic bovine leucosis: A prospective study in Japan. Prev. Vet. Med. 2020, 178, 104680. [Google Scholar] [CrossRef]
- Nishimori, A.; Andoh, K.; Matsuura, Y.; Kumagai, A.; Hatama, S. Establishment of a simplified inverse polymerase chain reaction method for diagnosis of enzootic bovine leukosis. Arch. Virol. 2021, 166, 841–851. [Google Scholar] [CrossRef]
- Sharifi, S.; Pakdel, A.; Ebrahimi, M.; Reecy, J.; Farsani, S.; Ebrahimie, E. Integration of machine learning and meta-analysis identifies the transcriptomic bio-signature of mastitis disease in cattle. PLoS ONE 2018, 13, e0191227. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, A.; Murakami, H.; Kakinuma, S.; Murao, K.; Ohmae, K.; Isobe, N.; Akamatsu, H.; Seto, T.; Hashimura, S.; Konda, K.; et al. Association between bovine leukemia virus proviral load and severity of clinical mastitis. J. Vet. Med. Sci. 2019, 81, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Somura, Y.; Sugiyama, E.; Fujikawa, H.; Murakami, K. Comparison of the copy numbers of bovine leukemia virus in the lymph nodes of cattle with enzootic bovine leukosis and cattle with latent infection. Arch. Virol. 2014, 159, 2693–2697. [Google Scholar] [CrossRef]
- Miura, S.; Inokuma, H. Evaluation of lactate dehydrogenase activity as an onset marker for enzootic bovine leukemia. Jpn. J. Large Anim. Clin. 2016, 6, 149–153. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Kent-Dennis, C.; Aschenbach, J.; Griebel, P.; Penner, G. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J. Dairy Sci. 2020, 103, 9587–9603. [Google Scholar] [CrossRef] [PubMed]
- Pareek, R.; Wellnitz, O.; Dorp, R.; Burton, J.; Kerr, D. Immunorelevantgene expression in LPS-challenged bovine mammary epithelial cells. J. Appl. Genet. 2005, 46, 171–177. [Google Scholar]
- Cheishvii, D.; Stefanska, B.; Yi, C.; Li, C.; Yu, P.; Arakelian, A.; Tanvir, I.; Khan, H.; Rabbani, M.; Szyf, M. A common promoter hypomethylation signature in invasive breast, liver and prostate cancer cell lines reveals novel targets involved in cancer invasiveness. Oncotarget 2015, 6, 33253–33268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Zhang, Y.; Xin, H. lncRNA SNHG3 facilitates acute myeloid leukemia cell growth via the regulation of miR-758-3p/SRGN axis. J. Cell Biochem. 2020, 121, 1023–1031. [Google Scholar] [CrossRef]
- Sharma, V.; Zhang, L. Interleukin-8 Expression in AIDS-associated lymphoma B-cell lines. Biochem. Biophys. Res. Commun. 2001, 282, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; Odelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, G.; Luo, Y.; Wang, Y.; Xie, C.; Jiang, W.; Xiao, Y.; Qian, G.; Wang, X. Downregulation of LAPTM5 suppresses cell proliferation and viability inducing cell cycle arrest at G0/G1 phase of bladder cancer cells. Int. J. Oncol. 2016, 50, 263–271. [Google Scholar] [CrossRef] [Green Version]
- RezanejadBardaji, H.; Asadi, M.; Yaghoobi, M. Long noncoding RNA VIM-AS1 promotes colorectal cancer progression and metastasis by inducing EMT. Eur. J. Cell Biol. 2018, 97, 279–288. [Google Scholar] [CrossRef]
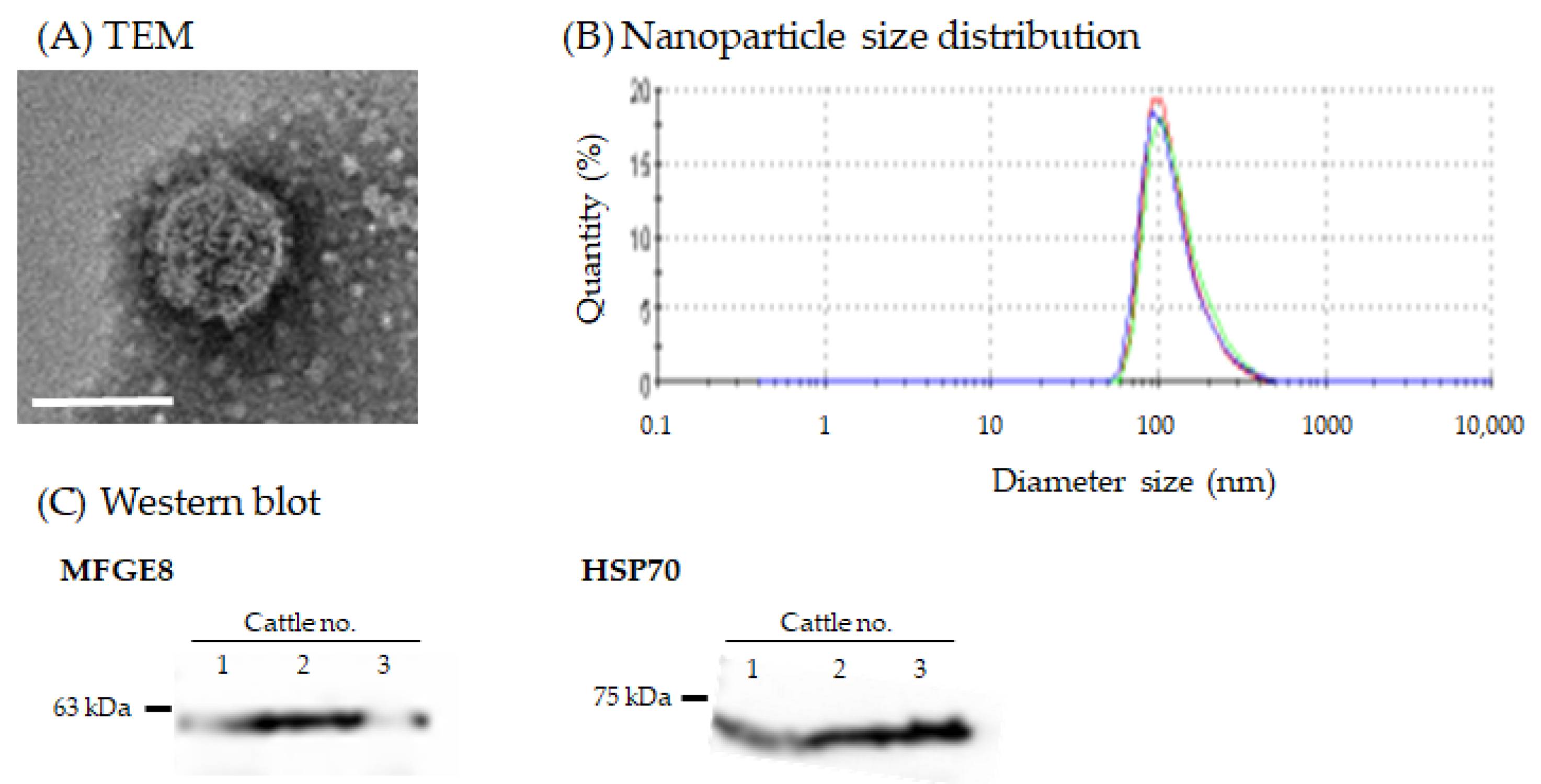
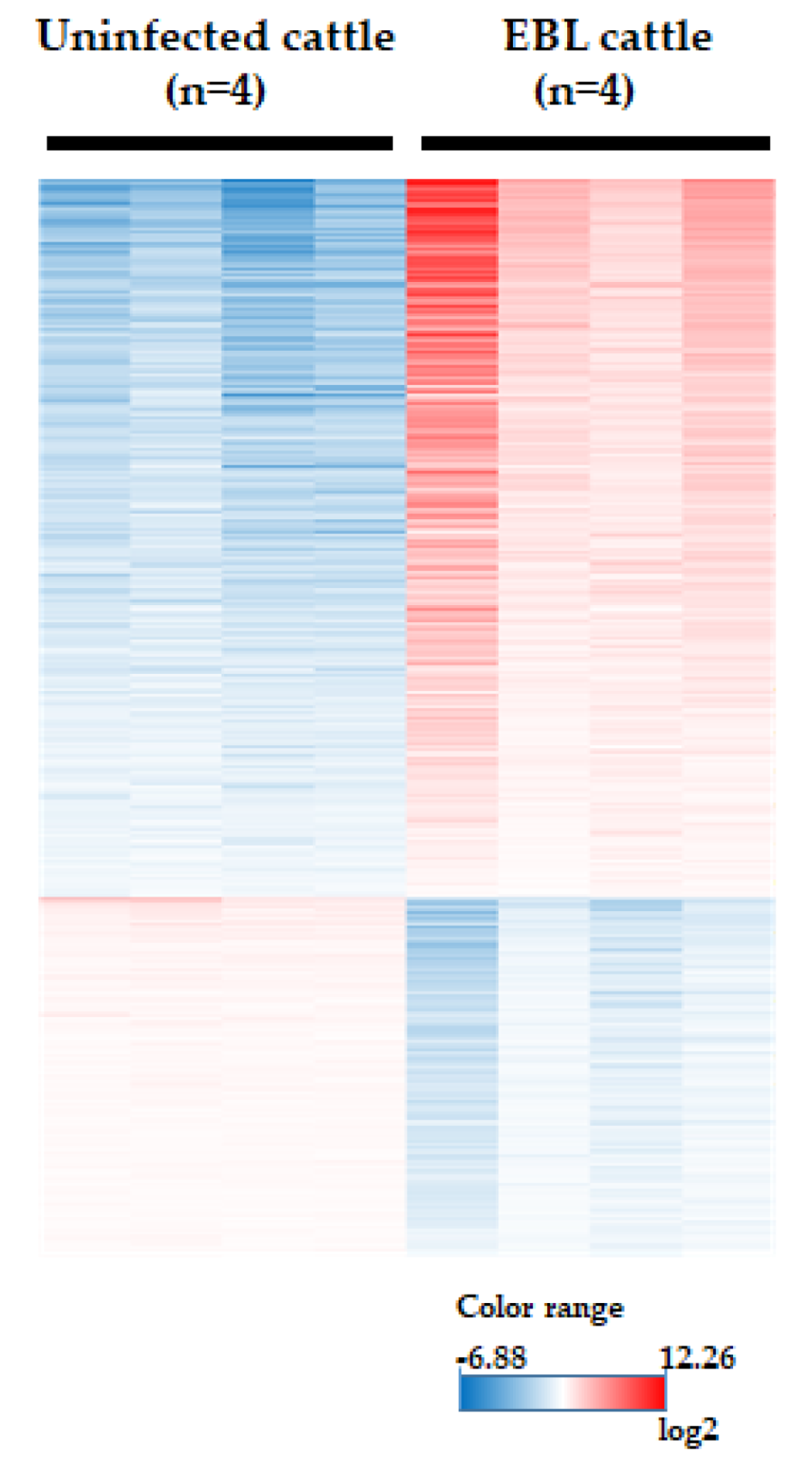
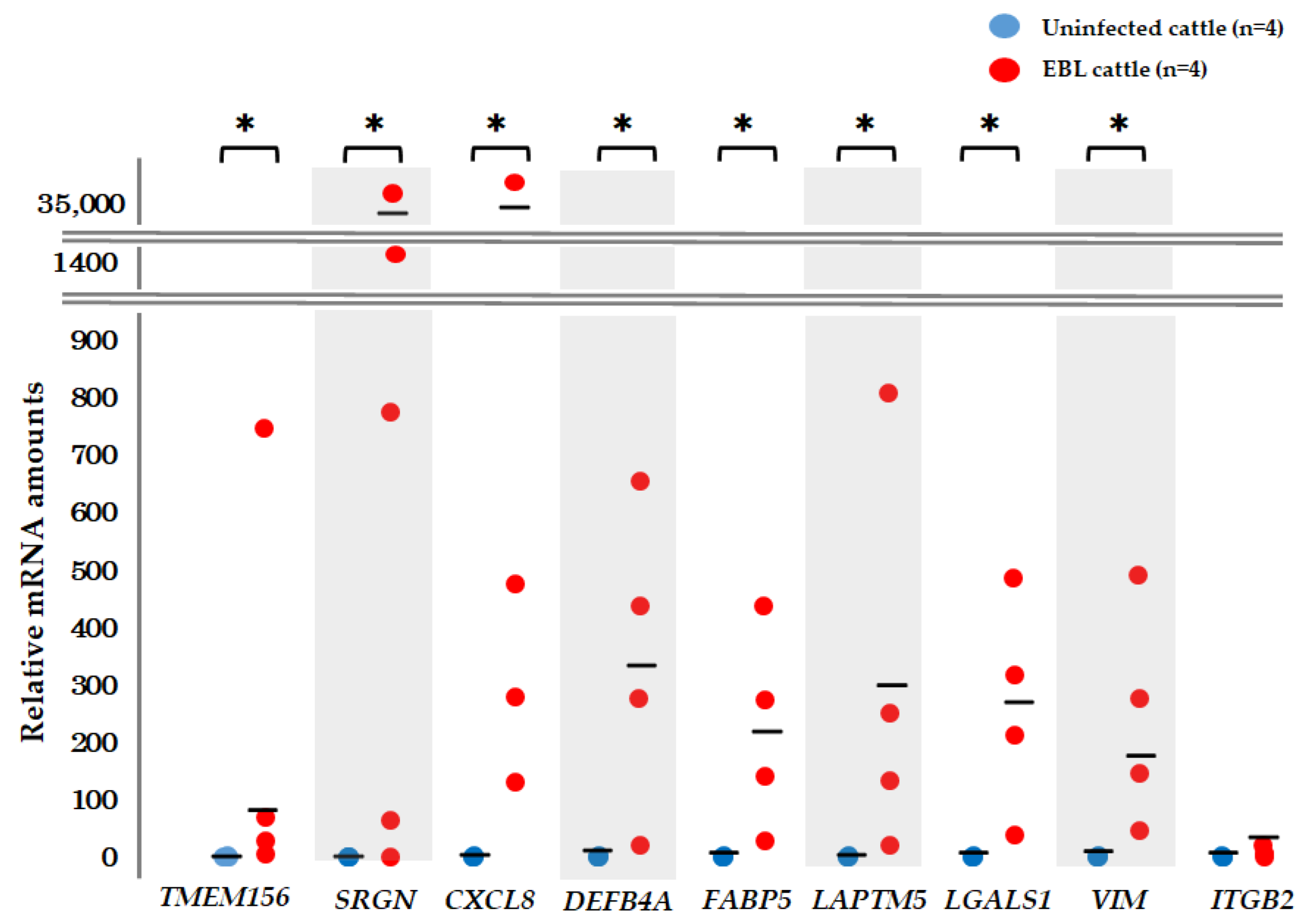
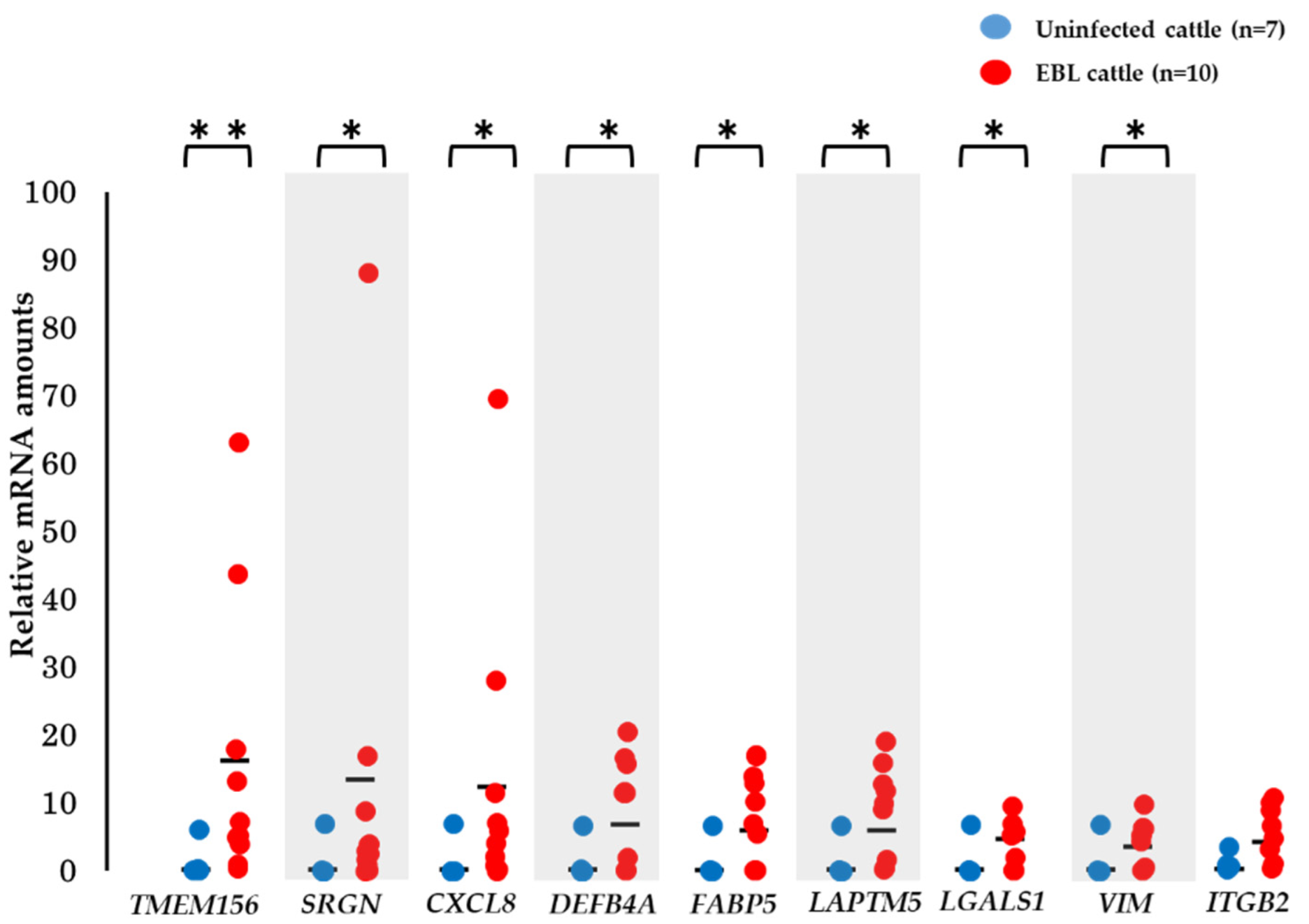

| Cattle No. | Age *2 (Month) | ELISA *3 Antibody | Nested PCR | Proviral Load *4 (/10⁵WBCs) | WBC *5 (/μL) | Lymphocyte (/μL) | Lymphocyte (%) | Key of EC *6 | Total LDH *7 (IU/l) | LDH Isozyme (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 2+3 | 4 | 5 | |||||||||||
| Experiment 1 | Uninfected cattle | |||||||||||||||
| 1 | 38 | − | − | NT | 8600 | 4200 | 48.5 | − | 1222 | 66.8 | 19.4 | 9.7 | 29.1 | 3.0 | 1.1 | |
| 2 | 25 | − | − | NT | 9100 | 4400 | 47.9 | − | 1080 | 61.8 | 21.5 | 11.9 | 33.4 | 3.6 | 1.2 | |
| 3 | 72 | − | − | NT | 6000 | 3100 | 52.4 | − | 1183 | 60.7 | 21.6 | 12.0 | 33.6 | 3.9 | 1.8 | |
| 4 | 33 | − | − | NT | 5400 | 2800 | 51.3 | − | 1304 | 65.5 | 18.3 | 10.2 | 28.5 | 3.8 | 2.2 | |
| EBL cattle | ||||||||||||||||
| 5 | 88 | + | + | 173,575 | 271,500 | 193,600 | 71.3 | + | 3795 | 22.0 | 28.9 | 29.8 | 58.7 | 14.6 | 4.7 | |
| 6 | 64 | + | + | 96,045 | 23,000 | 12,700 | 55.2 | + | 2201 | 36.0 | 31.8 | 22.0 | 53.8 | 7.6 | 2.6 | |
| 7 | 100 | + | + | 95,092 | 20,500 | 8900 | 43.6 | + | 3171 | 41.0 | 38.3 | 16.1 | 54.4 | 3.6 | 1.0 | |
| 8 | 172 | + | + | 56,434 | 11,700 | 4700 | 40.4 | − | 1881 | 31.2 | 31.9 | 23.0 | 54.9 | 9.8 | 4.1 | |
| Experiment 2 | Uninfected cattle | |||||||||||||||
| 9 | 29 | − | − | NT | 9100 | 4400 | 47.9 | − | 1080 | 61.8 | 21.5 | 11.9 | 33.4 | 3.6 | 1.2 | |
| 10 | 28 | − | − | NT | 6100 | 3200 | 53.2 | − | 1327 | 71.1 | 15.9 | 7.4 | 23.3 | 3.6 | 2.0 | |
| 11 | 53 | − | − | NT | 5400 | 2700 | 49.3 | − | 1190 | 72.2 | 14.9 | 6.9 | 21.8 | 2.1 | 3.4 | |
| 12 | 31 | − | − | NT | 4800 | 2100 | 42.9 | − | 1331 | 69.9 | 17.1 | 9.4 | 26.5 | 1.8 | 1.8 | |
| 13 | 77 | − | − | NT | 6000 | 3100 | 52.4 | − | 1183 | 60.7 | 21.6 | 12.0 | 33.6 | 3.9 | 1.8 | |
| 14 | 37 | − | − | NT | 5400 | 2800 | 51.3 | − | 1304 | 65.5 | 18.3 | 10.2 | 28.5 | 3.8 | 2.2 | |
| 15 | 42 | − | − | NT | 8600 | 4200 | 48.5 | − | 1222 | 66.8 | 19.4 | 9.7 | 29.1 | 3.0 | 1.1 | |
| EBL cattle | ||||||||||||||||
| 16 | 85 | + | + | 22,474 | 7200 | 4900 | 68.7 | − | 3001 | 49.6 | 32.2 | 13.3 | 45.5 | 3.9 | 1.0 | |
| 17 | 65 | + | + | 16,696 | 12,700 | 7100 | 55.6 | + | 1376 | 37.3 | 33.2 | 21.1 | 54.3 | 6.4 | 2.0 | |
| 18 | 59 | + | + | 132,721 | Over *8 | NT | NT | NT | 5439 | 30.7 | 32.1 | 17.6 | 49.7 | 6.2 | 13.4 | |
| 19 | 66 | NT | + | 45,139 | 8800 | 2500 | 28.3 | − | 2056 | 52.4 | 31.0 | 11.9 | 42.9 | 3.1 | 1.6 | |
| 20 | 66 | + | + | 1824 | 18,400 | 2600 | 14.3 | − | 814 | 44.9 | 18.1 | 21.6 | 39.7 | 10.3 | 5.1 | |
| 21 | 69 | NT | + | 8557 | 7000 | 3100 | 43.8 | − | 1536 | 35.4 | 30.0 | 23.0 | 53.0 | 8.7 | 2.9 | |
| 22 | 84 | + | + | 58,933 | 13,200 | 1600 | 12.4 | − | 1800 | 38.5 | 35.9 | 18.6 | 54.5 | 4.6 | 2.4 | |
| 23 | 77 | NT | + | NT | 13,200 | 1600 | 12.3 | − | 3369 | 42.6 | 28.6 | 17.2 | 45.8 | 5.1 | 6.5 | |
| 24 | 48 | + | + | 76,454 | 37,000 | 30,600 | 82.6 | + | 5000 | 38.4 | 35.4 | 17.7 | 53.1 | 5.4 | 2.8 | |
| 25 | 42 | NT | + | 863 | 13,100 | 1600 | 12.4 | − | 921 | 55.8 | 23.2 | 14.3 | 37.5 | 4.8 | 1.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiraoka, M.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. Identification of Potential mRNA Biomarkers in Milk Small Extracellular Vesicles of Enzootic Bovine Leukosis Cattle. Viruses 2022, 14, 1022. https://doi.org/10.3390/v14051022
Hiraoka M, Takashima S, Wakihara Y, Kamatari YO, Shimizu K, Okada A, Inoshima Y. Identification of Potential mRNA Biomarkers in Milk Small Extracellular Vesicles of Enzootic Bovine Leukosis Cattle. Viruses. 2022; 14(5):1022. https://doi.org/10.3390/v14051022
Chicago/Turabian StyleHiraoka, Mami, Shigeo Takashima, Yoshiko Wakihara, Yuji O. Kamatari, Kaori Shimizu, Ayaka Okada, and Yasuo Inoshima. 2022. "Identification of Potential mRNA Biomarkers in Milk Small Extracellular Vesicles of Enzootic Bovine Leukosis Cattle" Viruses 14, no. 5: 1022. https://doi.org/10.3390/v14051022
APA StyleHiraoka, M., Takashima, S., Wakihara, Y., Kamatari, Y. O., Shimizu, K., Okada, A., & Inoshima, Y. (2022). Identification of Potential mRNA Biomarkers in Milk Small Extracellular Vesicles of Enzootic Bovine Leukosis Cattle. Viruses, 14(5), 1022. https://doi.org/10.3390/v14051022







