TBK1 Mediates Innate Antiviral Immune Response against Duck Enteritis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Viral Infection and Overexpression Assay
2.3. TCID50 Assay
2.4. RNA Interference Assay
2.5. RNA Extraction and qRT-PCR
2.6. Western Blot Assay
2.7. Animal Infection Experiments
2.8. Drug Treatments
2.9. Statistical Analysis
3. Results
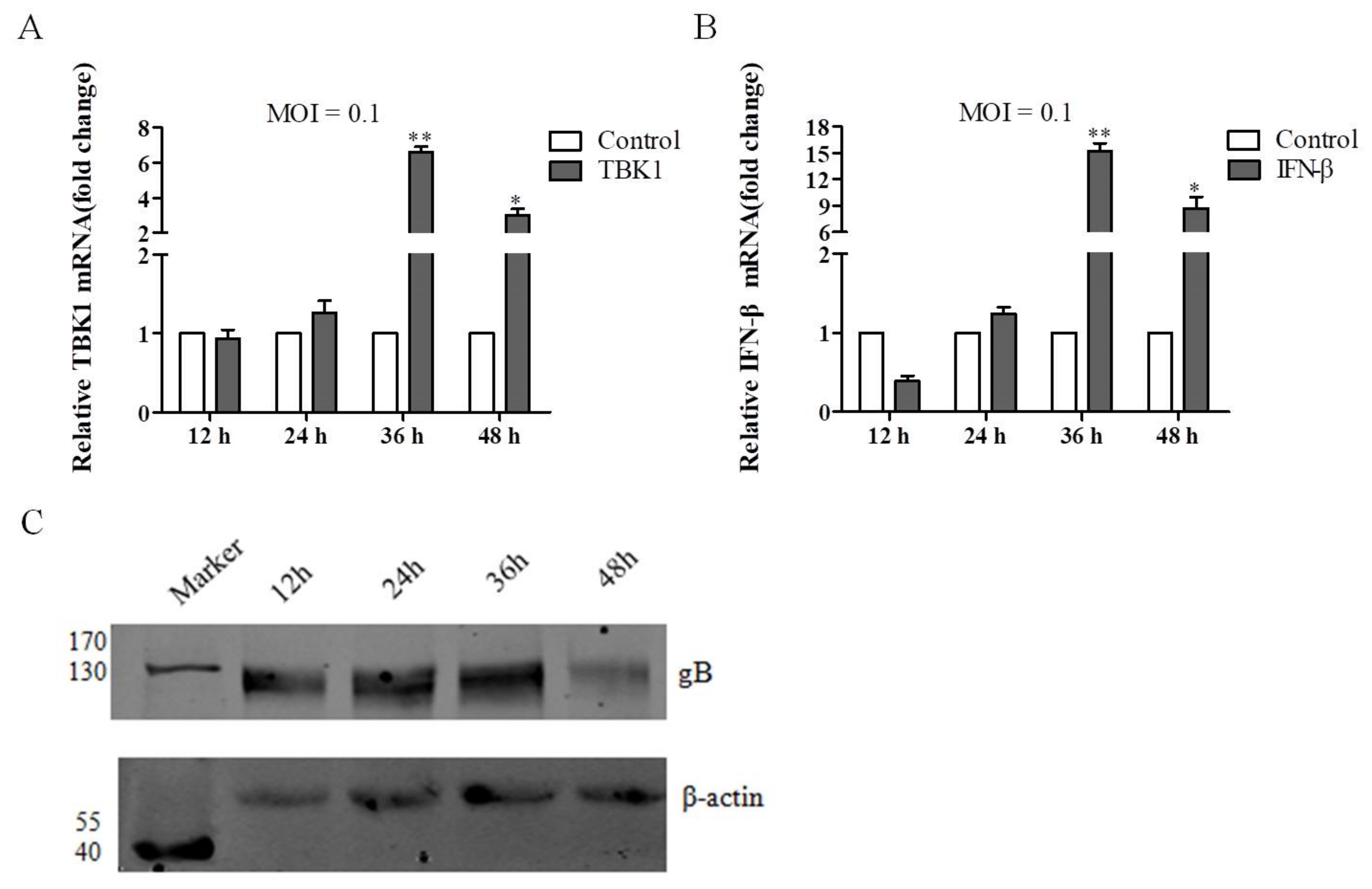
3.1. DEV Infection Results in the Increase of TBK1 and IFN-β Expression In Vitro
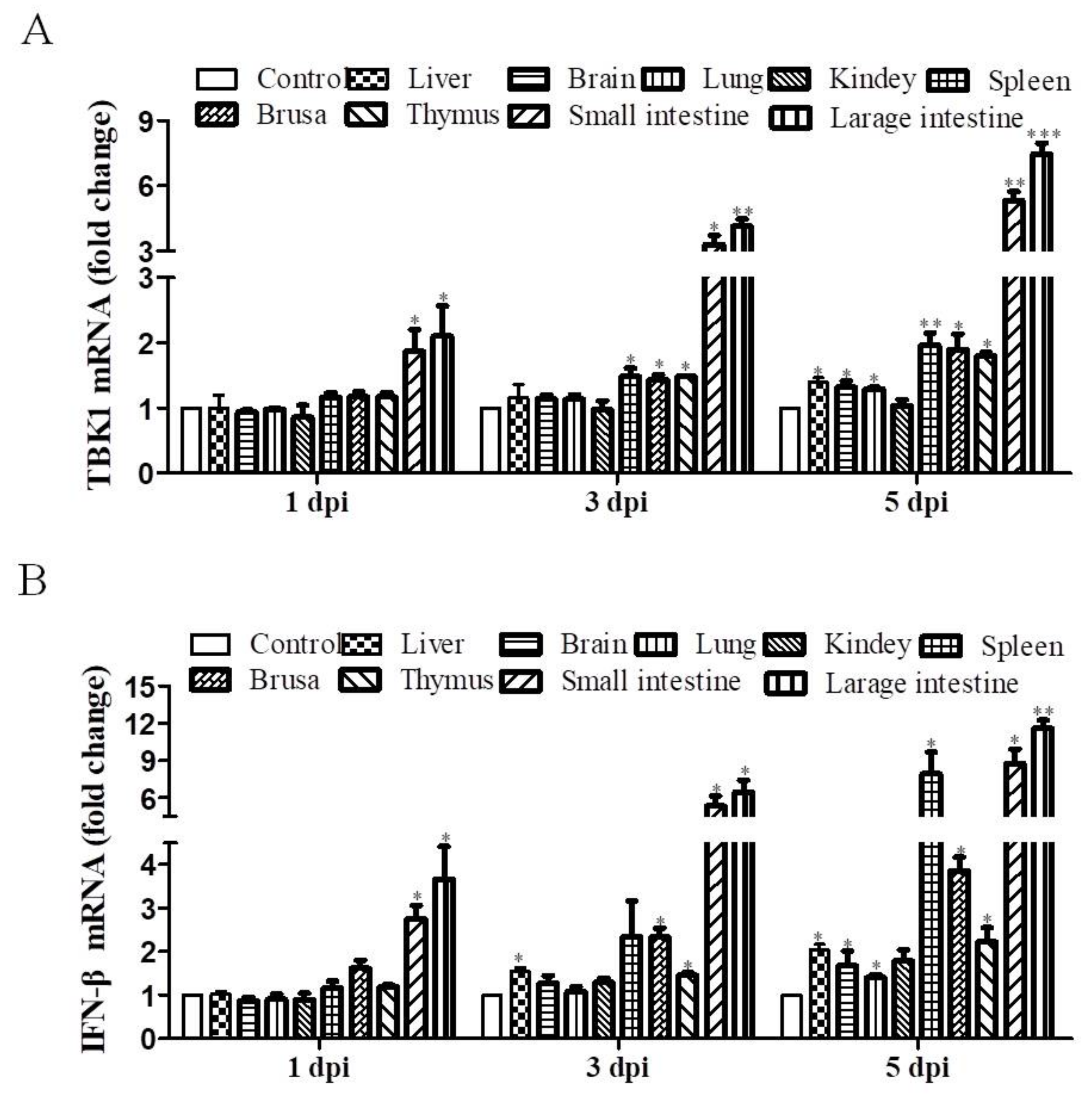
3.2. DEV Infection Induces TBK1 and IFN-β Expression In Vivo
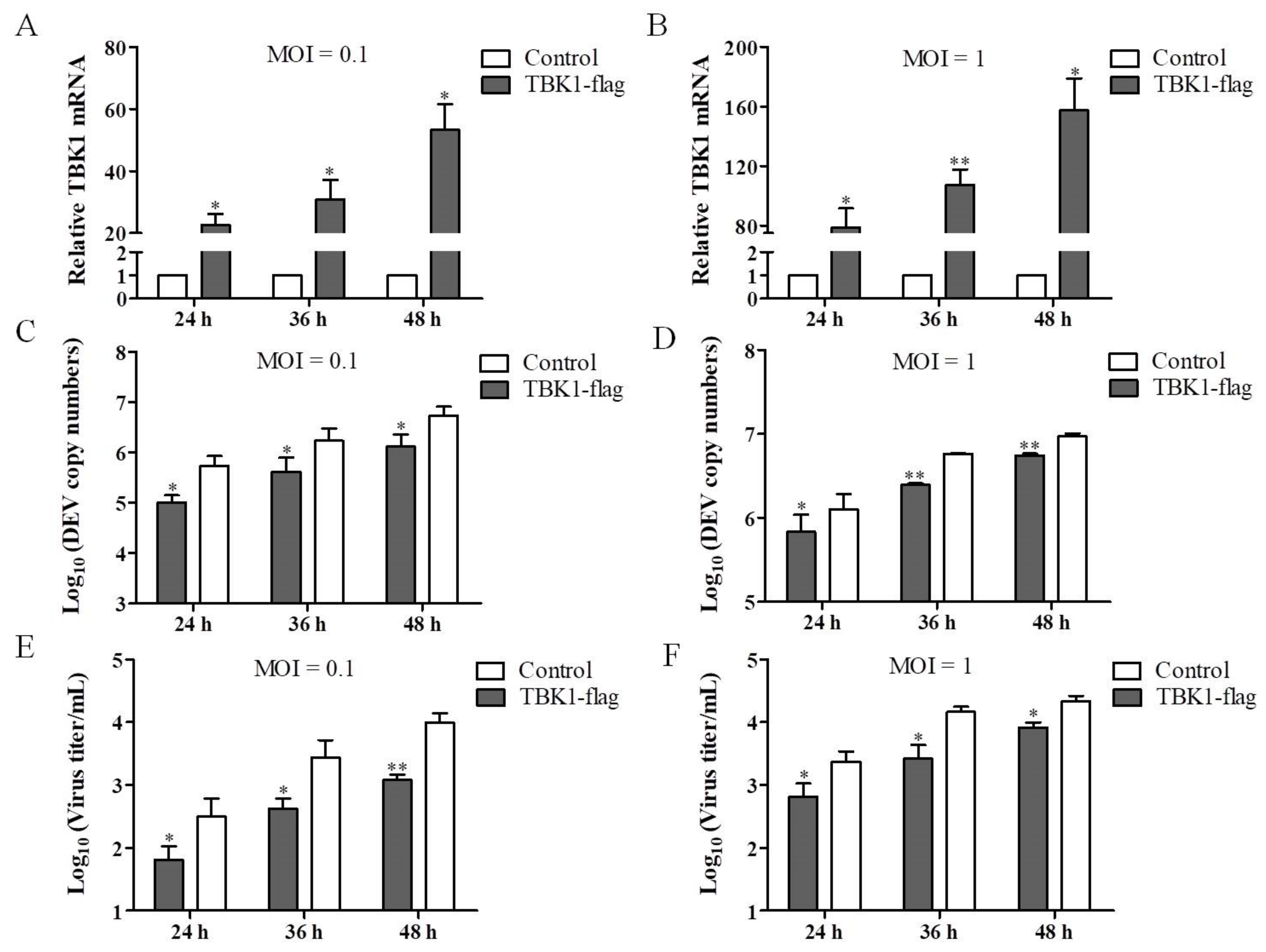
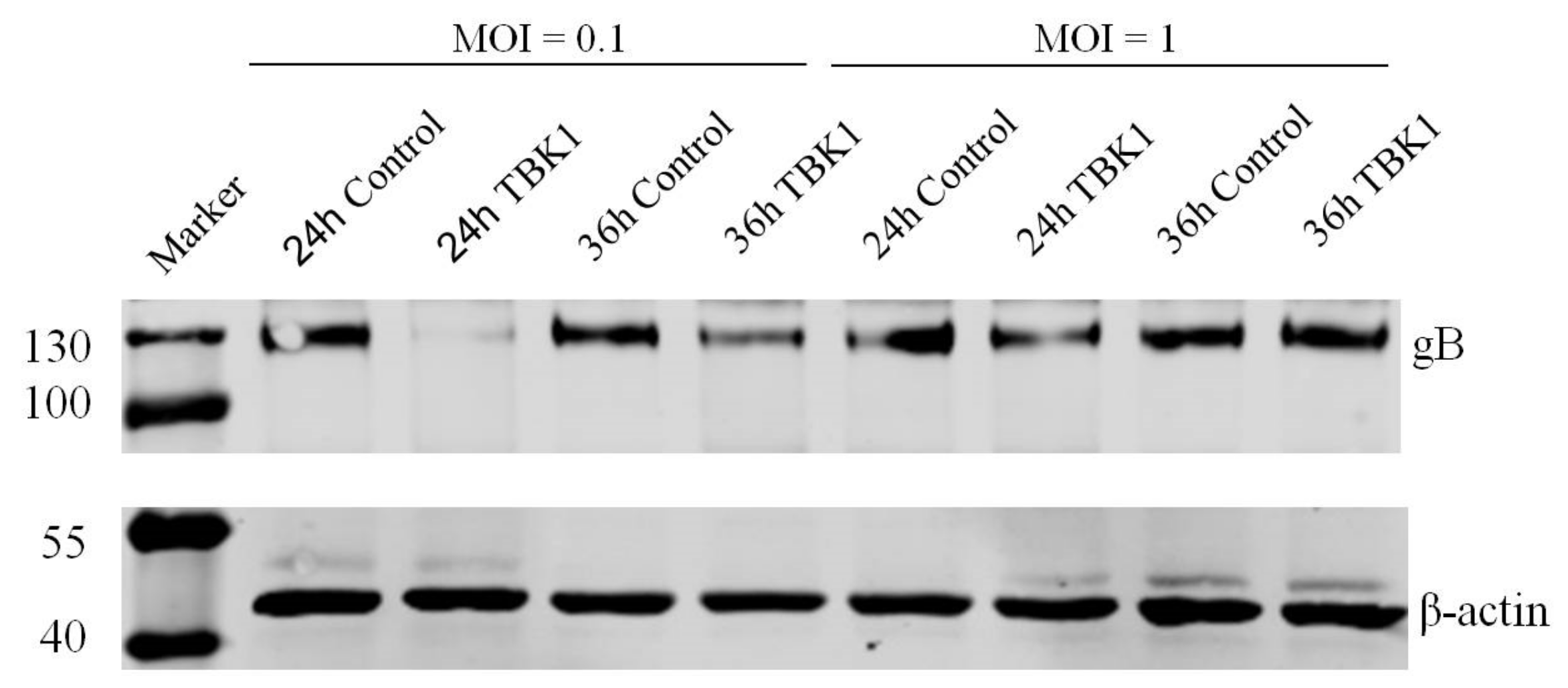
3.3. Overexpression of TBK1 Inhibits DEV Infection
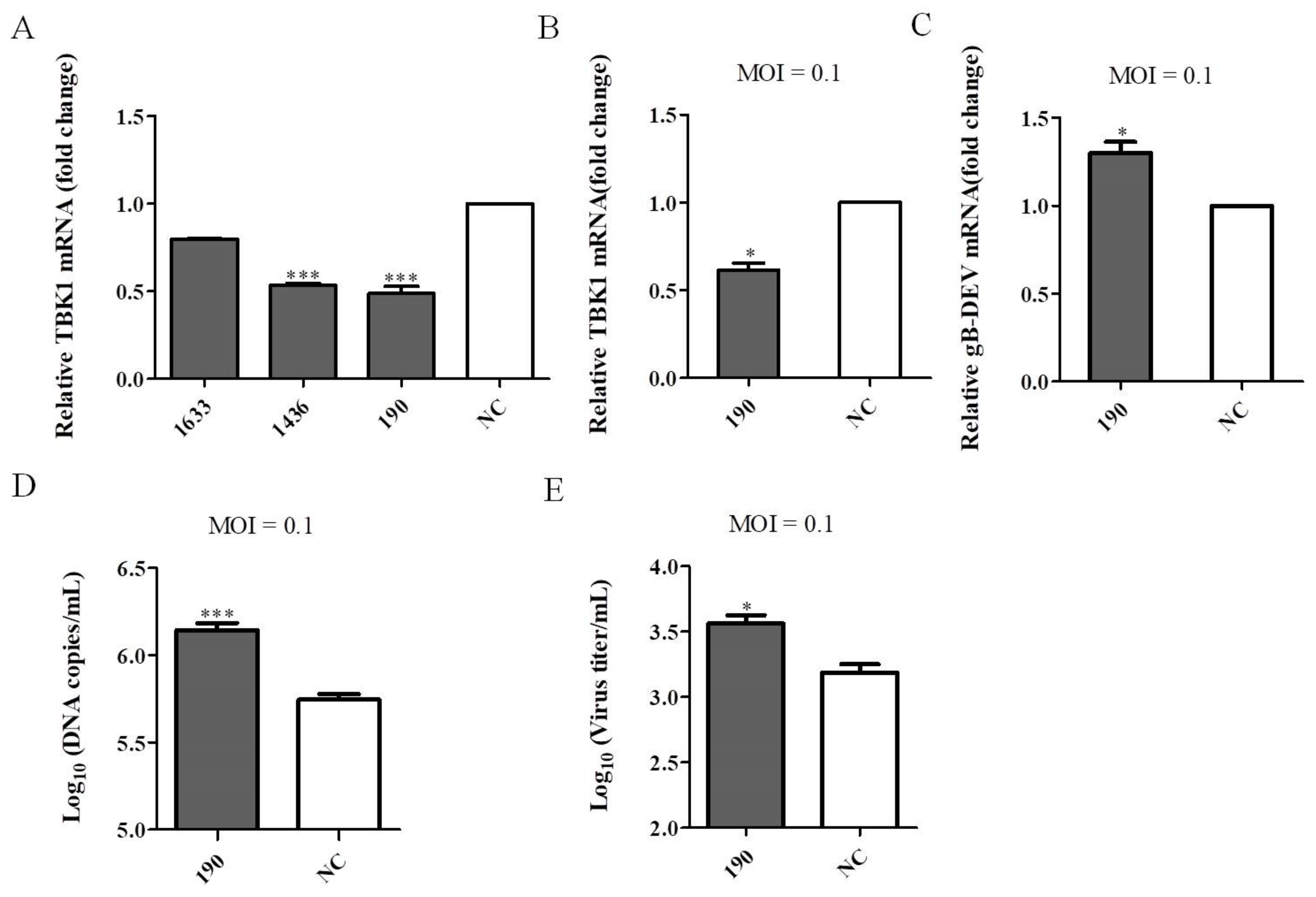
3.4. Knockdown of Endogenous TBK1 Expression Promotes DEV Infection
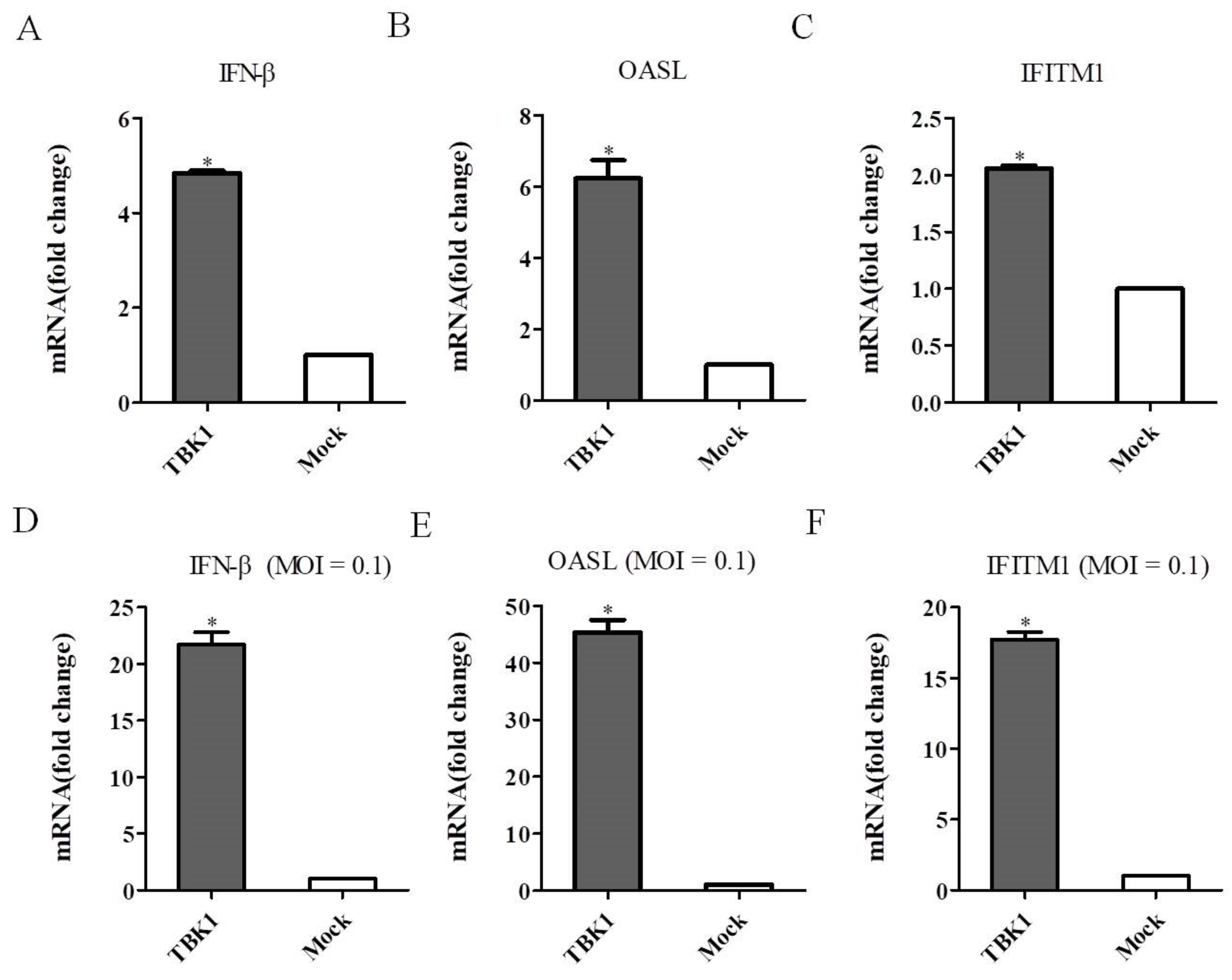
3.5. Overexpression of TBK1 Induces IFN-β and ISGs Expression
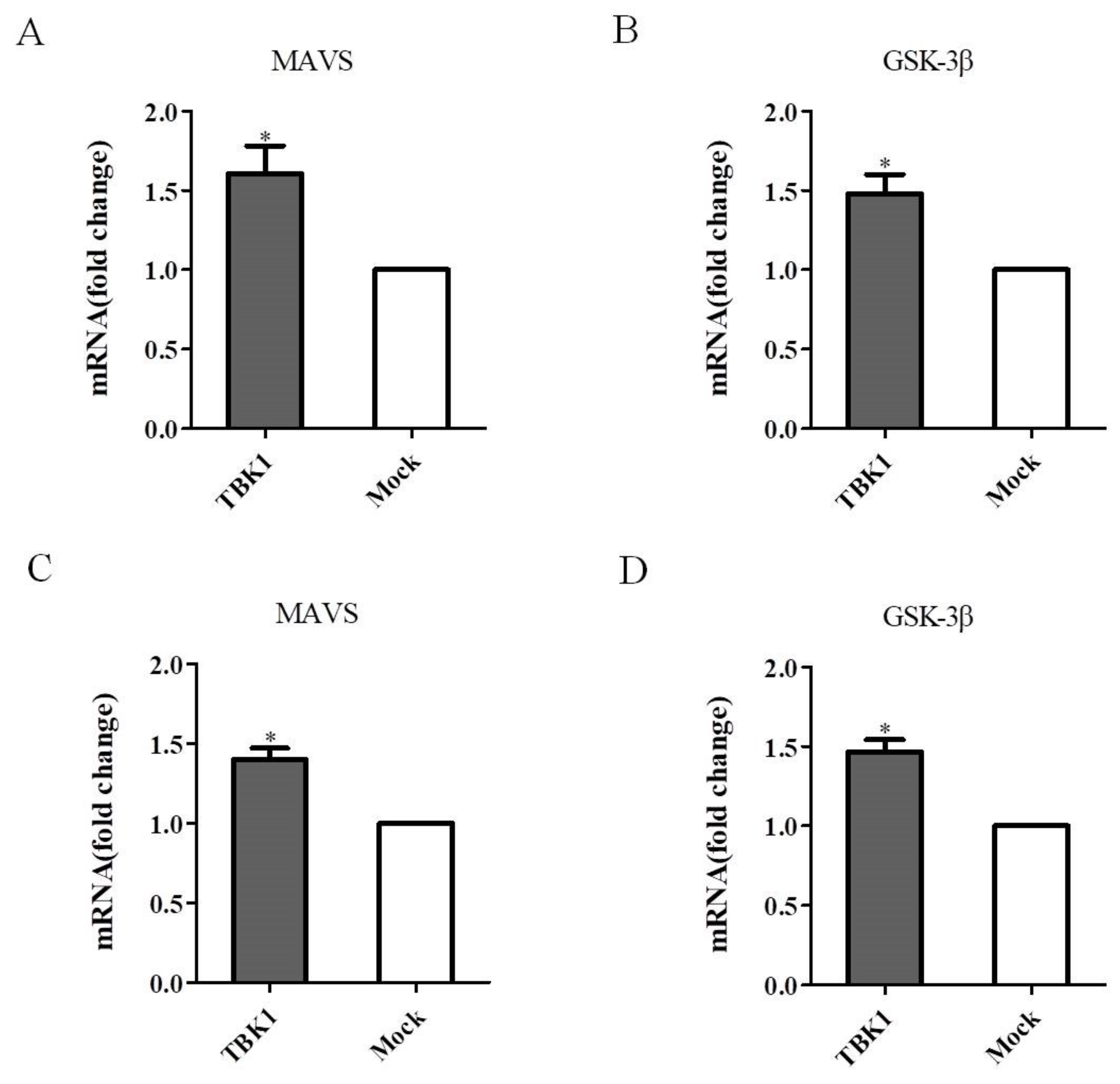
3.6. Overexpression of TBK1 Activates MAVS and GSK-3β in DEF Cells
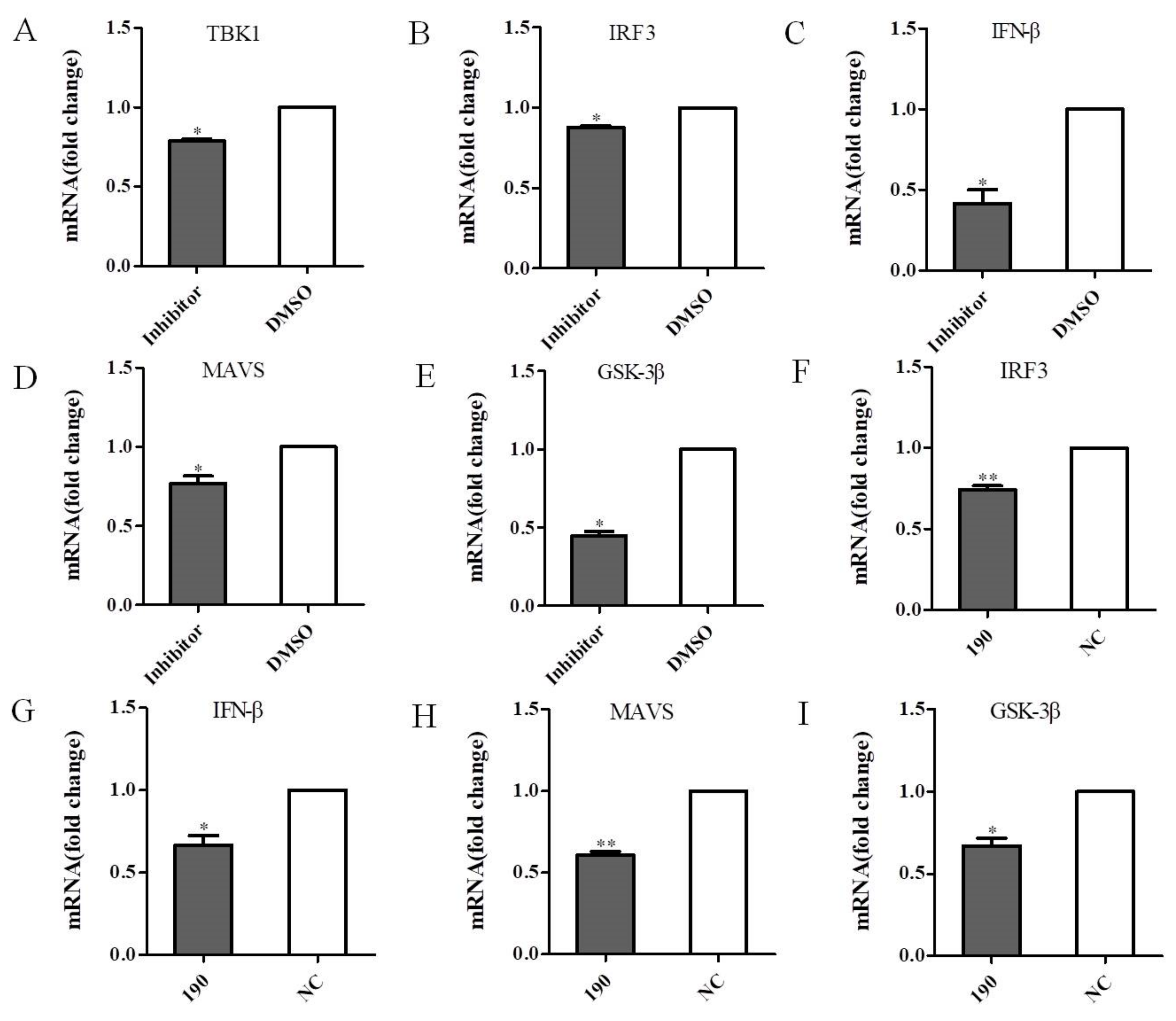
3.7. Inhibition of TBK1 Decreases the Antiviral Signaling
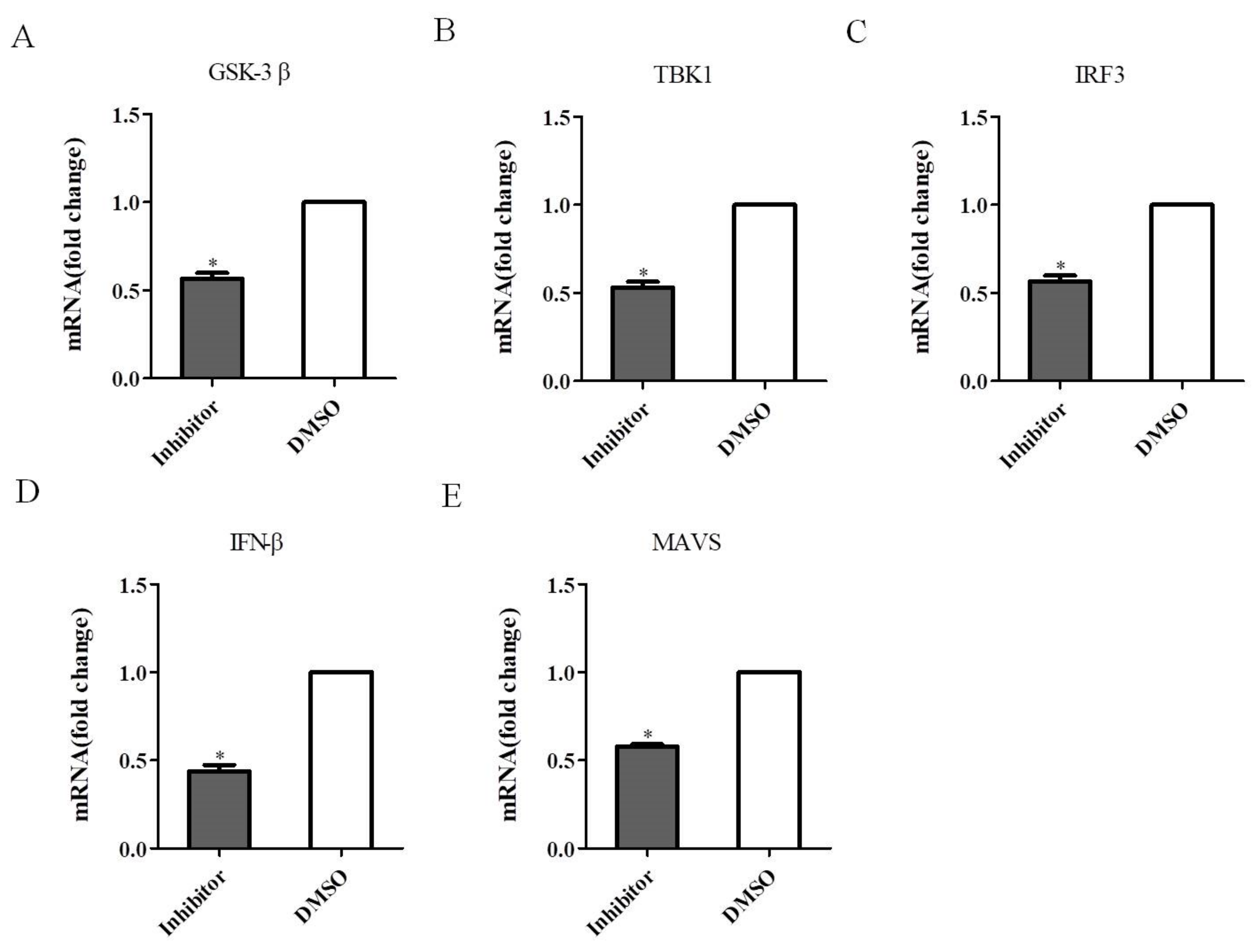
3.8. Effect of GSK-3β-Associated Signaling on TBK1 in Antiviral Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhama, K.; Kumar, N.; Saminathan, M.; Tiwari, R.; Karthik, K.; Kumar, M.A.; Palanivelu, M.; Shabbir, M.Z.; Malik, Y.S.; Singh, R.K. Duck virus enteritis (duck plague)—A comprehensive update. Vet. Q. 2017, 37, 57–80. [Google Scholar] [CrossRef]
- Gardner, R.; Wilkerson, J.; Johnson, J.C. Molecular characterization of the DNA of Anatid herpesvirus 1. Intervirology 1993, 36, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Sivakumar, M.; Selvaraj, P.; Yogeshpriya, S.; Veeraselvam, M. Duck viral enteritis in chronically infected and partially immune duck flock of nomadic. J. Entomol. Zool. Stud. 2018, 6, 2698–2700. [Google Scholar]
- King, A.M.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011; Volume 9. [Google Scholar]
- Kang, S.-W.; Rane, N.S.; Kim, S.J.; Garrison, J.L.; Taunton, J.; Hegde, R.S. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 2006, 127, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Kumar, J.; Dandapat, S.; Panickan, S.; Kumar, A.; Singh, M.; Bindu, S.; Dhama, K. Expression profiles of toll like receptors, MHC and cytokine genes along with viral load in organs of ducklings infected with an Indian isolate of duck enteritis virus. Microb. Pathog. 2022, 165, 105502. [Google Scholar] [CrossRef]
- Yang, F.; Xie, X.; Tang, C.; Jin, B.; Yue, H. Dynamics of IFN-α mRNA expression in liver of ducks infected with duck plague virus of different virulence. Chin. J. Prev. Vet. Med. 2008, 8, 647–650. [Google Scholar]
- Huo, H.; Wang, Y.; Wang, D.; Wang, Y.; Chen, X.; Zhao, L.; Chen, H. Duck RIG-I restricts duck enteritis virus infection. Vet. Microbiol. 2019, 230, 78–85. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Sanchez, D.J.; Cheng, G. New developments in the induction and antiviral effectors of type I interferon. Curr. Opin. Immunol. 2011, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Fish, E.N. The yin and yang of viruses and interferons. Trends Immunol. 2012, 33, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.-H.; Schneider, W.M.; Rice, C.M. Interferons and viruses: An evolutionary arms race of molecular interactions. Trends Immunol. 2015, 36, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Barbalat, R.; Ewald, S.E.; Mouchess, M.L.; Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 2011, 29, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U. Mechanisms of RIG-I-like receptor activation and manipulation by viral pathogens. J. Virol. 2014, 88, 5213–5216. [Google Scholar] [CrossRef]
- Porritt, R.A.; Hertzog, P.J. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 2015, 36, 150–160. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Xu, P. TBK1, a central kinase in innate immune sensing of nucleic acids and beyond. Acta Biochim. Biophys. Sin. 2020, 52, 757–767. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Hillesheim, A.; Nordhoff, C.; Boergeling, Y.; Ludwig, S.; Wixler, V. β-catenin promotes the type I IFN synthesis and the IFN-dependent signaling response but is suppressed by influenza A virus-induced RIG-I/NF-κB signaling. Cell Commun. Signal. 2014, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; TenOever, B.R.; Grandvaux, N.; Zhou, G.-P.; Lin, R.; Hiscott, J. Triggering the interferon antiviral response through an IKK-related pathway. Science 2003, 300, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Miyahira, A.; Sun, J.; Liu, Y.; Cheng, G.; Liang, H. Crystal structure of the ubiquitin-like domain of human TBK1. Protein Cell 2012, 3, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Li, Y.; Chen, H.; Ni, J.; Bi, D.; Luo, R.; Jin, H. Functional characterization of duck TBK1 in IFN-β induction. Cytokine 2018, 111, 325–333. [Google Scholar] [CrossRef]
- Byrne, K.M.; Horohov, D.W.; Kousoulas, K.G. Glycoprotein B of bovine herpesvirus-1 binds heparin. Virology 1995, 209, 230–235. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Littel-Van Den Hurk, S.V.D.; Liang, X.; Babiuk, L.A. Functional analysis of the transmembrane anchor region of bovine herpesvirus 1 glycoprotein gB. Virology 1997, 228, 39–54. [Google Scholar] [CrossRef][Green Version]
- Kühn, J.; Kramer, M.; Willenbacher, W.; Wieland, U.; Lorentzen, E.; Braun, R. Identification of herpes simplex virus type 1 glycoproteins interacting with the cell surface. J. Virol. 1990, 64, 2491–2497. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Lan, X.; Ye, F.; Tian, K.; Zhao, X.; Yin, H.; Li, D.; Xu, H.; Liu, Y.; et al. Molecular characterization, expression of chicken TBK1 gene and its effect on IRF3 signaling pathway. PLoS ONE 2017, 12, e0177608. [Google Scholar] [CrossRef]
- Hou, F.; Sun, L.; Zheng, H.; Skaug, B.; Jiang, Q.-X.; Chen, Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011, 146, 448–461. [Google Scholar] [CrossRef]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.-T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef]
- Lei, C.-Q.; Zhong, B.; Zhang, Y.; Zhang, J.; Wang, S.; Shu, H.-B. Glycogen synthase kinase 3β regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity 2010, 33, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Moller, M.; Wasel, J.; Schmetzer, J.; Weiss, U.; Meissner, M.; Schiffmann, S.; Weigert, A.; Moser, C.V.; Niederberger, E. The Specific IKKε/TBK1 Inhibitor Amlexanox Suppresses Human Melanoma by the Inhibition of Autophagy, NF-κaB and MAP Kinase Pathways. Int. J. Mol. Sci. 2020, 21, 4721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, M.; Yu, Z.; Tang, S.; Wang, L.; Cao, X.; Chen, T. The tyrosine kinase Src promotes phosphorylation of the kinase TBK1 to facilitate type I interferon production after viral infection. Sci. Signal. 2017, 10, eaae0435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Ding, X.J.; Dai, H.Y.; Peng, W.S.; Guo, N.F.; Zhang, Y.; Zhou, Q.L.; Chen, X.L. SB-216763, a GSK-3β inhibitor, protects against aldosterone-induced cardiac, and renal injury by activating autophagy. J. Cell. Biochem. 2018, 119, 5934–5943. [Google Scholar] [CrossRef]
- Nociari, M.; Ocheretina, O.; Murphy, M.; Falck-Pedersen, E. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J. Virol. 2009, 83, 4081–4091. [Google Scholar] [CrossRef][Green Version]
- Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007, 282, 15325–15329. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′-3′) | Accession Number | Target Region (bp) |
|---|---|---|---|
| TBK1-F | CAGACCATCGATATGCAGGACTTCAAATTA | XM_027456350.2 | 191–2375 |
| TBK1-R | ATTTGCGGCCGCGATACAGTCCACATTCC |
| siRNAs | Sense (5′–3′) | Antisense (5′–3′) | |
|---|---|---|---|
| siTBK1 | TBK1-190 | GCUGUUGUCUGACAUUCUATT | UAGAAUGUCAGACAACAGCTT |
| TBK1-1436 | GCCUGUAGAGUUGCCAGUUTT | AACUGGCAACUCUACAGGCTT | |
| TBK1-1633 | GGAAUCAUCAGAAGUGGAUTT | AUCCACUUCUGAUGAUUCCTT | |
| siNC | negative control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| Primer Name | Sequence (5′-3′) | Accession Number | Target Region (bp) |
|---|---|---|---|
| TBK1-qF | ACTGCTCACACCTGTCCTTG | KY963947.1 | 825–925 |
| TBK1-qR | TTCGGTGCAGGATGTCACTT | ||
| IFN-β-qF | AGATGGCTCCCAGCTCTACA | NM_001310827.2 | 213–422 |
| IFN-β-qR | AGTGGTTGAGCTGGTTGAGG | ||
| OASL-qF | AAGAAGATCAACAGCCGCCA | KU569293.1 | 1398–1508 |
| OASL-qR | CTTGGGCTGGACGTTGTAGT | ||
| IFITM1-qF | TCAAGGCCCGAGATAGGACA | KX811738.1 | 233–347 |
| IFITM1-qR | ATGATGGTGCCCACGATACC | ||
| GAPDH-qF | GCCTCTTGCACCACCAACT | AY436595.1 | 344–436 |
| GAPDH-qR | GGCATGGACAGTGGTCATAAGAC | ||
| DEV-qF | TGGGAAGGCTTTCGGTCGC | Reference [12] | |
| DEV-qR | CATTCGCGCCTTTGCTAAATTCTCT | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Huo, H.; Werid, G.M.; Ibrahim, Y.M.; Tang, L.; Wang, Y.; Chen, H. TBK1 Mediates Innate Antiviral Immune Response against Duck Enteritis Virus. Viruses 2022, 14, 1008. https://doi.org/10.3390/v14051008
Wang D, Huo H, Werid GM, Ibrahim YM, Tang L, Wang Y, Chen H. TBK1 Mediates Innate Antiviral Immune Response against Duck Enteritis Virus. Viruses. 2022; 14(5):1008. https://doi.org/10.3390/v14051008
Chicago/Turabian StyleWang, Dongfang, Hong Huo, Gebremeskel Mamu Werid, Yassein M. Ibrahim, Lijie Tang, Yue Wang, and Hongyan Chen. 2022. "TBK1 Mediates Innate Antiviral Immune Response against Duck Enteritis Virus" Viruses 14, no. 5: 1008. https://doi.org/10.3390/v14051008
APA StyleWang, D., Huo, H., Werid, G. M., Ibrahim, Y. M., Tang, L., Wang, Y., & Chen, H. (2022). TBK1 Mediates Innate Antiviral Immune Response against Duck Enteritis Virus. Viruses, 14(5), 1008. https://doi.org/10.3390/v14051008








