Abstract
Although surgical resection is available as a potentially curative therapy for hepatocellular carcinoma (HCC), high recurrence of HCC after surgery remains a serious obstacle for long-term patient survival. Therefore, the discovery of valuable prognostic biomarkers for HCC recurrence is urgently needed. Pre-S2 mutant is a mutant form of hepatitis B virus (HBV) large surface protein which is expressed from the HBV surface gene harboring deletion mutations spanning the pre-S2 gene segment. Pre-S2 mutant-positive HCC patients have been regarded as a high-risk population of HCC recurrence after resection surgery and display increased immune checkpoint programmed death ligand 1 (PD-L1) expression and pro-tumor regulatory T cells (Tregs) infiltration in tumor tissues. In this study, the association of higher levels of PD-L1 expression and Tregs infiltration in tumor tissues with post-operative HCC recurrence in pre-S2 mutant-positive HCC patients was evaluated. We found that patients with pre-S2 mutant in combination with higher levels of PD-L1 expression and Tregs infiltration in tumor tissues were independently associated with a higher risk of HCC recurrence (hazard ratio, 4.109; p value = 0.0011) and poorer recurrence-free survival (median, 8.2 versus 18.0 months; p value = 0.0004) than those of patients with either one or two of these three biomarkers. Furthermore, a combination of pre-S2 mutant, intra-tumoral PD-L1 expression, and tumor-infiltrating Tregs exhibited superior performance in identifying patients at a higher risk of HCC recurrence (area under the receiver operating characteristic curve, 0.8400). Collectively, this study suggests that higher levels of PD-L1 expression and Tregs infiltration in tumor tissues predicted a higher risk of HCC recurrence in pre-S2 mutant-positive HCC patients after curative surgical resection.
1. Introduction
As a predominant type of liver cancer, hepatocellular carcinoma (HCC) ranks as the sixth most frequent and third most fatal human cancer worldwide, resulting in over 800,000 deaths per year [1,2,3,4]. Among many therapeutic options for HCC patients, curative surgical resection is considered as one of the most potent treatments [5,6,7,8]. However, the HCC recurrence rate of up to 80% within 5 years after curative surgical resection remains a big challenge, responsible for a 5-year patient survival rate of only 30% [9,10,11]. Therefore, the discovery of potential biomarkers and therapeutic strategies for prediction and prevention of HCC recurrence is urgently needed to improve patient outcomes after resection surgery.
As a major etiological factor of HCC, chronic hepatitis B virus (HBV) infection accounts for nearly 50% of total HCC cases worldwide [4,12,13]. HBV is a lipid-enveloped hepatotropic virus and has a DNA genome incorporated into the viral nucleocapsid [14,15]. The HBV surface gene consists of three gene segments, namely the pre-S1, the pre-S2, and the S, and encodes three different sizes of surface proteins (small, middle, and large) from the S segment, the pre-S2 and S segments, and all three segments, respectively, which collectively constitute the envelope proteins of viral particles. Moreover, integration of HBV DNA into the host cell genomes is a frequent event occurred in HBV-related HCC, resulting in the induction of insertional mutagenesis and genomic instability and the persistent expression of viral proteins, particularly HBV surface and X proteins [16,17,18].
Several naturally occurring in-frame deletion mutations spanning the pre-S2 gene segment (predominantly nucleotides 1 to 54) with or without point mutations at the start codon of the pre-S2 gene segment of HBV surface gene have been clinically identified, leading to the expression of a mutant form of HBV large surface protein called the pre-S2 mutant and defective synthesis of the middle surface protein [19,20,21]. Because the region of nucleotides 1 to 54 in the pre-S2 gene segment overlaps the nucleocapsid-binding domain of HBV large surface protein, deletion of this region abrogates the assembly and secretion of viral particles [22,23]. Moreover, the region of nucleotides 1 to 54 in the pre-S2 gene segment coincides with the neutralization B cell and cytotoxic T cell epitopes of HBV large surface protein [24,25], suggesting that pre-S2 mutant may represent an immune escape mutant in chronic HBV infection. It has been well demonstrated that pre-S2 mutant is an important HBV oncoprotein that accumulates in the endoplasmic reticulum (ER) to induce ER stress and through which multiple oncogenic signaling pathways involved in cell proliferation are activated, including cell cycle progression, survival, metabolism, and genomic instability, to promote hepatocyte malignant transformation and HCC development [19,26,27,28,29,30,31,32,33,34,35,36,37,38]. Targeting at the pre-S2 mutant-activated oncogenic signals exhibits a preventive effect on HCC formation [31,39,40,41]. Furthermore, in patients with chronic hepatitis B and HBV-related HCC, the presence of pre-S2 mutant in blood and liver tissues serves as an independent biomarker for higher incidences of HCC development and recurrence after curative surgical resection, respectively [42,43,44,45,46,47,48,49]. Therefore, there is an urgent need to develop potential therapeutic strategies for treating the pre-S2 mutant-positive high-risk patient populations.
As a key hallmark of cancer, tumor cells can evade host immune surveillance through many mechanisms such as induction of immune checkpoint programmed death ligand 1 (PD-L1) expression and activation of pro-tumor regulatory T cells (Tregs) function to suppress cytotoxic T cells (CTLs)-mediated anti-tumor immune responses [50,51,52,53,54,55]. Multiple lines of evidence indicate that a higher level of PD-L1 expression or Tregs infiltration in tumor tissues of HCC patients is associated with poorer prognosis after curative surgical resection [56,57,58,59,60,61,62,63,64,65]. In addition, the pre-S2 mutant-positive HCC patients have been shown to display increased expression of intra-tumoral PD-L1 and elevated number of tumor-infiltrating Tregs compared with pre-S2 mutant-negative HCC patients [66,67]. However, whether the higher levels of PD-L1 expression and Tregs infiltration in tumor tissues of pre-S2 mutant-positive HCC patients predict a higher risk of HCC recurrence after curative surgical resection remain to be clarified.
In this study, 40 HBV-related HCC patients were enrolled and classified into pre-S2 mutant-positive and -negative groups of patients. The expression of PD-L1 and infiltration of Tregs in tumor tissues of patients were determined. The association between the levels of PD-L1 expression and Tregs infiltration in tumor tissues and the rate of HCC recurrence after curative surgical resection in pre-S2 mutant-positive HCC patients were statistically analyzed.
2. Materials and Methods
2.1. Collection of Patient Specimens
Pre-operative plasma and formalin-fixed and paraffin-embedded (FFPE) liver tissues were retrospectively collected from 40 HBV-related HCC patients who were positive for serum HBV surface antigen (HBsAg) and underwent curative surgical resection at China Medical University Hospital (Taichung, Taiwan) under the approval of the China Medical University & Hospital Research Ethics Committee (protocol No. CMUH107-REC1-080) from January 2006 to July 2017. Clinicopathological data of the patients were also retrieved from the hospital records. All research was conducted in accordance with the guidelines of the 1975 Declaration of Helsinki and informed consent was obtained from all participants.
2.2. Detection of Deletion Mutations Spanning the Pre-S2 Gene Segment in Plasma
Deletion mutations spanning the pre-S2 gene segment of HBV surface gene were detected in plasma of HBV-related HCC patients by using a next-generation sequencing (NGS)-based approach as previously described [68,69,70]. Briefly, plasma DNA was extracted by using the DNeasy Blood Kit (Qiagen, Valencia, CA, USA) and served as a template for amplification of the entire HBV pre-S gene (comprising the pre-S1 and pre-S2 gene segments) by polymerase chain reaction (PCR) using the high-fidelity Platinum SuperFi DNA polymerase (Invitrogen, Carlsbad, CA, USA). The resulting pre-S gene PCR products were subsequently analyzed by NGS using the NextSeq 500 System supplemented with the bcl2fastq Conversion Software v2.20 (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. Pre-S2 mutant-positive and -negative patients were defined according to the presence and absence of deletion mutations spanning the pre-S2 gene segment in plasma, respectively (Table S1).
2.3. Detection of Intratumoral PD-L1 and Tumor-Infiltrating Tregs in Liver Tissues
Intra-tumoral PD-L1 expression and tumor-infiltrating Tregs were detected in FFPE liver tissues of HBV-related HCC patients by using a fluorescent immunohistochemistry (IHC) staining-based approach as previously described [66,67]. Briefly, 4-μm-thick tissue sections were incubated with the primary antibodies mouse anti-PD-L1 (AM26531AF-N; Acris, Hiddenhausen, Germany) for detection of PD-L1 or rabbit anti-CD4 (ab133616; Abcam, Cambridge, UK) together with mouse anti-CD25 (Leica Biosystems, Buffalo Grove, IL, USA) for detection of Tregs, followed by the secondary antibodies Alexa Fluor 555-conjugated anti-mouse IgG (A-21424; Invitrogen) and/or Alexa Fluor 488-conjugated anti-rabbit IgG (A11008; Invitrogen). Nuclei were counterstained with 4ʹ, 6-diamidino-2-phenylindole (DAPI; Invitrogen). Additionally, histopathology of the tissue sections was examined by hematoxylin and eosin (H&E) staining to define the tumor regions. Whole-slide images of the fluorescent IHC- and H&E-stained tissue sections were obtained by using the 3DHistech Pannoramic SCAN II scanner (3DHistech, Budapest, Hungary) and Aperio CS2 scanner (Leica Biosystems), respectively. Total number of PD-L1-expressing cells or Tregs (CD4 and CD25 double-expressing cells) in 10 microscopic fields (original magnification, ×40; area, 1.566 mm2 per field) independently selected with the most abundant PD-L1-expressing cells or Tregs in the tumor region of each patient’s tissue section was counted and presented as the density (number of cells per mm2; cells/mm2) for statistical analysis (Figure S1).
2.4. Statistical Analysis
Clinicopathological correlation was analyzed by the chi-square test. Univariate and multivariate recurrence-free survival (RFS) analyses were conducted by the Cox proportional-hazards regression model. RFS curves were generated by the Kaplan–Meier method and compared by the log-rank test. Receiver operating characteristic (ROC) curves were established to differentiate patients with HCC recurrence from patients without HCC recurrence and the area under the ROC curve (AUC) was compared by the Hanley–McNeil test. A p value of < 0.05 was considered statistically significant.
3. Results
3.1. Association of HBV Pre-S2 Mutant-Positive HCC Patients with Higher Levels of PD-L1 Expression and Tregs Infiltration in Tumor Tissues
The clinicopathological profiles and correlation of the 40 HBV-related HCC patients enrolled in this study are summarized in Table 1. Among all patients, there were 34 men and six women and the median age was 40 years (range, 28–78). Of these patients, 36 out of 40 had detectable HBV DNA at a median level of 5.0 log10 copies/mL (range, 1.5–8.2) and 34 of 40 were negative for HBV e antigen (HBeAg). All patients had a median tumor size of 4.0 cm (range, 1.5–35.0) and received curative surgical resection, among whom 25 patients suffered post-operative HCC recurrence with a median RFS of 11.2 months (range, 1.5–72.3). According to fluorescent IHC staining-based analysis, the median densities of PD-L1-expressing cells and Tregs in tumor tissues of all patients were 14.37 cells/mm2 (range, 3.45–32.38) and 8.27 cells/mm2 (range, 0.89–19.60), respectively (Table 2). In addition, by NGS-based analysis, all patients were divided into 19 pre-S2 mutant-negative and 21 pre-S2 mutant-positive patients. The correlation between pre-S2 mutant and PD-L1 expression or Tregs infiltration in tumor tissues or other clinicopathological characteristics was evaluated and showed that the pre-S2 mutant-positive HCC patients were significantly associated with higher densities of PD-L1-expressing cells (median (range), 18.20 (6.96–32.38) versus 7.09 (3.45–18.07) cells/mm2; p value < 0.0001) and Tregs (median (range), 12.84 (8.05–19.60) versus 2.17 (0.89–6.96) cells/mm2; p value < 0.0001) in tumor tissues than the pre-S2 mutant-negative HCC patients (Table 2). The densities of PD-L1-expressing cells and Tregs in tumor tissues were further divided into high and low densities by using the median density as a cut-off value. Up to 18 (85%) and 20 (95%) of 21 pre-S2 mutant-positive patients had high densities of PD-L1-expressing cells and Tregs in tumor tissues but only two (10%) and none of 19 pre-S2 mutant-negative patients had high densities of PD-L1-expressing cells and Tregs, respectively (Table 2).

Table 1.
Clinicopathological profiles and correlation of the 40 HBV-related HCC patients.

Table 2.
Densities of PD-L1-expressing cells and Tregs in tumor tissues of the 40 HBV-related HCC patients.
3.2. Association of Higher Levels of PD-L1 Expression and Tregs Infiltration in Tumor Tissues of HBV Pre-S2 Mutant-Positive HCC Patients with a Higher Risk of HCC Recurrence and Poorer RFS after Curative Surgical Resection
The correlation of pre-S2 mutant, intra-tumoral PD-L1 expression, tumor-infiltrating Tregs, and other clinicopathological characteristics with HCC recurrence after curative surgical resection was assessed by univariate and multivariate analyses as well as RFS curves. In univariate analysis, the presence of deletion mutations spanning the pre-S2 gene segment in plasma or a high density of PD-L1-expressing cells or Tregs in tumor tissues was significantly associated with a higher risk of HCC recurrence (hazard ratio (HR) (95% confidence interval (CI)), 2.378 (1.015–5.573), p value = 0.0461; 3.379 (1.446–7.900), p value = 0.0049; 2.769 (1.180–6.498), p value = 0.0193, respectively) and poorer RFS (median (range), 7.7 (0.8–72.3) versus 18.5 (1.8–43.7) months, p value = 0.0396; 7.1 (0.8–72.3) versus 19.0 (2.1–61.1) months, p value = 0.0029; 8.2 (0.8–72.3) versus 18.0 (1.8–43.7) months, p value = 0.0146, respectively) than those of the absence of deletion mutations spanning the pre-S2 gene segment or a low density of PD-L1-expressing cells or Tregs (Table 3 and Figure 1A–C). A combination of the presence of deletion mutations spanning the pre-S2 gene segment and a high density of PD-L1-expressing cells or Tregs or a combination of high densities of PD-L1-expressing cells and Tregs was significantly associated with a higher risk of HCC recurrence (HR (95% CI), 3.315 (1.452–7.570), p value = 0.0044; 2.769 (1.180–6.498), p value = 0.0193; 3.928 (1.716–8.995), p value = 0.0012, respectively) and poorer RFS (median (range), 7.7 (0.8–72.3) versus 18.5 (1.8–61.1) months, p value = 0.0026; 8.2 (0.8–72.3) versus 18.0 (1.8–43.7) months, p value = 0.0146; 8.2 (0.8–72.3) versus 18.0 (1.8–61.1) months, p value = 0.0005, respectively) than those of other combinations (Table 3 and Figure 1D–F). Moreover, a combination of the presence of deletion mutations spanning the pre-S2 gene segment and high densities of PD-L1-expressing cells and Tregs was significantly associated with a higher risk of HCC recurrence (HR (95% CI), 4.109 (1.763–9.572), p value = 0.0011) and poorer RFS (median (range), 8.2 (0.8–72.3) versus 18.0 (1.8–43.7) months, p value = 0.0004) than those of other combinations (Table 3 and Figure 1G). When all patients were further classified into distinct groups according to their status at deletion mutations spanning the pre-S2 gene segment and densities of PD-L1-expressing cells and Tregs, 17 patients fell into Group 1 (absence of deletion mutations spanning the pre-S2 gene segment and low densities of PD-L1-expressing cells and Tregs), two patients fell into Group 2 (absence of deletion mutations spanning the pre-S2 gene segment, high density of PD-L1-expressing cells, and low density of Tregs), one patient fells into Group 3 (presence of deletion mutations spanning the pre-S2 gene segment, high density of PD-L1-expressing cells, and low density of Tregs), three patients fell into Group 4 (presence of deletion mutations spanning the pre-S2 gene segment, low density of PD-L1-expressing cells, and high density of Tregs), and 17 patients fell into Group 5 (presence of deletion mutations spanning the pre-S2 gene segment and high densities of PD-L1-expressing cells and Tregs). Only Group 5, rather than other groups of patients, had a significantly higher risk of HCC recurrence (HR (95% CI), 3.593 (1.456–8.862), p value = 0.0055) and poorer RFS (median (range), 8.2 (0.8–72.3) versus 19.0 (3.0–43.7) months, p value = 0.0032) than Group 1 (Table 3 and Figure 1H). Furthermore, in multivariate analysis, a high density of PD-L1-expressing cells, a combination of high densities of PD-L1-expressing cells and Tregs, and a combination of the presence of deletion mutations spanning the pre-S2 gene segment and high densities of PD-L1-expressing cells and Tregs, were shown as independent biomarkers for predicting a higher risk of HCC recurrence (HR (95% CI), 2.663 (1.069–6.632), p value = 0.0354; 2.580 (1.022–6.516), p value = 0.0449; 3.163 (1.309–7.644), p value = 0.0105, respectively) (Table 3). The Group 5 of patients were independently associated with a higher risk of HCC recurrence (HR (95% CI), 2.837 (1.048–7.679), p value = 0.0401) than that of the Group 1 of patients (Table 3).

Table 3.
Univariate and multivariate recurrence-free survival analyses of the 40 HBV-related HCC patients.
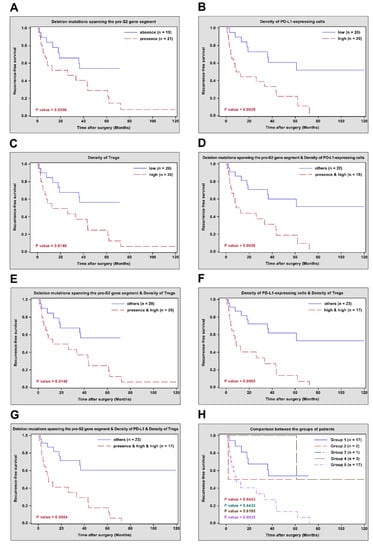
Figure 1.
RFS curves in different groups of HBV-related HCC patients after curative surgical resection. (A) RFS curves in patients with presence versus absence of deletion mutations spanning the pre-S2 gene segment. (B) RFS curves in patients with high versus low density of PD-L1-expressing cells. (C) RFS curves in patients with high versus low density of Tregs. (D) RFS curves in patients with the presence of deletion mutations spanning the pre-S2 gene segment and high density of PD-L1-expressing cells versus other combinations. (E) RFS curves in patients with the presence of deletion mutations spanning the pre-S2 gene segment and high density of Tregs versus other combinations. (F) RFS curves in patients with high densities of PD-L1-expressing cells and Tregs versus other combinations. (G) RFS curves in patients with the presence of deletion mutations spanning the pre-S2 gene segment and high densities of PD-L1-expressing cells and Tregs versus other combinations. (H) RFS curves in the Groups 2, 3, 4, and 5 of patients versus the Group 1 of patients, respectively. The number of patients in each group and p values between different groups of patients were indicated in the plots. Abbreviations: RFS, recurrence-free survival; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; PD-L1, programmed death ligand 1; Tregs, regulatory T cells; n, number.
3.3. Superior Performance of HBV Pre-S2 Mutant in Combination with Higher Levels of PD-L1 Expression and Tregs Infiltration in Tumor Tissues in Identifying Patients at Higher Risk of HCC Recurrence after Curative Surgical Resection
The prognostic performance of pre-S2 mutant, intra-tumoral PD-L1 expression, tumor-infiltrating Tregs, and a combination of either two or all of these three biomarkers for HCC recurrence after curative surgical resection was evaluated by ROC curves. As shown in Figure 2, a combination of deletion mutations spanning the pre-S2 gene segment, density of PD-L1-expressing cells, and density of Tregs had the highest AUC (0.8400, 95% CI 0.7467–0.9333) followed by a combination of density of PD-L1-expressing cells and density of Tregs (0.7867, 95% CI 0.6705–0.9028), a combination of deletion mutations spanning the pre-S2 gene segment and density of PD-L1-expressing cells (0.7533, 95% CI 0.6224–0.8843), a combination of deletion mutations spanning the pre-S2 gene segment and density of Tregs (0.7400, 95% CI 0.5997–0.8803), density of PD-L1-expressing cells (0.7400, 95% CI 0.5997–0.8803), density of Tregs (0.7400, 95% CI 0.5997–0.8803), and deletion mutations spanning the pre-S2 gene segment (0.7067, 95% CI 0.5579–0.8554).
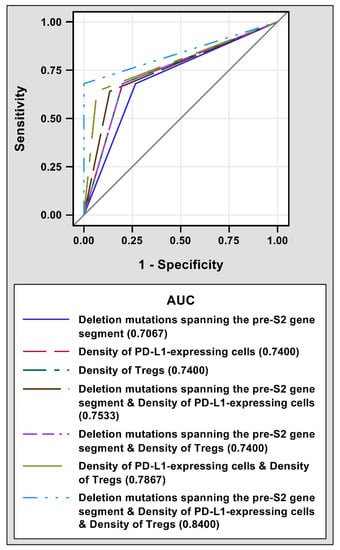
Figure 2.
ROC curves of different prognostic biomarkers for HCC recurrence after curative surgical resection. 25 patients with and 15 patients without post-operative HCC recurrence were analyzed. The AUCs for deletion mutations spanning the pre-S2 gene segment (solid dark blue line), density of PD-L1-expressing cells (dashed red line), density of Tregs (dashed dark green line), a combination of deletion mutations spanning the pre-S2 gene segment and density of PD-L1-expressing cells (dashed brown line), a combination of deletion mutations spanning the pre-S2 gene segment and density of Tregs (dashed purple line), a combination of density of PD-L1-expressing cells and density of Tregs (dashed light green line), and a combination of deletion mutations spanning the pre-S2 gene segment, density of PD-L1-expressing cells, and density of Tregs (dashed light blue line) were shown in the bottom of the plot. Abbreviations: ROC, receiver operating characteristic; HCC, hepatocellular carcinoma; AUC, area under the ROC curve; PD-L1, programmed death ligand 1; Tregs, regulatory T cells.
4. Discussion
Although surgical resection is considered as a potentially curative treatment for HCC, high recurrence rate of HCC after surgery is still a significant threat, responsible for poor patient outcomes [9,10,11]. Therefore, the development of useful biomarkers for identifying patients at a higher risk of post-operative HCC recurrence for better surveillance and management remains a key goal to improve patient survival. In this study, we found that higher levels of PD-L1 expression and Tregs infiltration in tumor tissues of HBV pre-S2 mutant-positive HCC patients were associated with a higher risk of HCC recurrence and poorer RFS after curative surgical resection. Furthermore, a combination of the presence of deletion mutations spanning the pre-S2 gene segment and high densities of PD-L1-expressing cells and Tregs was validated as an independent prognostic biomarker with better performance in predicting a higher risk of post-operative HCC recurrence than that of either one or a combination of either two of these three biomarkers.
As an important HBV oncoprotein, pre-S2 mutant can induce HCC development through activation of multiple oncogenic signaling pathways to promote hepatocyte malignant transformation, including endoplasmic reticulum stress, genomic instability, anchorage-independent cell growth, cell proliferation, cell cycle progression, cell survival, and glucose and lipid metabolism. [19,26,27,28,29,30,31,32,33,34,35,36,37,38]. Patients with pre-S2 mutant have been regarded as a high-risk population for HCC recurrence after curative surgical resection and the presence of deletion mutations spanning the pre-S2 gene segment in plasma represents an independent biomarker for predicting a higher risk of HCC recurrence after surgery [46,47,48,49]. However, there is still room for improvement in the prognostic performance of pre-S2 mutant serving as a biomarker for HCC recurrence. Recently, it has been shown that the presence of deletion mutations spanning the pre-S2 gene segment together with low serum albumin level exhibits better prognostic performance for HCC recurrence after curative surgical resection than that of either biomarker alone [71]. In this study, we further identified that patients with pre-S2 mutant in combination with higher levels of PD-L1 expression and Tregs infiltration in tumor tissues were associated with a higher risk of post-operative HCC recurrence than that of patients with either one or two of these three biomarkers.
PD-L1 and Tregs play important roles in the evasion of tumor cells from host immune surveillance in the tumor microenvironment, contributing to cancer development [50,51]. As one of the immune checkpoint molecules, PD-L1 expressed on tumor cells interacts with its receptor programmed death 1 (PD-1) expressed on CTLs, leading to inhibition of CTLs-mediated anti-tumor immune responses [52,53]. As one of the tumor-infiltrating lymphocytes, Tregs exert immunosuppressive and pro-tumor functions through suppression of the anti-tumor activities of CTLs, such as inhibition of their activation, proliferation, and secretion of cytotoxic proteins and cytokines [54,55]. Moreover, PD-L1 plays an essential role in promoting the development, expansion, and suppressive function of Tregs [72,73,74]. Consistent with the importance of PD-L1 and Tregs in tumor progression, a higher level of PD-L1 expression or Tregs infiltration in tumor tissues has been associated with poorer prognosis in HCC patients after curative surgical resection [56,57,58,59,60,61,62,63,64,65]. In this study, we further showed that a combination of higher levels of PD-L1 expression and Tregs infiltration in tumor tissues was associated with a higher risk of HCC recurrence and poorer RFS than those of either biomarker alone in pre-S2 mutant-positive HCC patients after curative surgical resection. Considering that the therapeutics targeting PD-L1 and Tregs either alone or in combination show great promise in the treatment of many cancers including HCC [75,76,77,78,79,80], the results of this study may therefore not only suggest the levels of PD-L1 expression and Tregs infiltration in tumor tissues as valuable prognostic biomarkers but also support PD-L1- and Tregs-targeted therapies as potential therapeutic options for pre-S2 mutant-positive HCC patients after curative surgical resection although the efficacy remains to be validated.
There are some limitations in this study. First, the number of patients analyzed is too small. Although the clinicopathological profiles of the patients correspond with the representative characteristics of a large population of HCC patients in Taiwan [81], a greater number of patients from different clinical centers is required to further evaluate the results of this study and its application in clinical practice. Next, the HBV genotypes of the patients enrolled in this study are mainly B and C. Although it has been shown that genotypes B and C are the most prevalent HBV strains in Asian countries including Taiwan [82] and there is no significant difference in the deletion patterns of pre-S2 gene segment between these two genotypes [83], whether the results of this study are applicable to other genotypes remains to be clarified. In addition, the underlying mechanisms of the close association between pre-S2 mutant and high densities of PD-L1-expressing cells and Tregs in HCC tissues remain unclear. Some overlap has been observed between the pre-S2 mutant-activated signaling pathways and the signaling pathways upregulating PD-L1 and Tregs such as vascular endothelial growth factor-A, transforming growth factor-β, mammalian target of rapamycin, and Myc [30,31,84,85,86,87], supporting that pre-S2 mutant may play a potential role in induction of intratumoral PD-L1 expression and Tregs infiltration.
5. Conclusions
In this study, we demonstrated that a combination of HBV pre-S2 mutant and higher levels of PD-L1 expression and Tregs infiltration in tumor tissues was an independent prognostic biomarker with greater performance in identifying patients at a higher risk of HCC recurrence after curative surgical resection than that of either one or a combination of either two of these three biomarkers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14061346/s1, Table S1: Pre-S2 gene deletion regions of the 21 HBV pre-S2 mutant-positive HCC patients; Figure S1: Representative images of PD-L1-expressing cells and Tregs in fluorescent IHC-stained HCC Tissues.
Author Contributions
Conceptualization, L.-B.J., T.-C.L., S.-C.H. and C.-F.T.; Formal analysis, L.-B.J. and T.-C.L.; Funding acquisition, L.-B.J., S.-C.H. and C.-F.T.; Investigation, L.-B.J., T.-C.L., S.-C.H. and C.-F.T.; Methodology, L.-B.J., T.-C.L., S.-C.H. and C.-F.T.; Supervision, C.-F.T.; Writing—original draft, C.-F.T.; Writing—review & editing, C.-F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Medical University Hospital, Taichung, Taiwan [grant numbers DMR-111-047].
Institutional Review Board Statement
This study was performed under the approval of the China Medical University & Hospital Research Ethics Committee (protocol No. CMUH107-REC1-080).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the paper and its supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Venook, A.P.; Papandreou, C.; Furuse, J.; de Guevara, L.L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010, 15 (Suppl. 4), 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Lin, W.-Y.; Liu, C.-S.; Lin, C.-C.; Lai, H.-C.; Lai, S.-W. Association of different types of liver disease with demographic and clinical factors. BioMedicine 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mortality, G.B.D.; Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Llovet, J.M.; Bruix, J. Novel advancements in the management of hepatocellular carcinoma in 2008. J. Hepatol. 2008, 48 (Suppl. 1), S20–S37. [Google Scholar] [CrossRef] [PubMed]
- Wall, W.J.; Marotta, P.J. Surgery and transplantation for hepatocellular cancer. Liver Transpl. 2000, 6 (Suppl. 2), S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Alsowmely, A.M.; Hodgson, H.J. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol. Ther. 2002, 16, 1–15. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Hargreaves, G.; Azoulay, D.; Bismuth, H. Hepatocellular carcinoma: Surgical indications and results. Crit. Rev. Oncol. Hematol. 2003, 47, 13–27. [Google Scholar] [CrossRef]
- Tung-Ping Poon, R.; Fan, S.T.; Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg. 2000, 232, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann. Surg. 1999, 229, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.P.; Hwang, L.Y. Hepatocellular carcinoma and hepatitis B virus. Semin. Liver Dis. 1984, 4, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Turati, F.; La Vecchia, C. Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925. [Google Scholar] [CrossRef]
- Sung, W.-K.; Zheng, H.; Li, S.; Chen, R.; Liu, X.; Li, Y.; Lee, N.P.; Lee, W.H.; Ariyaratne, P.N.; Tennakoon, C.; et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 2012, 44, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Lee, J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007, 252, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Bonilla Guerrero, R.; Roberts, L.R. The role of hepatitis B virus integrations in the pathogenesis of human hepatocellular carcinoma. J. Hepatol. 2005, 42, 760–777. [Google Scholar] [CrossRef]
- Wang, H.-C.; Wu, H.C.; Chen, C.-F.; Fausto, N.; Lei, H.-Y.; Su, I.-J. Different Types of Ground Glass Hepatocytes in Chronic Hepatitis B Virus Infection Contain Specific Pre-S Mutants that May Induce Endoplasmic Reticulum Stress. Am. J. Pathol. 2003, 163, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Su, I.-J.; Wang, H.-C.; Wu, H.C.; Huang, W.-Y. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J. Gastroenterol. Hepatol. 2008, 23 Pt 1, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-F.; Li, T.-C.; Huang, H.-Y.; Chan, W.-L.; Wu, H.-C.; Shyu, W.-C.; Su, I.-J.; Jeng, L.-B. Hepatitis B virus pre-S2 deletion (nucleotide 1 to 54) in plasma predicts recurrence of hepatocellular carcinoma after curative surgical resection. PLoS ONE 2020, 15, e0242748. [Google Scholar] [CrossRef] [PubMed]
- Le Seyec, J.; Chouteau, P.; Cannie, I.; Guguen-Guillouzo, C.; Gripon, P. Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity. J. Virol. 1998, 72, 5573–5578. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Sonnabend, J.; Seitz, S.; Urban, S. The Pre-S2 Domain of the Hepatitis B Virus Is Dispensable for Infectivity but Serves a Spacer Function for L-Protein-Connected Virus Assembly. J. Virol. 2010, 84, 3879–3888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thedja, M.D.; Muljono, D.H.; Ie, S.I.; Sidarta, E.; Turyadi; Verhoef, J.; Marzuki, S. Genogeography and Immune Epitope Characteristics of Hepatitis B Virus Genotype C Reveals Two Distinct Types: Asian and Papua-Pacific. PLoS ONE 2015, 10, e0132533. [Google Scholar]
- Hatazawa, Y.; Yano, Y.; Okada, R.; Tanahashi, T.; Hayashi, H.; Hirano, H.; Minami, A.; Kawano, Y.; Tanaka, M.; Fukumoto, T.; et al. Quasispecies variant of pre-S/S gene in HBV-related hepatocellular carcinoma with HBs antigen positive and occult infection. Infect. Agents Cancer 2018, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.-F.; Wu, H.C.; Shyu, W.-C.; Jeng, L.-B.; Su, I.-J. Pre-S2 Mutant-Induced Mammalian Target of Rapamycin Signal Pathways as Potential Therapeutic Targets for Hepatitis B Virus-Associated Hepatocellular Carcinoma. Cell Transplant. 2017, 26, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.-C.A.; Neo, J.C.; Wu, J.-C.; Chen, Y.-F.; Kao, C.-H.; Tsai, T.-F. Expression of a hepatitis B virus pre-S2 deletion mutant in the liver results in hepatomegaly and hepatocellular carcinoma in mice. J. Pathol. 2017, 241, 463–474. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Jeng, L.-B.; Chan, W.-L.; Su, I.-J.; Teng, C.-F. Hepatitis B Virus Pre-S Gene Deletions and Pre-S Deleted Proteins: Clinical and Molecular Implications in Hepatocellular Carcinoma. Viruses 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Su, I.J.; Lei, H.Y.; Wang, H.C.; Lin, W.C.; Chang, W.T.; Huang, W.; Chang, W.; Chang, Y.; Chen, C. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004, 279, 46384–46392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-C.; Teng, C.-F.; Wu, H.-C.; Tsai, H.-W.; Chuang, H.-C.; Tsai, T.-F.; Hsu, Y.-H.; Huang, W.; Wu, L.-W.; Su, I.-J. Enhanced expression of vascular endothelial growth factor-A in ground glass hepatocytes and its implication in hepatitis B virus hepatocarcinogenesis. Hepatology 2009, 49, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-F.; Hsieh, W.-C.; Wu, H.C.; Lin, Y.-J.; Tsai, H.-W.; Huang, W.; Su, I.-J. Hepatitis B Virus Pre-S2 Mutant Induces Aerobic Glycolysis through Mammalian Target of Rapamycin Signal Cascade. PLoS ONE 2015, 10, e0122373. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.-F.; Wu, H.-C.; Hsieh, W.-C.; Tsai, H.-W.; Su, I.-J. Activation of ATP Citrate Lyase by mTOR Signal Induces Disturbed Lipid Metabolism in Hepatitis B Virus Pre-S2 Mutant Tumorigenesis. J. Virol. 2015, 89, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-H.; Su, I.-J.; Wang, H.-C.; Tsai, J.-H.; Huang, Y.-J.; Chang, W.-W.; Lai, M.-D.; Lei, H.-Y.; Huang, W. Hepatitis B Virus Pre-S2 Mutant Surface Antigen Induces Degradation of Cyclin-Dependent Kinase Inhibitor p27Kip1 through c-Jun Activation Domain-Binding Protein 1. Mol. Cancer Res. 2007, 5, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-C.; Chang, W.-T.; Wu, H.-C.; Huang, W.; Lei, H.-Y.; Lai, M.-D.; Fausto, N.; Su, I.-J. Hepatitis B virus pre-S2 mutant upregulates cyclin A expression and induces nodular proliferation of hepatocytes. Hepatology 2005, 41, 761–770. [Google Scholar] [CrossRef]
- Hung, J.-H.; Teng, Y.-N.; Wang, L.H.-C.; Su, I.-J.; Wang, C.C.C.; Huang, W.; Lee, K.-H.; Lu, K.-Y.; Wang, L.-H. Induction of Bcl-2 Expression by Hepatitis B Virus Pre-S2 Mutant Large Surface Protein Resistance to 5-Fluorouracil Treatment in Huh-7 Cells. PLoS ONE 2011, 6, e28977. [Google Scholar] [CrossRef]
- Wang, L.H.-C.; Huang, W.; Lai, M.-D.; Su, I.-J. Aberrant cyclin A expression and centrosome overduplication induced by hepatitis B virus Pre-S2 mutants and its implication in hepatocarcinogenesis. Carcinogenesis 2011, 33, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, T.T.-C.; Yang, A.; Chiu, W.-T.; Li, T.-N.; Wang, L.-H.; Wu, Y.-H.; Wang, H.-C.; Chen, L.; Wang, W.-C.; Huang, W.; et al. Hepatitis B virus PreS2-mutant large surface antigen activates store-operated calcium entry and promotes chromosome instability. Oncotarget 2016, 7, 23346–23360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-H.; Chang, Y.-Y.; Su, I.-J.; Yen, C.-J.; Liu, Y.-R.; Liu, R.-J.; Hsieh, W.-C.; Tsai, H.-W.; Wang, L.H.-C.; Huang, W. Hepatitis B virus pre-S2 mutant large surface protein inhibits DNA double-strand break repair and leads to genome instability in hepatocarcinogenesis. J. Pathol. 2015, 236, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-H.; Su, I.-J.; Yen, C.-J.; Tsai, T.-F.; Tsai, H.-W.; Tsai, H.-N.; Huang, Y.-J.; Chen, Y.-Y.; Ai, Y.-L.; Kao, L.-Y.; et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid suppresses the pro-oncogenic effects induced by hepatitis B virus pre-S 2 mutant oncoprotein and represents a potential chemopreventive agent in high-risk chronic HBV patients. Carcinogenesis 2012, 34, 475–485. [Google Scholar] [CrossRef]
- TTeng, C.-F.; Yu, C.-H.; Chang, H.-Y.; Hsieh, W.-C.; Wu, T.-H.; Lin, J.-H.; Wu, H.C.; Jeng, L.-B.; Su, I.-J. Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model. Sci. Rep. 2019, 9, 10338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.-F.; Wu, H.-C.; Su, I.-J.; Jeng, L.-B. Hepatitis B Virus Pre-S Mutants as Biomarkers and Targets for the Development and Recurrence of Hepatocellular Carcinoma. Viruses 2020, 12, 945. [Google Scholar] [CrossRef]
- Chen, C.H.; Hung, C.H.; Lee, C.M.; Hu, T.H.; Wang, J.H.; Wang, J.C.; Lu, S.; Changchien, C. Pre-S deletion and complex mutations of hepatitis B virus related to advanced liver disease in HBeAg-negative patients. Gastroenterology 2007, 133, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Choi, M.S.; Gwak, G.-Y.; Paik, Y.-H.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Pre-S Mutation Is a Significant Risk Factor for Hepatocellular Carcinoma Development: A Long-Term Retrospective Cohort Study. Am. J. Dig. Dis. 2012, 58, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-C.; Su, I.-J.; Wu, H.-C.; Hsieh, Y.-H.; Yao, W.-J.; Young, K.-C.; Chang, T.-C.; Hsieh, H.-C.; Tsai, H.-N.; Huang, W. A pre-S gene chip to detect pre-S deletions in hepatitis B virus large surface antigen as a predictive marker for hepatoma risk in chronic hepatitis B virus carriers. J. Biomed. Sci. 2009, 16, 84. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Lu, C.; Chen, W.; Yao, W.; Wang, H.; Chang, T.; Lei, H.; Shiau, A.; Su, I. Prevalence and significance of hepatitis B virus (HBV) pre-S mutants in serum and liver at different replicative stages of chronic HBV infection. Hepatology 2001, 33, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-W.; Lin, Y.-J.; Lin, P.-W.; Wu, H.-C.; Hsu, K.-H.; Yen, C.-J.; Chan, S.-H.; Huang, W.; Su, I.-J. A clustered ground-glass hepatocyte pattern represents a new prognostic marker for the recurrence of hepatocellular carcinoma after surgery. Cancer 2011, 117, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-W.; Lin, Y.-J.; Wu, H.C.; Chang, T.-T.; Wu, I.-C.; Cheng, P.-N.; Yen, C.-J.; Chan, S.-H.; Huang, W.; Su, I.-J. Resistance of ground glass hepatocytes to oral antivirals in chronic hepatitis B patients and implication for the development of hepatocellular carcinoma. Oncotarget 2016, 7, 27724–27734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.J.; Ai, Y.L.; Tsai, H.W.; Chan, S.H.; Yen, C.S.; Cheng, K.H.; Lee, Y.; Kao, C.; Wang, Y.; Chen, Y.; et al. Hepatitis B virus surface gene pre-S2 mutant as a high-risk serum marker for hepatoma recurrence after curative hepatic resection. Hepatology 2018, 68, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.-F.; Li, T.-C.; Huang, H.-Y.; Lin, J.-H.; Chen, W.-S.; Shyu, W.-C.; Wu, H.-C.; Peng, C.-Y.; Su, I.-J.; Jeng, L.-B. Next-Generation Sequencing-Based Quantitative Detection of Hepatitis B Virus Pre-S Mutants in Plasma Predicts Hepatocellular Carcinoma Recurrence. Viruses 2020, 12, 796. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ning, Q.; Yang, L.; Mo, Z.; Tang, S. Mechanisms of immune escape in the cancer immune cycle. Int. Immunopharmacol. 2020, 86, 106700. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Shevach, E.M.; McHugh, R.S.; Piccirillo, C.A.; Thornton, A.M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 2001, 182, 58–67. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.-S.; Gu, X.; Xiong, W.; Guo, W.; Han, L.; Bai, Y.; Peng, C.; Cui, M.; Xie, M. Increased programmed death ligand-1 expression predicts poor prognosis in hepatocellular carcinoma patients. OncoTargets Ther. 2016, 9, 4805–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.I.; Jeong, D.; Ji, S.; Ahn, T.; Bae, S.H.; Chin, S.; Chung, J.C.; Kim, H.C.; Lee, M.S.; Baek, M.-J. Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. Treat. 2017, 49, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.; Tang, Z. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Tanaka, F.; Mimori, K.; Inoue, H.; Kai, S.; Shibata, K.; Ohta, M.; Kitano, S.; Mori, M. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur. J. Surg. Oncol. (EJSO) 2008, 34, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Z.; Chen, K.-J.; Xu, Z.-Y.; Chen, H.; Zhou, L.; Xie, H.-Y.; Zheng, S.-S. Prediction of Recurrence and Survival in Hepatocellular Carcinoma Based on Two Cox Models Mainly Determined by FoxP3+ Regulatory T Cells. Cancer Prev. Res. 2013, 6, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Kryczek, I.; Chen, L.; Zou, W.; Welling, T.H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009, 69, 8067–8075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semaan, A.; Dietrich, D.; Bergheim, D.; Dietrich, J.; Kalff, J.C.; Branchi, V.; Matthaei, H.; Kristiansen, G.; Fischer, H.-P.; Goltz, D. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch. 2016, 470, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased Regulatory T Cells Correlate with CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Liao, W.; Yang, H.; Xu, H.; Du, S.; Zhao, H.; Lu, X.; Sang, X.; Mao, Y. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: A meta-analysis. Oncotarget 2017, 8, 39658–39672. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.-F.; Li, T.-C.; Wang, T.; Wu, T.-H.; Wang, J.; Wu, H.-C.; Shyu, W.-C.; Su, I.-J.; Jeng, L.-B. Increased Expression of Programmed Death Ligand 1 in Hepatocellular Carcinoma of Patients with Hepatitis B Virus Pre-S2 Mutant. J. Hepatocell. Carcinoma 2020, 7, 385–401. [Google Scholar] [CrossRef]
- Teng, C.-F.; Li, T.-C.; Wang, T.; Liao, D.-C.; Wen, Y.-H.; Wu, T.-H.; Wang, J.; Wu, H.-C.; Shyu, W.-C.; Su, I.-J.; et al. Increased infiltration of regulatory T cells in hepatocellular carcinoma of patients with hepatitis B virus pre-S2 mutant. Sci. Rep. 2021, 11, 1136. [Google Scholar] [CrossRef]
- Teng, C.-F.; Huang, H.-Y.; Li, T.-C.; Shyu, W.-C.; Wu, H.C.; Lin, C.-Y.; Su, I.-J.; Jeng, L.-B. A Next-Generation Sequencing-Based Platform for Quantitative Detection of Hepatitis B Virus Pre-S Mutants in Plasma of Hepatocellular Carcinoma Patients. Sci. Rep. 2018, 8, 14816. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Jeng, L.-B.; Su, I.-J.; Teng, C.-F. Approaches for Detection of Hepatitis B Virus Pre-S Gene Deletions and Pre-S Deleted Proteins and Their Application in Prediction of Higher Risk of Hepatocellular Carcinoma Development and Recurrence. Viruses 2022, 14, 428. [Google Scholar] [CrossRef]
- Teng, C.-F.; Tsai, H.-W.; Li, T.-C.; Wang, T.; Wang, J.; Shyu, W.-C.; Wu, H.C.; Su, I.-J.; Jeng, L.-B. Detection of hepatitis B virus pre-S mutants in plasma by a next-generation sequencing-based platform determines their patterns in liver tissues. PLoS ONE 2020, 15, e0234773. [Google Scholar] [CrossRef]
- Jeng, L.-B.; Li, T.-C.; Hsu, S.-C.; Chan, W.-L.; Teng, C.-F. Association of Low Serum Albumin Level with Higher Hepatocellular Carcinoma Recurrence in Patients with Hepatitis B Virus Pre-S2 Mutant after Curative Surgical Resection. J. Clin. Med. 2021, 10, 4187. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Han, Y.; Huang, Y.; Jiang, S.; Huang, Z.; Chen, R.; Yu, Z.; Yu, K.; Zhang, S. PD-L1 Is Expressed and Promotes the Expansion of Regulatory T Cells in Acute Myeloid Leukemia. Front. Immunol. 2020, 11, 1710. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra20. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-T.; Jiang, M.-J.; Deng, Z.-J.; Li, L.; Huang, J.-L.; Liu, Z.-X.; Zhong, J.-H. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Current Progresses and Challenges. Front. Oncol. 2021, 11, 737497. [Google Scholar] [CrossRef]
- Yin, X.; Wu, T.; Lan, Y.; Yang, W. Current progress of immune checkpoint inhibitors in the treatment of advanced hepatocellular carcinoma. Biosci. Rep. 2022, 42, BSR20212304. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Zeng, S.; Shen, H. From bench to bed: The tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 396. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Arce Vargas, F.; Furness, A.J.S.; Solomon, I.; Joshi, K.; Mekkaoui, L.; Lesko, M.H.; Rota, E.M. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 2017, 46, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Zhulai, G.; Oleinik, E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand. J. Immunol. 2022, 95, e13129. [Google Scholar] [CrossRef]
- Liu, P.-H.; Hsu, C.-Y.; Hsia, C.-Y.; Lee, Y.-H.; Su, C.-W.; Huang, Y.-H.; Lee, F.-Y.; Lin, H.-C.; Huo, T.-I. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J. Hepatol. 2016, 64, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Q.; Jia, J. Hepatitis B virus genotypes: Epidemiological and clinical relevance in Asia. Hepatol. Int. 2016, 10, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liang, X.; Chen, S.; Wang, H.; Li, H.; Fang, M.; Bai, X.; Wang, Z.; Wang, M.; Zhu, S.; et al. Next-generation sequencing revealed divergence in deletions of the preS region in the HBV genome between different HBV-related liver diseases. J. Gen. Virol. 2017, 98, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.F.; Clay, T.M.; Hobeika, A.C.; Lyerly, H.; Morse, M.A. Vascular endothelial growth factor and immunosuppression in cancer: Current knowledge and potential for new therapy. Expert Opin. Biol. Ther. 2007, 7, 449–460. [Google Scholar] [CrossRef]
- Tian, M.; Neil, J.R.; Schiemann, W.P. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal 2011, 23, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Lastwika, K.J.; Wilson, W., 3rd; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).