Intensive-Dose Tinzaparin in Hospitalized COVID-19 Patients: The INTERACT Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Study Population
3.2. Tinzaparin Dose and Duration
3.3. Tinzaparin Effectiveness and Safety
3.4. Association of Laboratory and Clinical Parameters with Thrombosis
3.5. Evolution of Laboratory and Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. INTERACT Study Group
References
- Levi, M.; Thachil, J. Coronavirus Disease 2019 Coagulopathy: Disseminated Intravascular Coagulation and Thrombotic Microangiopathy-Either, Neither, or Both. Semin. Thromb. Hemost. 2020, 46, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed on 10 January 2022).
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response. JAMA J. Am. Med. Assoc. 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [Green Version]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Northwell COVID-19 Research Consortium; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Liu, V.X.; Jackson, M.L.; Schmidt, M.A.; Jewell, B.L.; Flores, J.P.; Jentz, C.; Northrup, G.R.; Mahmud, A.; Reingold, A.L.; et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: Prospective cohort study. BMJ 2020, 369, m1923. [Google Scholar] [CrossRef]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, Diagnosis, and Treatment of VTE in Patients with Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival among Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Beckman, J.A.; Tomlinson, L.; Gellad, W.F.; Alcorn, C.; Kidwai-Khan, F.; Skanderson, M.; Brittain, E.; King, J.T., Jr.; Ho, Y.L.; et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ 2021, 372, n311. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 117, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.; MacDonald, S.; Bull, T.; Hayman, M.; de Monteverde-Robb, R.; Sapsford, D.; Lavinio, A.; Varley, J.; Johnston, A.; Besser, M.; et al. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis 2020, 50, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Fegan, C.D. Tinzaparin as an antithrombotic: An overview. Hosp. Med. 1998, 59, 145–148. [Google Scholar]
- Linhardt, R.J.; Gunay, N.S. Production and chemical processing of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25 (Suppl. S3), 5–16. [Google Scholar]
- Mätzsch, T.; Bergqvist, D.; Hedner, U.; Ostergaard, P. Effects of an enzymatically depolymerized heparin as compared with conventional heparin in healthy volunteers. Thromb. Haemost. 1987, 57, 97–101. [Google Scholar] [CrossRef]
- Padilla, A.; Gray, E.; Pepper, D.S.; Barrowcliffe, T.W. Inhibition of thrombin generation by heparin and low molecular weight (LMW) heparins in the absence and presence of platelet factor 4 (PF4). Br. J. Haematol. 1992, 82, 406–413. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Bajaj, M.S.; Bajaj, S.P. Tissue factor pathway inhibitor: Potential therapeutic applications. Thromb. Haemost. 1997, 78, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.; Hoppensteadt, D.A.; Fareed, J. Tissue factor pathway inhibitor: An update of potential implications in the treatment of cardiovascular disorders. Expert Opin. Investig. Drugs 2001, 10, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Mohamed, S. Anti-angiogenic mechanisms and efficacy of the low molecular weight heparin, tinzaparin: Anti-cancer efficacy. Oncol. Rep. 2004, 12, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, J.; Puskulluoglu, M.; Krzemieniecki, K. The antineoplastic effect of low-molecular-weight heparins—A literature review. Contemp. Oncol. 2013, 17, 6–13. [Google Scholar] [CrossRef]
- Mousa, S.A.; Petersen, L.J. Anti-cancer properties of low-molecular-weight heparin: Preclinical evidence. Thromb. Haemost. 2009, 102, 258–267. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Estrada, Y.M.R.M.; Oldham, S.A. CTPA as the gold standard for the diagnosis of pulmonary embolism. Int. J. Comput. Assist. Radiol. Surg. 2011, 6, 557–563. [Google Scholar] [CrossRef]
- Zierler, B.K. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004, 109, I9–I14. [Google Scholar] [CrossRef] [Green Version]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-associated thrombosis: An overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Schulman, S.; Angeras, U.; Bergqvist, D.; Eriksson, B.; Lassen, M.R.; Fisher, W.; Subcommittee on Control of Anticoagulation of the Scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010, 8, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Stals, M.; Grootenboers, M.; van Guldener, C.; Kaptein, F.; Braken, S.; Chen, Q.; Chu, G.; van Driel, E.M.; Iglesias Del Sol, A.; de Jonge, E.; et al. Risk of thrombotic complications in influenza versus COVID-19 hospitalized patients. Res. Pract. Thromb. Haemost. 2021, 5, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, G.; Manzocchi-Besson, S.; Longchamp, A.; Righini, M.; Robert-Ebadi, H.; Blondon, M. Proximal deep vein thrombosis and pulmonary embolism in COVID-19 patients: A systematic review and meta-analysis. Thromb. J. 2021, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Lorente-Ros, M.; Aguilar-Gallardo, J.S.; Lizardo, C.P.; Narasimhan, H.; Morton, C.; Donahue, K.R.; Aronow, W.S. Anticoagulation in COVID-19: A review of current literature and guidelines. Hosp. Pract. 2021, 49, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators; Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Lopes, R.D.; de Barros, E.S.P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; de Aquino Martins, P.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Ainle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- INSPIRATION Investigators; Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Gupta, S.; Leaf, R.K.; Wang, W.; Rosovsky, R.P.; Brenner, S.K.; Hayek, S.S.; Berlin, H.; Kapoor, R.; Shaefi, S.; et al. Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients With COVID-19. Ann. Intern. Med. 2021, 174, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.K.; Ogunnaike, R.O.; Shah, R.; Stroever, S.; Cedeno, H.V.; Thapa, S.K.; Chronakos, J.J.; Jimenez, E.J.; Petrini, J.; Hegde, A. Clinical Outcomes with the Use of Prophylactic Versus Therapeutic Anticoagulation in Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0309. [Google Scholar] [CrossRef] [PubMed]
- Meizlish, M.L.; Goshua, G.; Liu, Y.; Fine, R.; Amin, K.; Chang, E.; DeFilippo, N.; Keating, C.; Liu, Y.; Mankbadi, M.; et al. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis. Am. J. Hematol. 2021, 96, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jonmarker, S.; Hollenberg, J.; Dahlberg, M.; Stackelberg, O.; Litorell, J.; Everhov, A.H.; Jarnbert-Pettersson, H.; Soderberg, M.; Grip, J.; Schandl, A.; et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care 2020, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Katneni, U.K.; Alexaki, A.; Hunt, R.C.; Schiller, T.; DiCuccio, M.; Buehler, P.W.; Ibla, J.C.; Kimchi-Sarfaty, C. Coagulopathy and Thrombosis as a Result of Severe COVID-19 Infection: A Microvascular Focus. Thromb. Haemost. 2020, 120, 1668–1679. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Barco, S.; Valerio, L.; Zuliani, G.; Zonzin, P.; Konstantinides, S.V. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur. J. Intern. Med. 2020, 82, 29–37. [Google Scholar] [CrossRef]
- Rostami, M.; Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Expert Rev. Hematol. 2020, 13, 1265–1275. [Google Scholar] [CrossRef]
- Gungor, B.; Atici, A.; Baycan, O.F.; Alici, G.; Ozturk, F.; Tugrul, S.; Asoglu, R.; Cevik, E.; Sahin, I.; Barman, H.A. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 39, 173–179. [Google Scholar] [CrossRef]
- Ikeagwulonu, R.C.; Ugwu, N.I.; Ezeonu, C.T.; Ikeagwulonu, Z.C.; Uro-Chukwu, H.C.; Asiegbu, U.V.; Obu, D.C.; Briggs, D.C. C-Reactive Protein and Covid-19 Severity: A Systematic Review. West Afr. J. Med. 2021, 38, 1011–1023. [Google Scholar]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Delshad, M.; Safaroghli-Azar, A.; Pourbagheri-Sigaroodi, A.; Poopak, B.; Shokouhi, S.; Bashash, D. Platelets in the perspective of COVID-19; pathophysiology of thrombocytopenia and its implication as prognostic and therapeutic opportunity. Int. Immunopharmacol. 2021, 99, 107995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Li, Y.; Liu, F.; Zhou, Q.; Peng, Z. Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: A two-center observational study. PLoS ONE 2021, 16, e0248671. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef]
- Fareed, J.; Jeske, W.; Fareed, D.; Clark, M.; Wahi, R.; Adiguzel, C.; Hoppensteadt, D. Are all low molecular weight heparins equivalent in the management of venous thromboembolism? Clin. Appl. Thromb. Hemost. 2008, 14, 385–392. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Petropoulou, A.D.; Verdy, E.; Samama, M.M.; Elalamy, I. Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular-weight heparins upon the phases of thrombin generation. J. Thromb. Haemost. 2007, 5, 955–962. [Google Scholar] [CrossRef]
- Nader, H.B.; Walenga, J.M.; Berkowitz, S.D.; Ofosu, F.; Hoppensteadt, D.A.; Cella, G. Preclinical differentiation of low molecular weight heparins. Semin. Thromb. Hemost. 1999, 25 (Suppl. S3), 63–72. [Google Scholar] [PubMed]
- Crowther, M.A.; Berry, L.R.; Monagle, P.T.; Chan, A.K. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br. J. Haematol. 2002, 116, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A. The low molecular weight heparin, tinzaparin, in thrombosis and beyond. Cardiovasc. Drug Rev. 2002, 20, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, S.A. Are low molecular weight heparins the same? Methods Mol. Med. 2004, 93, 49–59. [Google Scholar] [CrossRef]
- Johansen, K.B.; Balchen, T. Tinzaparin and other low-molecular-weight heparins: What is the evidence for differential dependence on renal clearance? Exp. Hematol. Oncol. 2013, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Mousa, S.A.; Fareed, J.; Iqbal, O.; Kaiser, B. Tissue factor pathway inhibitor in thrombosis and beyond. Methods Mol. Med. 2004, 93, 133–155. [Google Scholar] [CrossRef]
- Belen-Apak, F.B.; Sarialioglu, F. The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV-2 by inhibition of host cell proteases? Med. Hypotheses 2020, 142, 109743. [Google Scholar] [CrossRef]
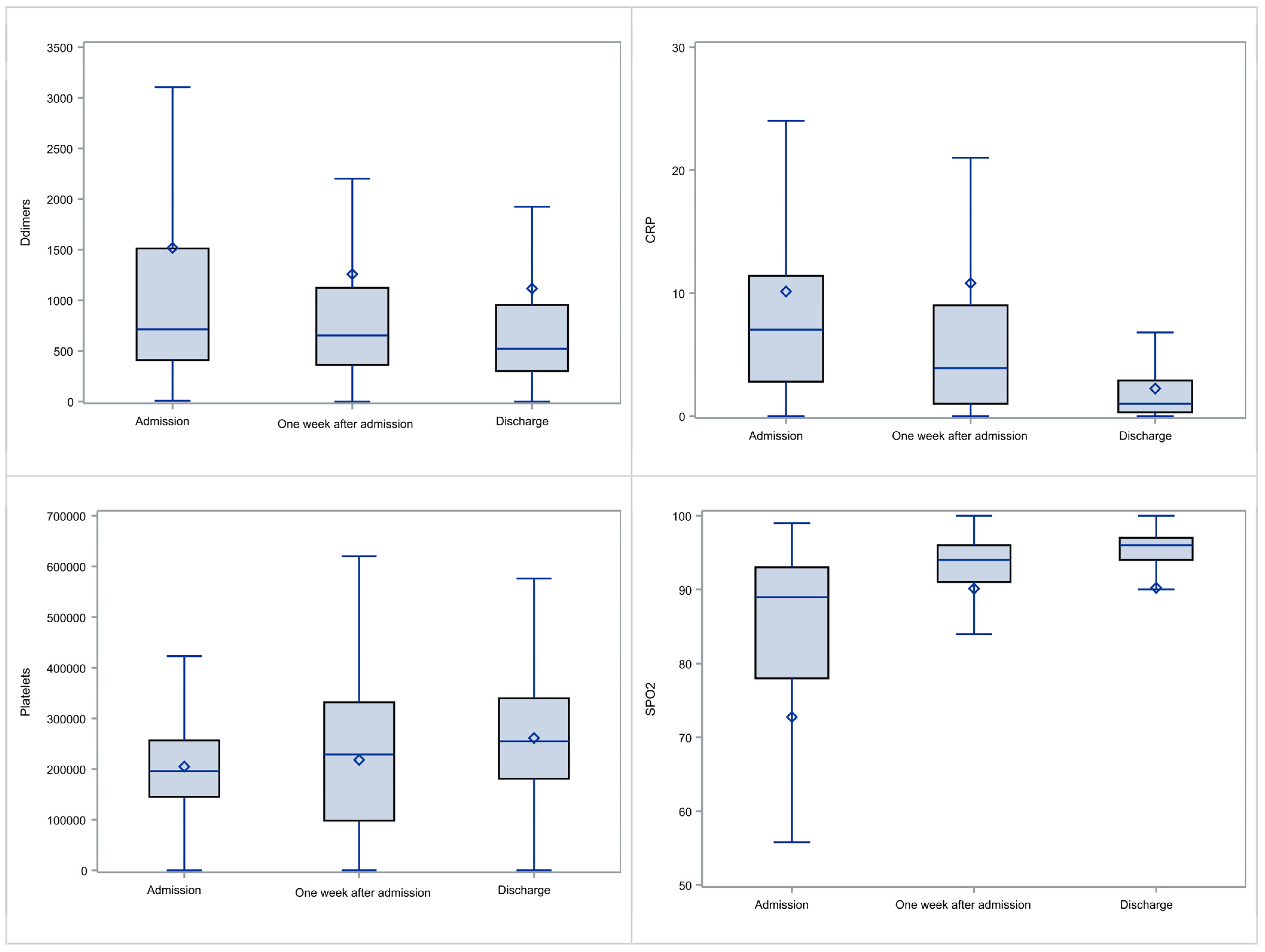
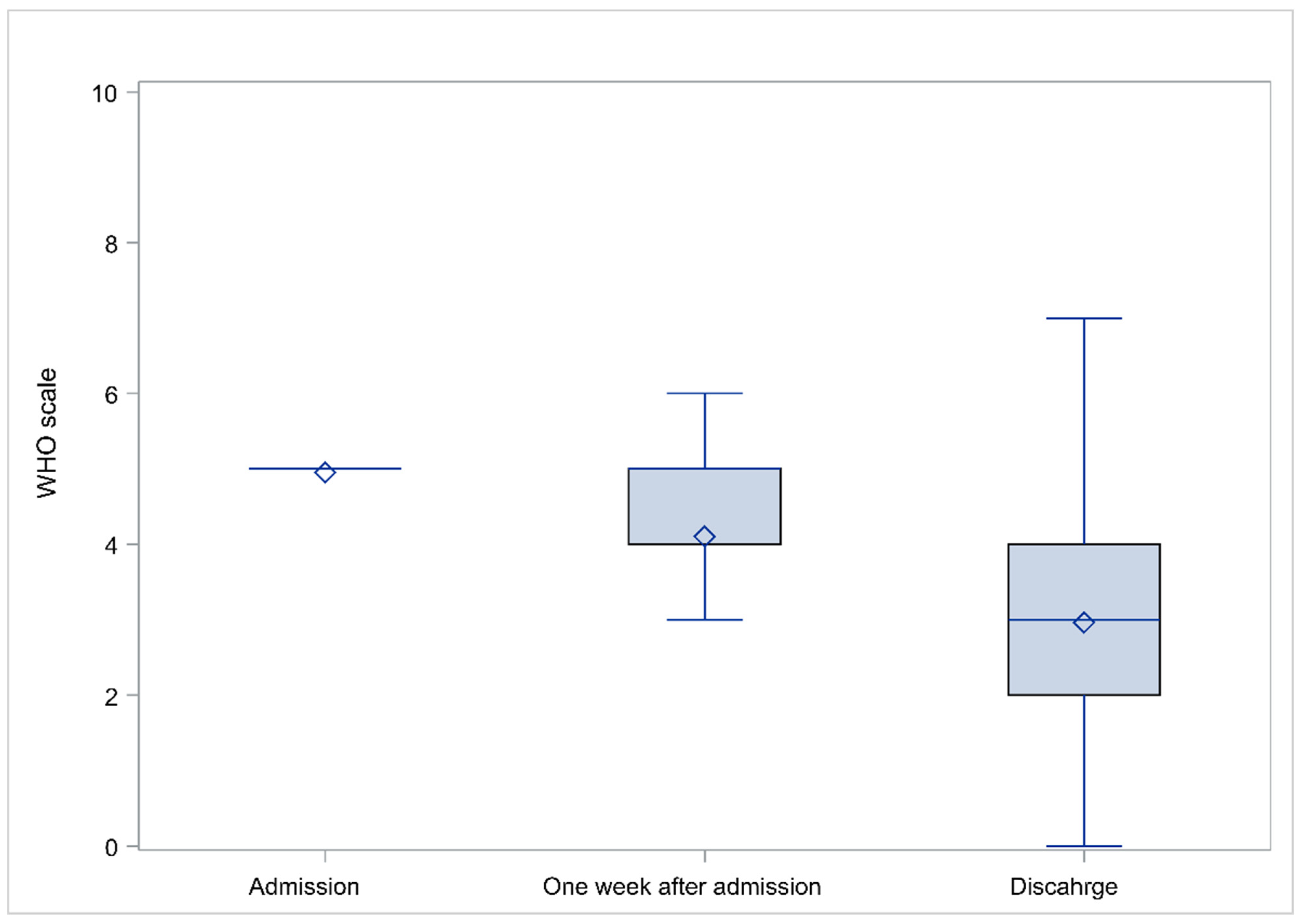
| Characteristic | Measure |
|---|---|
| Age (years) median (Q1–Q3 range) | 62 (49–72.5) |
| Gender (male) N (%) | 390 (55.2%) |
| Weight (Kgr) median (Q1–Q3 range) | 80 (72–89) |
| Height (m) median (Q1–Q3 range) | 1.7 (1.65–1.78) |
| BMI (Kgr/m2) median (Q1–Q3 range) | 27.3 (25.2–30.1) |
| Smoking N (%) | 107 (26.2%) |
| Thrombosis history N (%) | 30 (5%) |
| Heart attack | 11 |
| Stroke | 10 |
| Deep Venous Thrombosis (DVT) | 4 |
| Arterial Thrombotic Events (ATE) | 2 |
| Superficial Vein Thrombosis (SVT) | 2 |
| Type not reported | 1 |
| Bleeding history N (%) | 2 (0.4%) |
| Immobility history N (%) | 37 (8%) |
| Varicose veins N (%) | 17 (3.7%) |
| Family history of thrombosis N (%) | 2 (0.4%) |
| History of central catheter placement N (%) | 5 (1.1%) |
| Inherited thrombophilia N (%) | 1 (0.3%) |
| Recent surgery N (%) | 5 (1.2%) |
| Heart disease N (%) | 122 (18.1%) |
| Hypertension N (%) | 261 (41.5%) |
| Diabetes N (%) | 158 (25.4%) |
| Renal insufficiency N (%) | 24 (3.4%) |
| Liver disease | None |
| Inflammatory bowel disease N (%) | 18 (2.9%) |
| Endocrine disorders N (%) | 80 (12.9%) |
| Respirator problems N (%) | 58 (8.7%) |
| Malignancies N (%) | 11 (2.2%) |
| Other comorbidities N (%) | 100 (18.3%) |
| Long-term use of DOAC or acenocoumarol N (%) | 22 (5.0%) |
| Long-term use of heparins (history) N (%) | 3 (0.1%) |
| Long-term use of antiplatelet or aspirin N (%) | 56 (14.9%) |
| Primary Outcome | N | % | |
|---|---|---|---|
| Efficacy | Symptomatic distal deep vein thrombosis (DVT) | 11 | 1.6% |
| Symptomatic or incidental pulmonary embolism (PE) | 3 | 0.4% | |
| Both DVT and PE | 0 | - | |
| Fatal PE | 0 | - | |
| Total | 14 | 2.0% | |
| Safety | Major | 1 | 0.1% |
| Clinically relevant non-major bleeding (CRNMB) | 0 | - | |
| Minor | 3 | 0.4% | |
| Total | 4 | 0.6% | |
| In hospital deaths | 12 | 1.7% |
| Patients with Thrombosis (n = 14) | Patients without Thrombosis (n = 691) | |||
|---|---|---|---|---|
| Characteristic | Median (Q1–Q3) | Median (Q1–Q3) | p | |
| Demographics | Age (years) | 74.5 (62–79) | 61.9 (49–72) | 0.0149 |
| Weight (Kgr) | 74.5 (70–97) | 80 (72–89) | 0.9255 | |
| Height (meter) | 1.7 (1.6–1.8) | 1.7 (1.7–1.8) | 0.5368 | |
| BMI (Kg/m2) | 29.2 (24.2–33.2) | 27.3 (25.2–30.1) | 0.3417 | |
| Admission | CRP (mg/dL) | 7.3 (3.7–13.6) | 7.0 (2.8–11.4) | 0.6487 |
| D-dimers (µg/L) | 2490 (1580–6480) | 700 (400–1475) | <0.0001 | |
| Ferritin (ng/mL) | 429 (297–722) | 508 (278–870) | 0.7281 | |
| Hemoglobin (gm/dL) | 12.9 (11.4–14.5) | 13.6 (12.2–14.6) | 0.2871 | |
| PLTs (Count/mcL) | 221,500 (164,000–340,000) | 195,500 (145,000–255,000) | 0.1357 | |
| SpO2 (%) | 91 (87–96) | 89 (78–93) | 0.2258 | |
| Tinzaparin dose (Anti-Xa IU) | 11,000 (10.000–14,000) | 10,000 (8000–14,000) | 0.0398 | |
| WHO progression scale | 5 (5–5) | 5 (5–5) | 0.5311 | |
| One week ± two days after admission | CRP (mg/dL) | 6.7 (4–12.5) | 3.9 (1–9) | 0.0704 |
| D-dimers (µg/L) | 3510 (1458–9500) | 619 (352–1054.5) | <0.0001 | |
| Ferritin (ng/mL) | 770 (274–1047) | 542 (299–942) | 0.4253 | |
| Hemoglobin (gm/dL) | 12.3 (10.9–13.5) | 13.5 (12.1–14.2) | 0.0327 | |
| PLTs (Count/mcL) | 266,500 (130,500–366,000) | 227,000 (98,000–332,000) | 0.4367 | |
| SpO2 (%) | 92 (88.5–97) | 94 (91–96) | 0.6408 | |
| Tinzaparin dose (Anti-Xa IU) | 14,000 (10,000–18,000) | 10,000 (10,000–14,000) | 0.0608 | |
| WHO progression scale | 5 (5–5) | 5 (4–5) | 0.0769 | |
| Discharge | CRP (mg/dL) | 0.6 (0.2–2.6) | 1 (0.3–2.9) | 0.6361 |
| D-dimers (µg/L) | 1618.5 (1010–2255) | 500 (294–918) | <0.0001 | |
| Ferritin (ng/mL) | 634 (454.5–845) | 410 (210–645) | 0.0397 | |
| Hemoglobin (gm/dL) | 12.6 (10.7–14.1) | 13.3 (12–14.2) | 0.2379 | |
| PLTs (Count/mcL) | 176,000 (127,000–280,000) | 255,000 (183,000–340,000) | 0.0318 | |
| SpO2 (%) | 96 (92–97) | 96 (94–97) | 0.9332 | |
| Tinzaparin dose (Anti-Xa IU) | 14,000 (14,000–18,000) | 10,000 (10,000–14,000) | 0.0224 | |
| WHO progression scale | 4 (4–5) | 3 (2–4) | 0.0073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinosoglou, K.; Savopoulos, C.; Pouliakis, A.; Triantafyllidis, C.; Markatis, E.; Golemi, F.; Liontos, A.; Vadala, C.; Papanikolaou, I.C.; Dimakopoulou, V.; et al. Intensive-Dose Tinzaparin in Hospitalized COVID-19 Patients: The INTERACT Study. Viruses 2022, 14, 767. https://doi.org/10.3390/v14040767
Akinosoglou K, Savopoulos C, Pouliakis A, Triantafyllidis C, Markatis E, Golemi F, Liontos A, Vadala C, Papanikolaou IC, Dimakopoulou V, et al. Intensive-Dose Tinzaparin in Hospitalized COVID-19 Patients: The INTERACT Study. Viruses. 2022; 14(4):767. https://doi.org/10.3390/v14040767
Chicago/Turabian StyleAkinosoglou, Karolina, Christos Savopoulos, Abraham Pouliakis, Charalampos Triantafyllidis, Eleftherios Markatis, Foteini Golemi, Angelos Liontos, Charikleia Vadala, Ilias C. Papanikolaou, Vasiliki Dimakopoulou, and et al. 2022. "Intensive-Dose Tinzaparin in Hospitalized COVID-19 Patients: The INTERACT Study" Viruses 14, no. 4: 767. https://doi.org/10.3390/v14040767
APA StyleAkinosoglou, K., Savopoulos, C., Pouliakis, A., Triantafyllidis, C., Markatis, E., Golemi, F., Liontos, A., Vadala, C., Papanikolaou, I. C., Dimakopoulou, V., Xarras, P., Varela, K., Kaiafa, G., Mitsianis, A., Chatzistamati, A., Randou, E., Savvanis, S., Pavlaki, M., Efraimidis, G., ... on behalf of the INTERACT Study Group. (2022). Intensive-Dose Tinzaparin in Hospitalized COVID-19 Patients: The INTERACT Study. Viruses, 14(4), 767. https://doi.org/10.3390/v14040767








