New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Cohorts
2.2.1. Postmortem Cohorts
COVID-19 Autopsy Cohort
Non-COVID-19 Autopsy Cohort
Postmortem External and Internal Examination
Postmortem Diagnosis of Venous Thromboembolism
Clinical Data Collection—Postmortem Cohort
2.2.2. Ante Mortem Cohort
Ante Mortem COVID-19 Intensive Care Unit Cohort
Microbiological Examination
Ante Mortem Diagnosis of Venous Thromboembolism
Clinical Data Collection—Ante Mortem Cohort
2.2.3. Anticoagulation Regimes
2.2.4. Statistical Analysis
3. Result
3.1. Study Population
3.2. Postmortem Prevalence of VTE in Consecutive COVID-19 Decedents Compared to Non-COVID-19 Decedents
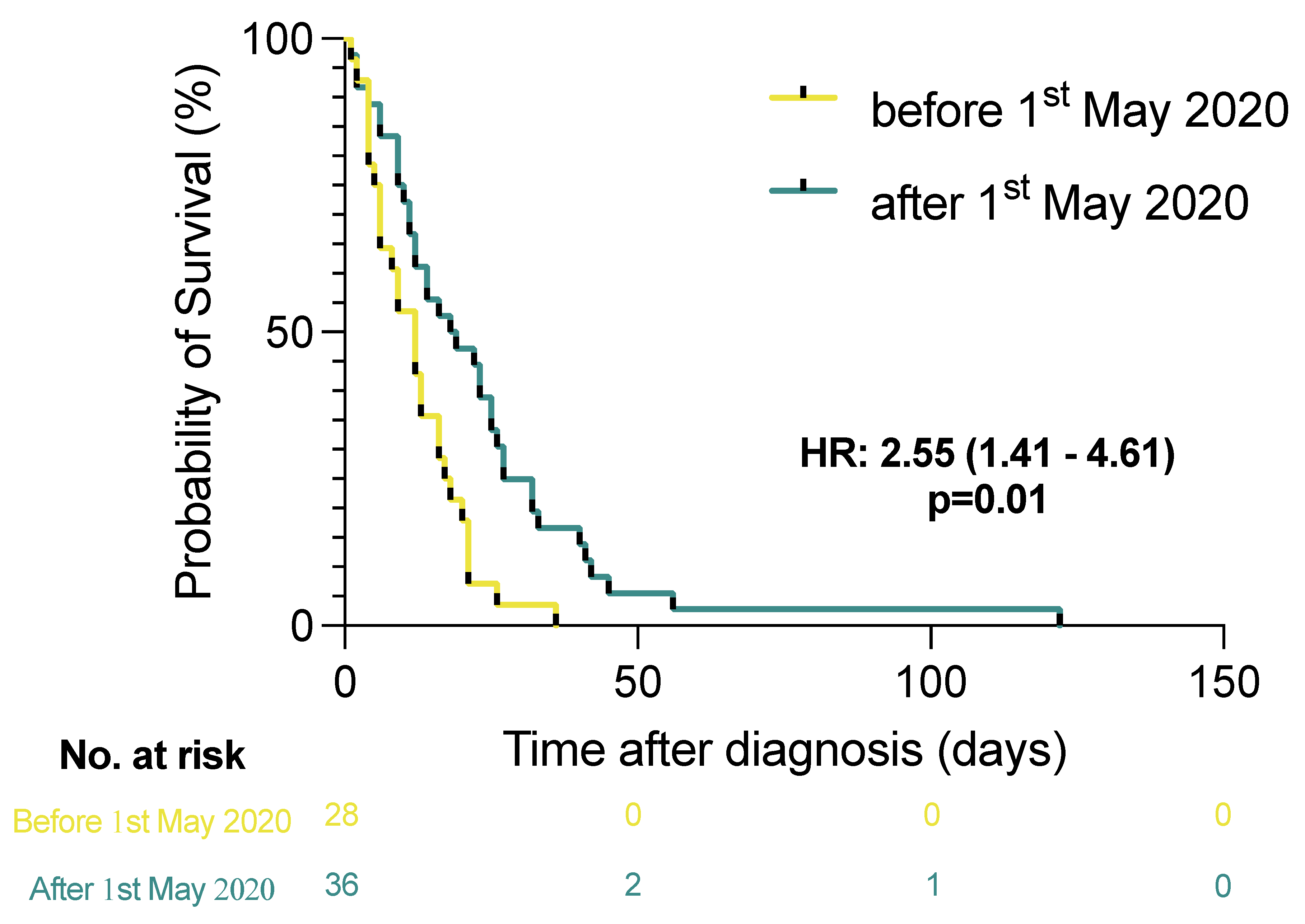
3.3. Effect of Local Guideline Changes on Anticoagulant Therapy
3.4. Ante Mortem Clinical Incidence, the Likelihood of Death, and the Effect of Anticoagulant Regimens on the Occurrence of VTE in Consecutive Critically Ill COVID-19 Patients Admitted to an ICU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Map—COVID-19. Available online: https://covid19.who.int/ (accessed on 3 January 2022).
- Roedl, K.; Jarczak, D.; Thasler, L.; Bachmann, M.; Schulte, F.; Bein, B.; Weber, C.F.; Schäfer, U.; Veit, C.; Hauber, H.-P.; et al. Mechanical ventilation and mortality among 223 critically ill patients with COVID-19—A multicentric study in Germany. Aust. Crit. Care 2020, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10,021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Gungor, B.; Atici, A.; Baycan, O.F.; Alici, G.; Ozturk, F.; Tugrul, S.; Asoglu, R.; Cevik, E.; Sahin, I.; Barman, H.A. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 39, 173–179. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Janssens, U.; Spinner, C.D.; Pfeifer, M.; Marx, G.; Karagiannidis, C. Clinical Practice Guideline: Recommendations on Inpatient Treatment of Patients with COVID-19. Dtsch. Arztebl. Int. 2021, 118, 375. [Google Scholar] [CrossRef]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; Moghadam, K.G.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, P.R.; Berger, J.S.; Gong, M.N.; Carrier, M.; Reynolds, H.R.; Kumar, A.; Turgeon, A.F.; et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef]
- Fitzek, A.; Schädler, J.; Dietz, E.; Ron, A.; Gerling, M.; Kammal, A.L.; Lohner, L.; Falck, C.; Möbius, D.; Goebels, H.; et al. Prospective postmortem evaluation of 735 consecutive SARS-CoV-2-associated death cases. Sci. Rep. 2021, 11, 19342. [Google Scholar] [CrossRef]
- Heinrich, F.; Nentwich, M.F.; Bibiza-Freiwald, E.; Nörz, D.; Roedl, K.; Christner, M.; Hoffmann, A.; Olearo, F.; Kluge, S.; Aepfelbacher, M.; et al. SARS-CoV-2 blood RNA load predicts outcome in critically ill COVID-19 patients. Open Forum Infect. Dis. 2021, 8, ofab509. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results from a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef]
- Lewis, T.C.; Cortes, J.; Altshuler, D.; Papadopoulos, J. Venous Thromboembolism Prophylaxis: A Narrative Review with a Focus on the High-Risk Critically Ill Patient. J. Intensive Care Med. 2019, 34, 877–888. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA 2020, 324, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Bompard, F.; Monnier, H.; Saab, I.; Tordjman, M.; Abdoul, H.; Fournier, L.; Sanchez, O.; Lorut, C.; Chassagnon, G.; Revel, M.-P. Pulmonary embolism in patients with COVID-19 pneumonia. Eur. Respir. J. 2020, 56, 2001365. [Google Scholar] [CrossRef] [PubMed]
- Edler, C.; Schröder, A.S.; Aepfelbacher, M.; Fitzek, A.; Heinemann, A.; Heinrich, F.; Klein, A.; Langenwalder, F.; Lütgehetmann, M.; Meißner, K.; et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Leg. Med. 2020, 134, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jiang, C.; Han, B.; Guan, C.; Fang, G.; Yan, S.; Wang, K.; Liu, L.; Conlon, C.P.; Xie, R.; et al. High prevalence of occult thrombosis in cases of mild/moderate COVID-19. Int. J. Infect. Dis. 2021, 104, 77–82. [Google Scholar] [CrossRef]
- Minet, C.; Potton, L.; Bonadona, A.; Hamidfar-Roy, R.; Somohano, C.A.; Lugosi, M.; Cartier, J.-C.; Ferretti, G.; Schwebel, C.; Timsit, J.-F. Venous thromboembolism in the ICU: Main characteristics, diagnosis and thromboprophylaxis. Crit. Care 2015, 19, 287. [Google Scholar] [CrossRef] [Green Version]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. JTH 2021, 19, 2225–2234. [Google Scholar] [CrossRef]
- Lopes, R.D.; de Barros, E.S.P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; de Aquino Martins, P.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Wang, H.F.; Yin, P.; Li, D.; Wang, D.L.; Peng, P.; Wang, W.H.; Wang, L.; Yuan, X.W.; Xie, J.Y.; et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: A multicenter retrospective study. J. Thromb. Haemost. JTH 2021, 19, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Mouhat, B.; Besutti, M.; Bouiller, K.; Grillet, F.; Monnin, C.; Ecarnot, F.; Behr, J.; Capellier, G.; Soumagne, T.; Pili-Floury, S.; et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur. Respir. J. 2020, 56, 2001811. [Google Scholar] [CrossRef]
- Heinz, C.; Miesbach, W.; Herrmann, E.; Sonntagbauer, M.; Raimann, F.J.; Zacharowski, K.; Weber, C.F.; Adam, E.H. Greater Fibrinolysis Resistance but No Greater Platelet Aggregation in Critically Ill COVID-19 Patients. Anesthesiology 2021, 134, 457–467. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef] [Green Version]
- Maltezou, H.C.; Raftopoulos, V.; Vorou, R.; Papadima, K.; Mellou, K.; Spanakis, N.; Kossyvakis, A.; Gioula, G.; Exindari, M.; Froukala, E.; et al. Association between upper respiratory tract viral load, comorbidities, disease severity and outcome of patients with SARS-CoV-2 infection. J. Infect Dis. 2021, 223, 1132–1138. [Google Scholar] [CrossRef]
- Bitker, L.; Dhelft, F.; Chauvelot, L.; Frobert, E.; Folliet, L.; Mezidi, M.; Trouillet-Assant, S.; Belot, A.; Lina, B.; Wallet, F.; et al. Protracted viral shedding and viral load are associated with ICU mortality in COVID-19 patients with acute respiratory failure. Ann. Intensive Care 2020, 10, 167. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Gonzalez-Rivera, M.; Almansa, R.; Micheloud, D.; Tedim, A.P.; Dominguez-Gil, M.; Resino, S.; Martin-Fernandez, M.; Ryan Murua, P.; Perez-Garcia, F.; et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care 2020, 24, 691. [Google Scholar] [CrossRef]
- Hagman, K.; Hedenstierna, M.; Gille-Johnson, P.; Hammas, B.; Grabbe, M.; Dillner, J.; Ursing, J. Severe Acute Respiratory Syndrome Coronavirus 2 RNA in Serum as Predictor of Severe Outcome in Coronavirus Disease 2019: A Retrospective Cohort Study. Clin. Infect. Dis. 2020, 73, e2995–e3001. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Ghandili, S.; Pfefferle, S.; Roedl, K.; Sonnemann, P.; Karagiannis, P.; Boenisch, O.; Kluge, S.; Schmiedel, S.; Ittrich, H.; Rohde, H.; et al. Challenges in treatment of patients with acute leukemia and COVID-19: A series of 12 patients. Blood Adv. 2020, 4, 5936–5941. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Heidenreich, S.; Pfefferle, S.; Jarczak, D.; Urbanowicz, T.T.; Norz, D.; Aepfelbacher, M.; Kroger, N.; Kluge, S.; Lutgehetmann, M.; et al. Viral Dynamics of SARS-CoV-2 in Critically Ill Allogeneic Hematopoietic Stem Cell Transplant Recipients and Immunocompetent Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 242–245. [Google Scholar] [CrossRef]

| Overall Patients | Pulmonary Embolism | Univariate Logistic Regression | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | |||
| n = 64 | n = 19 | 95% CI | 95% CI | |||
| Sociodemographic variables | ||||||
| Age | 72.5 (63.5–79.0) | 72.0 (57.0–79.0) | 0.99 (0.94–1.03) | 0.49 | 0.96 (0.91–1.01) | 0.13 |
| Sex (Ref: Female) | 47 (73.4%) | 14 (73.7%) | 1.02 (0.30–3.44) | 0.98 | 1.27 (0.21–7.49) | 0.80 |
| BMI | 27.7 (22.6–32.8) | 29.6 (24.7–36.8) | 1.02 (0.98–1.07) | 0.35 | 1.02 (0.96–1.09) | 0.53 |
| Pre-existing medical conditions | ||||||
| Type II diabetes mellitus | 18 (28.1%) | 4 (21.1%) | 0.59 (0.17–2.10) | 0.42 | 0.49 (0.10–2.38) | 0.38 |
| Arterial hypertension | 33 (51.6%) | 9 (47.4%) | 0.79 (0.27–2.31) | 0.66 | 0.42 (0.08–2.12) | 0.29 |
| Chronic lung disease | 20 (31.2%) | 6 (31.6%) | 1.02 (0.32–3.24) | 0.97 | 0.96 (0.24–3.89) | 0.95 |
| Chronic kidney disease | 13 (20.3%) | 3 (15.8%) | 0.66 (0.16–2.71) | 0.56 | 0.56 (0.09–3.54) | 0.54 |
| ICU-related therapies | ||||||
| Mechanical ventilation | 50 (78.1%) | 17 (89.5%) | 3.09 (0.62–15.42) | 0.02 | 2.89 (0.19–43.41) | 0.44 |
| ECMO | 11 (17.2%) | 3 (15.8%) | 0.87 (0.20–3.70) | 0.85 | 0.21 (0.04–1.13) | 0.107 |
| RRT | 35 (54.7%) | 15 (78.9%) | 4.69 (1.34–16.46) | 0.02 | 11.22 (2.36–53.30) | 0.002 |
| COVID-19-related therapies | ||||||
| Remdesivir | 1 (1.6%) | 0 (0.0%) | ND | ND | ND | ND |
| Dexamethasone | 19 (29.7%) | 4 (21.1%) | 0.53 (0.15–1.89) | 0.33 | 0.76 (0.18–3.19) | 0.71 |
| Tocilizumab | 0 (0%) | 0 (0%) | ND | ND | ND | ND |
| COVID-19 disease severity | ||||||
| ARDS | 48 (75.0%) | 15 (78.9%) | 1.36 (0.38–4.93) | 0.64 | 0.57 (0.09–43.63) | 0.55 |
| Overall Patients | Non-Survivors | Univariate Logistic Regression | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | |||
| n = 170 | n = 71 | 95% CI | 95% CI | |||
| Sociodemographic variables | ||||||
| Age | 63.0 (55.0–73.0) | 66.0 (58.0–76.0) | 1.03 (1.00–1.05) | 0.04 | 1.03 (0.98–1.08) | 0.24 |
| Sex (Ref: Female) | 112 (65.9%) | 49 (69.0%) | 0.79 (0.41–1.50) | 0.47 | 0.61 (0.16–2.33) | 0.47 |
| BMI | 27.2 (24.2–31.9) | 26.3 (23.9–32.7) | 0.99 (0.95–1.04) | 0.78 | 0.88 (0.80–0.96) | 0.01 |
| Charlson comorbidity index | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | 1.09 (0.96–1.24) | 0.17 | 1.50 (1.02–2.21) | 0.04 |
| Pre-existing medical conditions | ||||||
| Type II diabetes mellitus | 57 (33.5%) | 22 (31.0%) | 0.82 (0.43–1.57) | 0.55 | 0.69 (0.19–2.57) | 0.58 |
| Arterial hypertension | 97 (57.1%) | 42 (59.2%) | 1.16 (0.62–2.15) | 0.64 | 1.75 (0.39–7.89) | 0.47 |
| Chronic lung disease | 24 (14.1%) | 11 (15.5%) | 1.21 (0.51–2.89) | 0.66 | 0.47 (0.12–1.95) | 0.30 |
| Chronic kidney disease | 27 (15.9%) | 10 (14.1%) | 0.79 (0.34–1.85) | 0.59 | 0.15 (0.01–2.05) | 0.16 |
| ICU-related therapies | ||||||
| Nasal oxygen therapy | 60 (35.3%) | 24 (33.8%) | 0.89 (0.47–1.69) | 0.73 | 0.54 (0.12–2.44) | 0.43 |
| NIV | 41 (24.1%) | 19 (26.8%) | 1.28 (0.63–2.59) | 0.50 | 0.79 (0.13–4.62) | 0.79 |
| MV | 120 (70.6%) | 65 (91.5%) | 8.67 (2.43–21.87) | <0.0001 | 0.98 (0.03–34.31) | 0.99 |
| MV time (days) | 8.0 (0.0–21.5) | 12.0 (5.0–22.0) | 1.00 (0.98–1.01) | 0.68 | 0.91 (0.87–0.96) | <0.0001 |
| ECMO | 49 (28.8%) | 33 (46.5%) | 4.50 (2.22–9.16) | <0.0001 | 41.33 (5.54–308.31) | <0.0001 |
| RRT | 79 (46.5%) | 51 (71.8%) | 6.47 (3.28–12.73) | <0.0001 | 10.55 (2.59–43.02) | 0.001 |
| Catecholamines | 132 (77.6%) | 67 (94.4%) | 8.76 (2.94–26.08) | <0.0001 | 0.70 (0.03–16.54) | 0.82 |
| COVID-19-related therapies | ||||||
| Remdesivir | 32 (18.8%) | 10 (14.1%) | 0.57 (0.25–1.30) | 0.18 | 0.49 (0.10–2.46) | 0.38 |
| Dexamethasone | 73 (42.9%) | 35 (49.3%) | 1.56 (0.84–2.89) | 0.16 | 1.48 (0.29–7.65) | 0.64 |
| Tocilizumab | 3 (1.8%) | 3 (4.2%) | ND | ND | ND | ND |
| TPE | 6 (3.5%) | 3 (4.2%) | 1.41 (0.28–7.21) | 0.68 | 2.37 (0.38–14.71) | 0.36 |
| COVID-19 disease severity | ||||||
| ARDS | 113 (66.5%) | 64 (90.1%) | 9.33 (3.89–22.36) | <0.0001 | 7.59 (0.27–211.19) | 0.23 |
| ARDS severity | 3.0 (0.0–3.0) | 3.0 (3.0–3.0) | 2.29 (1.70–2.08) | <0.0001 | 1.79 (0.54–5.96) | 0.34 |
| SAPS II—on admission | 40.0 (33.0–48.0) | 43.0 (37.0–52.0) | 1.07 (1.03–1.10) | <0.0001 | 1.05 (0.98–1.13) | 0.17 |
| SOFA score—on admission | 7.0 (3.0–12.0) | 10.0 (6.0–13.0) | 1.12 (1.04–1.19) | 0.001 | 0.92 (0.79–1.07) | 0.27 |
| No Pulmonary Embolism | Pulmonary Embolism | Overall Patients | Comparative Statistics (p-Value) | |
|---|---|---|---|---|
| n = 158 | n = 12 | n = 170 | ||
| Sociodemographic variables | ||||
| Age | 63.0 (53.0–73.0) | 62.5 (59.0–74.5) | 63.0 (55.0–73.0) | 0.54 |
| Sex | ||||
| Male | 104 (65.8%) | 8 (66.7%) | 112 (65.9%) | 0.95 |
| Female | 54 (34.2%) | 4 (33.3%) | 58 (34.1%) | . |
| BMI | 27.2 (24.2–31.9) | 27.0 (23.7–31.8) | 27.2 (24.2–31.9) | 0.66 |
| Charlson comorbidity index | 2.0 (1.0–3.0) | 2.5 (0.5–4.5) | 2.0 (1.0–3.0) | 0.58 |
| Pre-existing medical conditions | ||||
| Type II diabetes mellitus | 49 (31.0%) | 8 (66.7%) | 57 (33.5%) | 0.01 |
| Arterial hypertension | 91 (57.6%) | 6 (50.0%) | 97 (57.1%) | 0.61 |
| Chronic lung disease | 22 (13.9%) | 2 (16.7%) | 24 (14.1%) | 0.79 |
| Chronic kidney disease | 24 (15.2%) | 3 (25.0%) | 27 (15.9%) | 0.37 |
| ICU-related therapy | ||||
| Nasal oxygen therapy | 56 (35.4%) | 4 (33.3%) | 60 (35.3%) | 0.88 |
| NIV | 36 (22.8%) | 5 (41.7%) | 41 (24.1%) | 0.14 |
| MV | 109 (69.0%) | 11 (91.7%) | 120 (70.6%) | 0.10 |
| MV time | 8.0 (0.0–19.5) | 18.5 (4.5–58.0) | 8.0 (0.0–21.5) | 0.04 |
| ECMO | 45 (28.5%) | 4 (33.3%) | 49 (28.8%) | 0.72 |
| RRT | 67 (42.4%) | 12 (100.0%) | 79 (46.5%) | <0.0001 |
| Catecholamines | 121 (76.6%) | 11 (91.7%) | 132 (77.6%) | 0.23 |
| COVID-19-related therapy | ||||
| Remdesivir | 30 (19.0%) | 2 (16.7%) | 32 (18.8%) | 0.84 |
| Dexamethasone | 67 (42.4%) | 6 (50.0%) | 73 (42.9%) | 0.61 |
| Tocilizumab | 3 (1.9%) | 0 (0.0%) | 3 (1.8%) | 0.63 |
| TPE therapy | 6 (3.8%) | 0 (0.0%) | 6 (3.5%) | 0.49 |
| COVID-19 disease severity | ||||
| ARDS | 55 (34.8%) | 2 (16.7%) | 57 (33.5%) | 0.20 |
| ARDS severity | 2.0 (0.0–3.0) | 3.0 (3.0–3.0) | 3.0 (0.0–3.0) | 0.04 |
| SAPSII | 40.0 (33.0–48.0) | 40.0 (34.0–52.5) | 40.0 (33.0–48.0) | 0.70 |
| SOFA score | 7.0 (3.0–12.0) | 7.0 (2.5–11.5) | 7.0 (3.0–12.0) | 0.80 |
| Overall Patients | Non-Survivors | Univariate Logistic Regression | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|
| n = 170 | n = 71 | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Pulmonary embolism | 12 (7.1%) | 9 (12.7%) | 4.65 (1.21–17.83) | 0.03 | 2.21 (0.40–12.08) | 0.36 |
| Low-molecular-weight heparin | ||||||
| Low dose | 23 (13.5%) | 5 (7%) | 0.14 (0.48–0.43) | <0.0001 | 0.08 (0.02–0.43) | 0.003 |
| Intermediate dose | 31 (18.2%) | 5 (7%) | 0.10 (0.03–0.29) | <0.0001 | 0.06 (0.01–0.29) | 0.001 |
| High dose | 31 (18.2%) | 5 (7%) | 0.10 (0.03–0.29) | <0.0001 | 0.06 (0.01–0.28) | <0.0001 |
| Unfractionated heparin | ||||||
| Low dose | 7 (4.1%) | 6 (8.5%) | 14.06 (1.63–121.58) | 0.02 | 1.65 (0.14–19.85) | 0.69 |
| High dose | 56 (32.9%) | 33 (46.5%) | 3.36 (1.71–6.60) | <0.0001 | 0.39 (0.10–1.61) | 0.19 |
| Factor-IIa antagonist | 9 (5.3%) | 7 (9.9%) | 5.30 (1.07–26.35) | 0.04 | 0.84 (0.10–6.81) | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrich, F.; Roedl, K.; Jarczak, D.; Goebels, H.-L.; Heinemann, A.; Schäfer, U.; Ludwig, F.; Bachmann, M.; Bein, B.; Weber, C.F.; et al. New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis. Viruses 2022, 14, 811. https://doi.org/10.3390/v14040811
Heinrich F, Roedl K, Jarczak D, Goebels H-L, Heinemann A, Schäfer U, Ludwig F, Bachmann M, Bein B, Weber CF, et al. New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis. Viruses. 2022; 14(4):811. https://doi.org/10.3390/v14040811
Chicago/Turabian StyleHeinrich, Fabian, Kevin Roedl, Dominik Jarczak, Hanna-Lisa Goebels, Axel Heinemann, Ulrich Schäfer, Frank Ludwig, Martin Bachmann, Berthold Bein, Christian Friedrich Weber, and et al. 2022. "New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis" Viruses 14, no. 4: 811. https://doi.org/10.3390/v14040811
APA StyleHeinrich, F., Roedl, K., Jarczak, D., Goebels, H.-L., Heinemann, A., Schäfer, U., Ludwig, F., Bachmann, M., Bein, B., Weber, C. F., Sydow, K., Bota, M., Paschen, H.-R., de Weerth, A., Veit, C., Detsch, O., Brand, P.-A., Kluge, S., Ondruschka, B., & Wichmann, D. (2022). New Insights in the Occurrence of Venous Thromboembolism in Critically Ill Patients with COVID-19—A Large Postmortem and Clinical Analysis. Viruses, 14(4), 811. https://doi.org/10.3390/v14040811






