Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection
Abstract
:1. Introduction
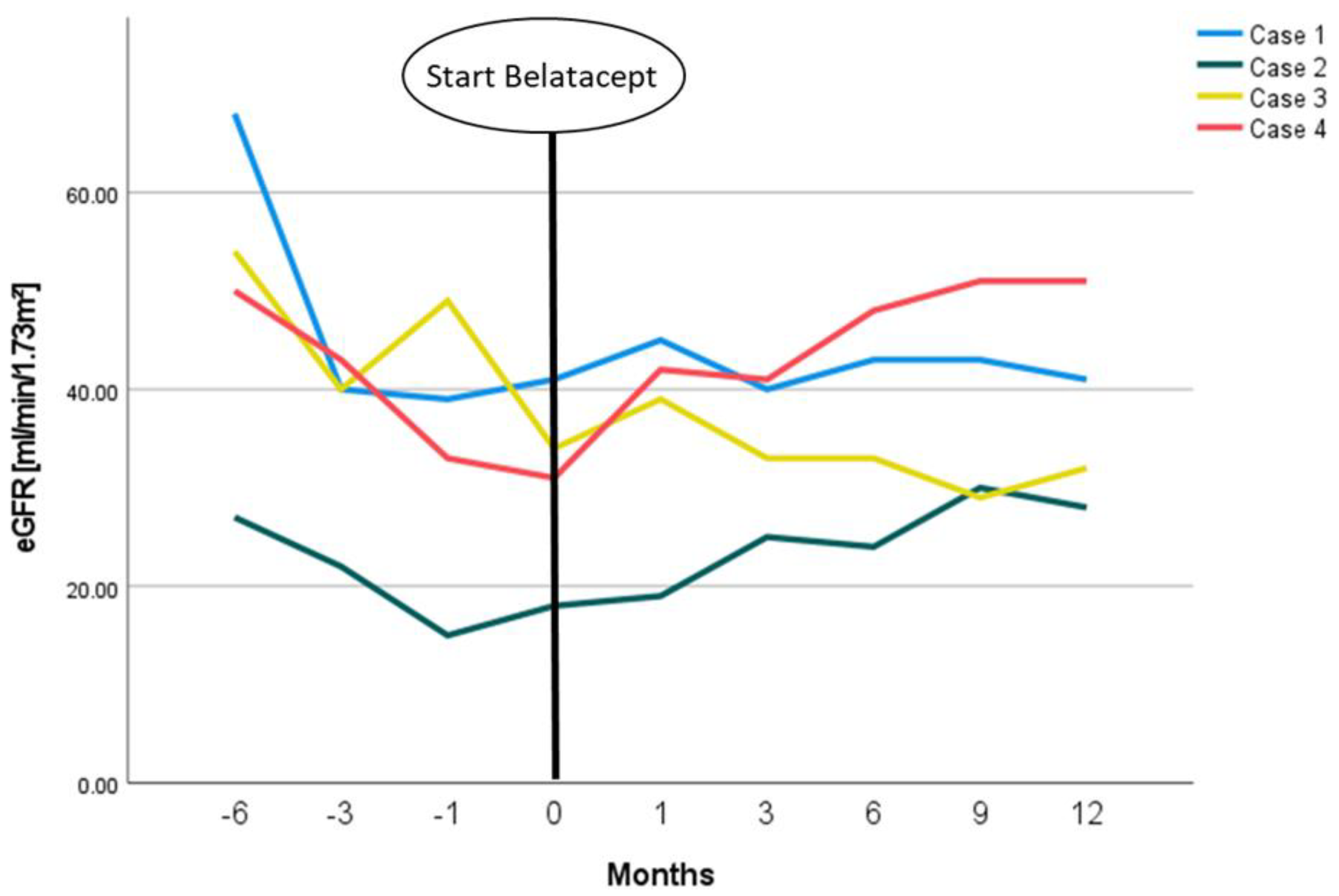
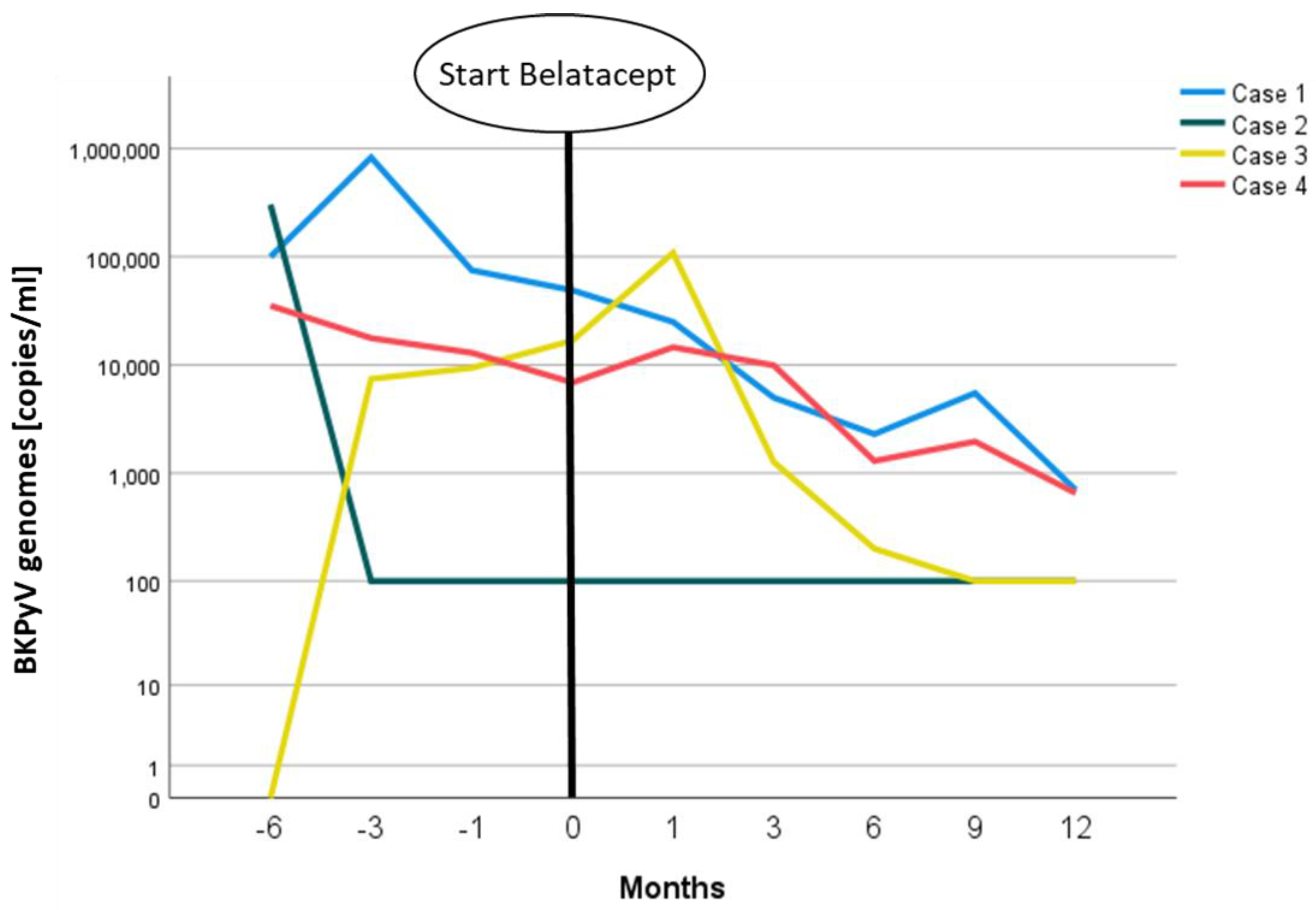
2. Case Presentations
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Knowles, W.A.; Pipkin, P.; Andrews, N.; Vyse, A.; Minor, P.; Brown, D.W.G.; Miller, E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 2003, 71, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P. BK virus in solid organ transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 4), S136–S146. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 2009, 199, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginevri, F.; de Santis, R.; Comoli, P.; Pastorino, N.; Rossi, C.; Botti, G.; Fontana, I.; Nocera, A.; Cardillo, M.; Ciardi, M.R.; et al. Polyomavirus BK infection in pediatric kidney-allograft recipients: A single-center analysis of incidence, risk factors, and novel therapeutic approaches. Transplantation 2003, 75, 1266–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, D.C.; Agha, I.; Bohl, D.L.; Schnitzler, M.A.; Hardinger, K.L.; Lockwood, M.; Torrence, S.; Schuessler, R.; Roby, T.; Gaudreault-Keener, M.; et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 2005, 5, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P. BK polyomavirus in solid organ transplantation. Am. J. Transplant. 2013, 13 (Suppl. 4), 179–188. [Google Scholar] [CrossRef]
- Kuypers, D.R.J. Management of polyomavirus-associated nephropathy in renal transplant recipients. Nat. Rev. Nephrol. 2012, 8, 390–402. [Google Scholar] [CrossRef]
- Bussalino, E.; Marsano, L.; Parodi, A.; Russo, R.; Massarino, F.; Ravera, M.; Gaggero, G.; Fontana, I.; Garibotto, G.; Zaza, G.; et al. Everolimus for BKV nephropathy in kidney transplant recipients: A prospective, controlled study. J. Nephrol. 2021, 34, 531–538. [Google Scholar] [CrossRef]
- Liacini, A.; Seamone, M.E.; Muruve, D.A.; Tibbles, L.A. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: Toward a new therapy for BK virus infection. Transplantation 2010, 90, 1450–1457. [Google Scholar] [CrossRef]
- Sommerer, C.; Suwelack, B.; Dragun, D.; Schenker, P.; Hauser, I.A.; Witzke, O.; Hugo, C.; Kamar, N.; Merville, P.; Junge, M.; et al. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019, 96, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.P.; Sommerer, C.; Witzke, O.; Tedesco, H.; Chadban, S.; Mulgaonkar, S.; Qazi, Y.; de Fijter, J.W.; Oppenheimer, F.; Cruzado, J.M.; et al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am. J. Transplant. 2019, 19, 3018–3034. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Jouve, T.; Janbon, B.; Rostaing, L.; Malvezzi, P. Belatacept in kidney transplantation and its limitations. Expert Rev. Clin. Immunol. 2019, 15, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.A.; Gebel, H.M.; Townsend, R.; Roberts, M.E.; Polinsky, M.; Yang, L.; Meier-Kriesche, H.-U.; Larsen, C.P. De novo donor-specific antibodies in belatacept-treated vs. cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am. J. Transplant. 2018, 18, 1783–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Durrbach, A.; Pestana, J.M.; Pearson, T.; Vincenti, F.; Garcia, V.D.; Campistol, J.; Del Rial, M.C.; Florman, S.; Block, A.; Di Russo, G.; et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am. J. Transplant. 2010, 10, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Terrec, F.; Jouve, T.; Malvezzi, P.; Janbon, B.; Naciri Bennani, H.; Rostaing, L.; Noble, J. Belatacept Use after Kidney Transplantation and Its Effects on Risk of Infection and COVID-19 Vaccine Response. J. Clin. Med. 2021, 10, 5159. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.; Kamar, N.; Agarwal, A.; de Fijter, J.; Rostaing, L.; Berger, S.; Djamali, A.; et al. Conversion from Calcineurin Inhibitor to Belatacept-based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021. [Google Scholar] [CrossRef]
- Rostaing, L.; Massari, P.; Garcia, V.D.; Mancilla-Urrea, E.; Nainan, G.; Del Carmen Rial, M.; Steinberg, S.; Vincenti, F.; Shi, R.; Di Russo, G.; et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: A randomized phase II study. Clin. J. Am. Soc. Nephrol. 2011, 6, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Chavarot, N.; Divard, G.; Scemla, A.; Amrouche, L.; Aubert, O.; Leruez-Ville, M.; Timsit, M.O.; Tinel, C.; Zuber, J.; Legendre, C.; et al. Increased incidence and unusual presentations of CMV disease in kidney transplant recipients after conversion to belatacept. Am. J. Transplant. 2021, 21, 2448–2458. [Google Scholar] [CrossRef]
- Kleiboeker, H.L.; Jorgenson, M.R.; Smith, J.A. Belatacept may result in profound and persistent loss of cytomegalovirus-specific cell-mediated immunity: A case report. Transpl. Infect. Dis. 2022, e13795. [Google Scholar] [CrossRef]
- Bassil, N.; Rostaing, L.; Mengelle, C.; Kallab, S.; Esposito, L.; Guitard, J.; Cardeau-Desangles, I.; Weclawiak, H.; Izopet, J.; Kamar, N. Prospective monitoring of cytomegalovirus, Epstein-Barr virus, BK virus, and JC virus infections on belatacept therapy after a kidney transplant. Exp. Clin. Transplant. 2014, 12, 212–219. [Google Scholar] [PubMed]
- Simon, T.A.; Dong, L.; Winthrop, K.L. Risk of opportunistic infections in patients with rheumatoid arthritis initiating abatacept: Cumulative clinical trial data. Arthritis Res. Ther. 2021, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Powell, J.T.; Patel, M.; Tsapepas, D. Risk of posttransplant lymphoproliferative disorder associated with use of belatacept. Am. J. Health Syst. Pharm. 2013, 70, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Ruiss, R.; Jochum, S.; Wanner, G.; Reisbach, G.; Hammerschmidt, W.; Zeidler, R. A virus-like particle-based Epstein-Barr virus vaccine. J. Virol. 2011, 85, 13105–13113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühne, J.F.; Neudörfl, C.; Beushausen, K.; Keil, J.; Malysheva, S.; Wandrer, F.; Haller, H.; Messerle, M.; Blume, C.; Neuenhahn, M.; et al. Differential effects of Belatacept on virus-specific memory versus de novo allo-specific T cell responses of kidney transplant recipients and healthy donors. Transpl. Immunol. 2020, 61, 101291. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Wilhelm, M.; Wilk, S.; Hirsch, H.H. BK polyomavirus-specific antibody and T-cell responses in kidney transplantation: Update. Curr. Opin. Infect. Dis. 2019, 32, 575–583. [Google Scholar] [CrossRef]
- Binggeli, S.; Egli, A.; Dickenmann, M.; Binet, I.; Steiger, J.; Hirsch, H.H. BKV replication and cellular immune responses in renal transplant recipients. Am. J. Transplant. 2006, 6, 2218–2219. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwun, J.; Gibby, A.C.; Hong, J.J.; Farris, A.B.; Iwakoshi, N.N.; Villinger, F.; Kirk, A.D.; Knechtle, S.J. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am. J. Transplant. 2014, 14, 59–69. [Google Scholar] [CrossRef]
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sex | male | male | male | male |
| Age at start belatacept (years) | 57.3 | 59.4 | 80.5 | 52.9 |
| Time since KTx (months) | 8.5 | 8.0 | 7.0 | 11.8 |
| PRA % | 0 | 0 | 0 | 0 |
| Donation type | ABO-I living donor KTx | postmortal | postmortal | ABO-I living donor KTx |
| Time on dialysis before KTx (months) | 12 | 118 | 49 | 10 |
| Previous KTx | none | none | none | none |
| CMV mismatch | D−/R+ | D−/R− | D−/R+ | D−/R+ |
| Induction therapy | ATG + RTX | Basiliximab | ATG | ATG + RTX |
| Initial immunosuppressive regimen | Tac/MMF/Pred | Tac/MMF/Pred | Tac/MMF/Pred | Tac/MMF/Pred |
| Diagnosis of ESRD | Hypertensive nephropathy | Medullary sponge kidneys | Hypertensive nephropathy | IgA nephropathy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehn, U.; Siam, S.; Wiening, V.; Pavenstädt, H.; Reuter, S. Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection. Viruses 2022, 14, 1005. https://doi.org/10.3390/v14051005
Jehn U, Siam S, Wiening V, Pavenstädt H, Reuter S. Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection. Viruses. 2022; 14(5):1005. https://doi.org/10.3390/v14051005
Chicago/Turabian StyleJehn, Ulrich, Sami Siam, Vanessa Wiening, Hermann Pavenstädt, and Stefan Reuter. 2022. "Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection" Viruses 14, no. 5: 1005. https://doi.org/10.3390/v14051005
APA StyleJehn, U., Siam, S., Wiening, V., Pavenstädt, H., & Reuter, S. (2022). Belatacept as a Treatment Option in Patients with Severe BK Polyomavirus Infection and High Immunological Risk—Walking a Tightrope between Viral Control and Prevention of Rejection. Viruses, 14(5), 1005. https://doi.org/10.3390/v14051005






