Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-like Illness in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Nasopharyngeal and Oropharyngeal Swabs
2.3. Analysis of Viruses by MLPA
2.4. Statistical Analysis
3. Results
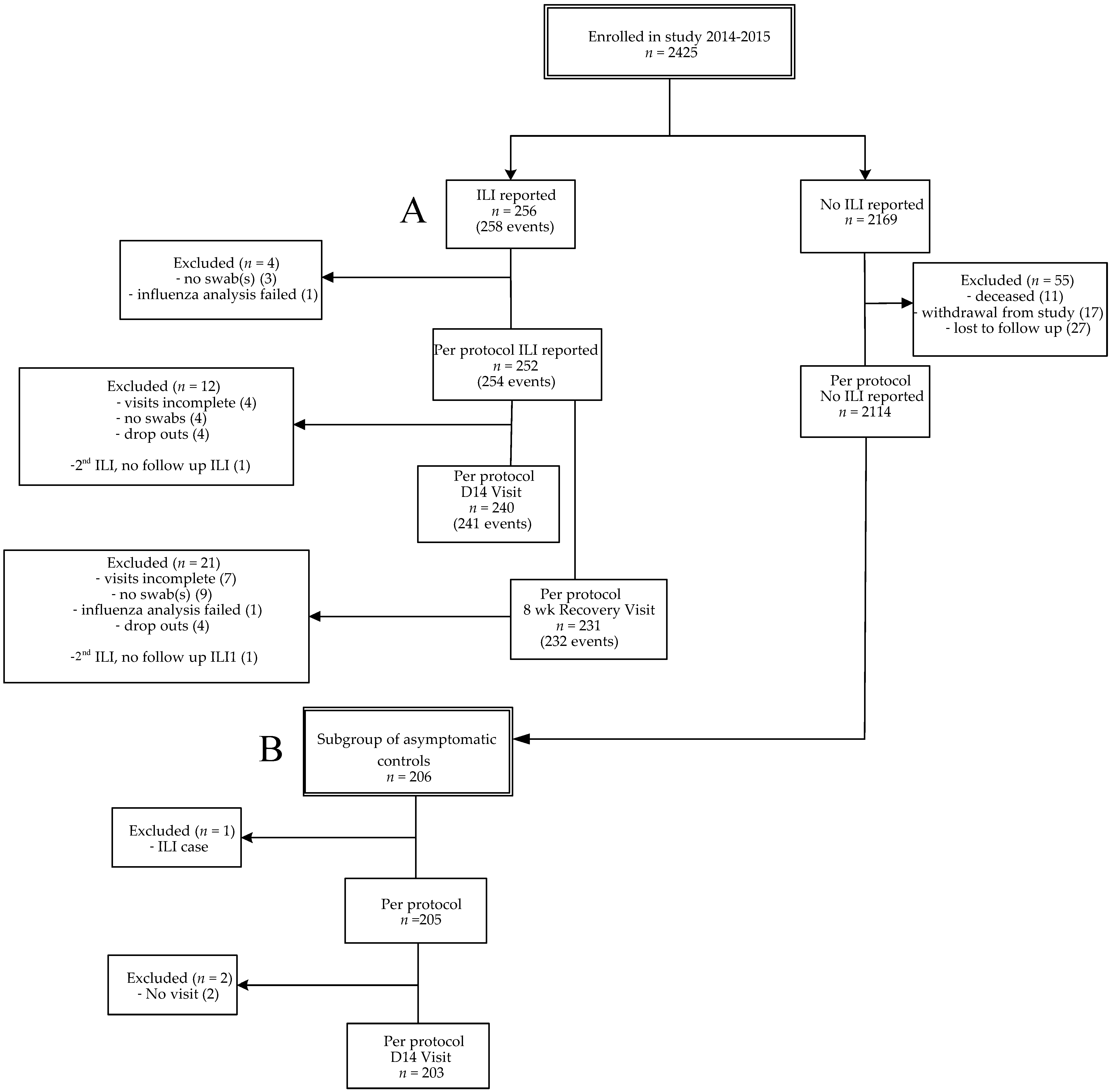
3.1. Study Cohort and ILI Incidence
3.2. Influenza Virus Infection in ILI Cases and Influenza Vaccine Effectiveness
3.3. Other Respiratory Viruses Detected in ILI Cases from Season 2014–2015
3.4. Persistence of Viruses in ILI Cases
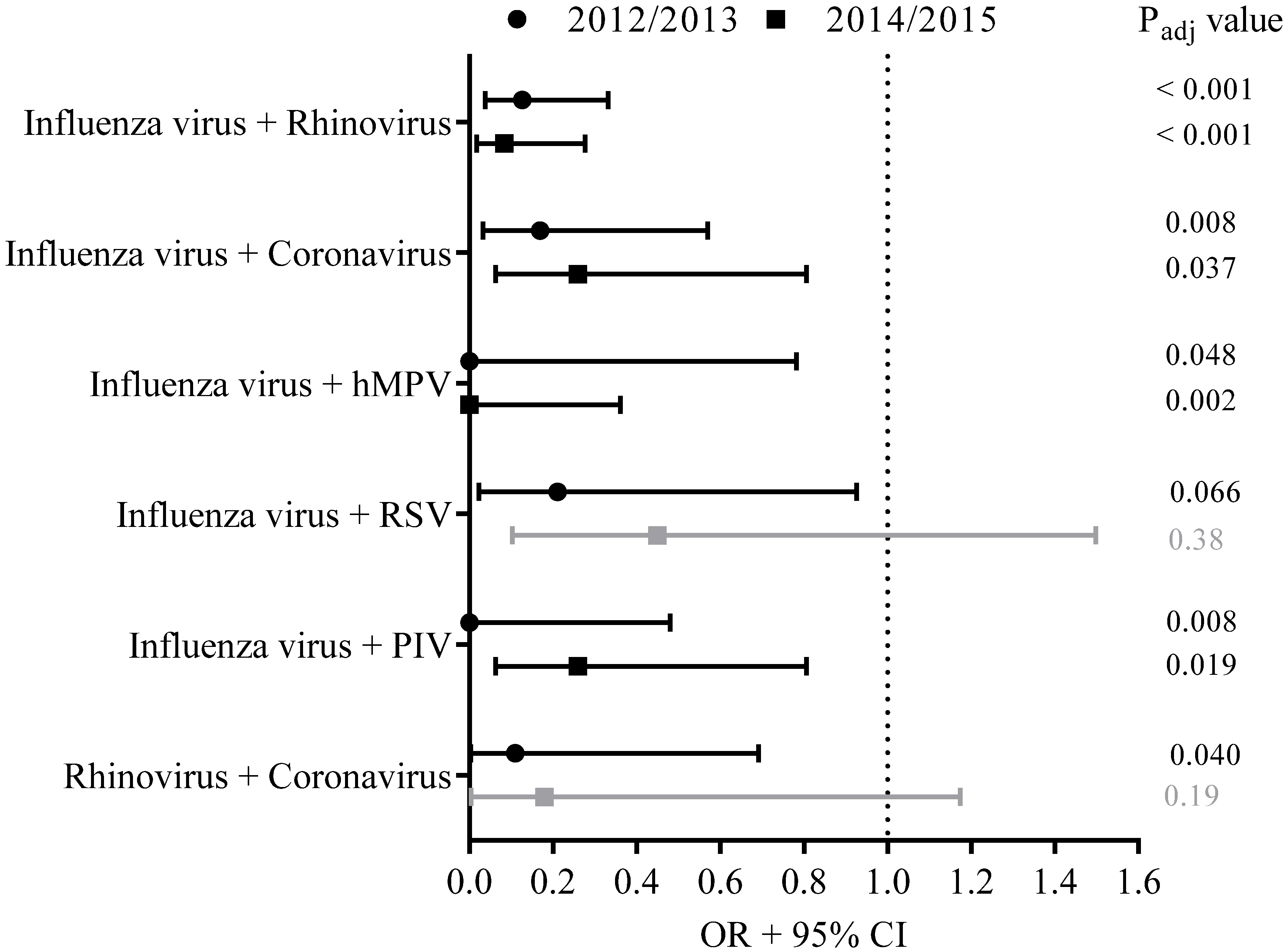
3.5. Viral Co-Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Fact Sheet N°211: Influenza (Seasonal). Available online: http://www.who.int/mediacentre/factsheets/fs211/en/index.html (accessed on 5 April 2022).
- van Beek, J.; Veenhoven, R.H.; Bruin, J.P.; van Boxtel, R.A.J.; de Lange, M.M.A.; Meijer, A.; Sanders, E.A.M.; Rots, N.Y.; Luytjes, W. Influenza-like Illness Incidence Is Not Reduced by Influenza Vaccination in a Cohort of Older Adults, Despite Effectively Reducing Laboratory-Confirmed Influenza Virus Infections. J. Infect. Dis. 2017, 216, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; McElhaney, J.E.; Beran, J.; van Essen, G.A.; Duval, X.; Esen, M.; Galtier, F.; Gervais, P.; Hwang, S.J.; Kremsner, P.; et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J. Infect. Dis. 2014, 209, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Sakamoto, T.; Tanaka, K. Influenza vaccine effectiveness among elderly persons living in the community during the 2003–2004 season. Vaccine 2008, 26, 6477–6480. [Google Scholar] [CrossRef]
- Nicholson, K.G.; Kent, J.; Hammersley, V.; Cancio, E. Acute viral infections of upper respiratory tract in elderly people living in the community: Comparative, prospective, population based study of disease burden. BMJ 1997, 315, 1060–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gageldonk-Lafeber, A.B.; Heijnen, M.L.; Bartelds, A.I.; Peters, M.F.; van der Plas, S.M.; Wilbrink, B. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin. Infect. Dis. 2005, 41, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Cilla, G.; Onate, E.; Perez-Yarza, E.G.; Montes, M.; Vicente, D.; Perez-Trallero, E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008, 80, 1843–1849. [Google Scholar] [CrossRef]
- Franz, A.; Adams, O.; Willems, R.; Bonzel, L.; Neuhausen, N.; Schweizer-Krantz, S.; Ruggeberg, J.U.; Willers, R.; Henrich, B.; Schroten, H.; et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J. Clin. Virol. 2010, 48, 239–245. [Google Scholar] [CrossRef]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple versus single virus respiratory infections: Viral load and clinical disease severity in hospitalized children. Influenza Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef]
- Pel, J.Z.S. Proefonderzoek naar de frequentie en de aetiologie van griepachtige ziekten in de winter 1963–1964. Huisarts Wet. 1965, 86, 321. [Google Scholar]
- Meijer, A.; Beerens, A.; Claas, E.; Hermans, M.; de Jong, A.; Molenkamp, R.; Niesters, H.; Overduin, P.; Rossen, J.; Schuurman, R.; et al. Preparing the outbreak assistance laboratory network in the Netherlands for the detection of the influenza virus A(H1N1) variant. J. Clin. Virol. 2009, 45, 179–184. [Google Scholar] [CrossRef]
- Biere, B.; Bauer, B.; Schweiger, B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J. Clin. Microbiol. 2010, 48, 1425–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Hoek, W.; Dijkstra, F.; de Lange, M.M.; Donker, G.A.; Meijer, A.; van der Sande, M.A. Letter to the editor: Influenza vaccine effectiveness: Heterogeneity in estimates for the 2012/13 season. Euro Surveill. 2013, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Kissling, E.; Emborg, H.D.; Larrauri, A.; McMenamin, J.; Pozo, F.; Trebbien, R.; Mazagatos, C.; Whitaker, H.; Valenciano, M.; et al. Interim 2019/20 influenza vaccine effectiveness: Six European studies, September 2019 to January 2020. Euro Surveill. 2020, 25, 2000153. [Google Scholar] [CrossRef] [Green Version]
- Pebody, R.; Warburton, F.; Andrews, N.; Ellis, J.; von Wissmann, B.; Robertson, C.; Yonova, I.; Cottrell, S.; Gallagher, N.; Green, H.; et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill. 2015, 20, 30013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenciano, M.; Kissling, E.; Reuss, A.; Rizzo, C.; Gherasim, A.; Horvath, J.K.; Domegan, L.; Pitigoi, D.; Machado, A.; Paradowska-Stankiewicz, I.A.; et al. Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co-circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE Multicentre Case-Control Study, Europe 2014/15. Euro Surveill. 2016, 21, 30139. [Google Scholar] [CrossRef] [PubMed]
- Belazi, S.; Olsen, S.J.; Brown, C.; Green, H.K.; Mook, P.; Nguyen-Van-Tam, J.; Penttinen, P.; Lansbury, L. Spotlight influenza: Laboratory-confirmed seasonal influenza in people with acute respiratory illness: A literature review and meta-analysis, WHO European Region, 2004 to 2017. Euro Surveill. 2021, 26, 2000343. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, K.; Hallmann-Szelinska, E.; Kondratiuk, K.; Brydak, L.B. Antigenic Drift of A/H3N2/Virus and Circulation of Influenza-Like Viruses during the 2014/2015 Influenza Season in Poland. Adv. Exp. Med. Biol. 2016, 905, 33–38. [Google Scholar] [CrossRef]
- Petat, H.; Gajdos, V.; Angoulvant, F.; Vidalain, P.O.; Corbet, S.; Marguet, C.; Brouard, J.; Vabret, A.; Gouilh, M.A. High Frequency of Viral Co-Detections in Acute Bronchiolitis. Viruses 2021, 13, 990. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 16, 27142–27150. [Google Scholar] [CrossRef] [Green Version]
- Baillie, V.L.; Moore, D.P.; Mathunjwa, A.; Baggett, H.C.; Brooks, A.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.C.; Knoll, M.D.; Kotloff, K.L.; et al. Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study. Viruses 2021, 13, 1249. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [PubMed]
- Lindenmann, J. From interference to interferon: A brief historical introduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 299, 3–6. [Google Scholar] [PubMed]
- DaPalma, T.; Doonan, B.P.; Trager, N.M.; Kasman, L.M. A systematic approach to virus-virus interactions. Virus Res. 2010, 149, 1–9. [Google Scholar] [CrossRef]
- Callahan, Z.Y.; Smith, T.K.; Ingersoll, C.; Gardner, R.; Korgenski, E.K.; Sloan, C.D. Comparative Seasonal Respiratory Virus Epidemic Timing in Utah. Viruses 2020, 12, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casalegno, J.S.; Ottmann, M.; Duchamp, M.B.; Escuret, V.; Billaud, G.; Frobert, E.; Morfin, F.; Lina, B. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin. Microbiol. Infect. 2010, 16, 326–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anestad, G.; Nordbo, S.A. Virus interference. Did rhinoviruses activity hamper the progress of the 2009 influenza A (H1N1) pandemic in Norway? Med. Hypotheses 2011, 77, 1132–1134. [Google Scholar] [CrossRef]
- Casalegno, J.S.; Ottmann, M.; Bouscambert-Duchamp, M.; Valette, M.; Morfin, F.; Lina, B. Impact of the 2009 influenza A(H1N1) pandemic wave on the pattern of hibernal respiratory virus epidemics, France, 2009. Euro Surveill. 2010, 15, 19485. [Google Scholar] [CrossRef]
- Hirsh, S.; Hindiyeh, M.; Kolet, L.; Regev, L.; Sherbany, H.; Yaary, K.; Mendelson, E.; Mandelboim, M. Epidemiological changes of respiratory syncytial virus (RSV) infections in Israel. PLoS ONE 2014, 9, e90515. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the Respiratory Tract: Viral Competition for Resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef]
- ECDC. Influenza Case Definitions. Available online: http://ecdc.europa.eu/en/healthtopics/influenza/surveillance/Pages/influenza_case_definitions.aspx (accessed on 1 March 2022).
- WHO. ILI SARI Surveillance Case Definition. Available online: http://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/ (accessed on 1 March 2022).
- CDC. Overview of Influenza Surveillance in the United States. Available online: http://www.cdc.gov/flu/weekly/overview.htm (accessed on 1 March 2022).

| 2014/2015 | ||||||
|---|---|---|---|---|---|---|
| All (n = 2366) | ILI (n = 252) | No ILI (n = 2114) | Asymptomatic Controls (n = 205) | p Value ILI vs. No ILI | p Value ILI vs. Asymptomic Controls | |
| Male sex | 1205 (50.9%) | 121 (48.0%) | 1084 (51.3%) | 107 (52.2%) | NS | NS |
| Age, y, mean (range) | 70.9 (60–94) | 69.6 (60–88) | 71.1 (60–94) | 71.4 (60–88) | 0.001 | 0.001 |
| Influenza vaccination 2014/2015 | 1614 (68.2%) | 168 (66.7%) | 1446 (68.4%) | 165 (80.5%) | NS | 0.001 |
| ILI (n = 252) | Asymptomatic Controls (n = 205) | p Value | |
|---|---|---|---|
| Any chronic illness * | 113 (44.8%) | 85 (41.5%) | NS |
| % vaccinated with any chronic illness | 90 (79.6%) | 75 (88.2%) | |
| % vaccinated without chronic illness | 78 (56.1%) | 90 (75%) | |
| p value (Pearson χ2 test) | 0.0001 | 0.02 |
| Vaccinated | Non-Vaccinated | p Value | |
|---|---|---|---|
| (n = 168 Participants) | (n = 84 Participants) | ||
| Influenza virus | 69 (41.1%) | 31 (36.9%) | NS |
| Influenza virus A | 57 (33.9%) | 19 (22.6%) | NS |
| A(H3N2) | 52 (31.0%) | 16 (19.0%) | NS |
| 31 (18.4%) | 8 (9.5%) | NS |
| 14 (8.3%) | 8 (9.5%) | NS |
| A(H1N1)pdm09 | 5 (3.0%) | 3 (3.6%) | NS |
| Influenza virus B | 12 (7.1%) | 12 (14.3%) | NS |
| |||
| Coronavirus | 16 (9.5%) | 9 (10.7%) | NS |
| hMPV | 13 (7.7%) | 3 (3.6%) | NS |
| RSV | 9 (5.4%) | 8 (9.5%) | NS |
| Rhinoviruses | 28 (16.7%) | 16 (19.0%) | NS |
| Parainfluenza virus | 6 (3.6%) | 5 (6.0%) | NS |
| n | Odds Ratio (95% CI) | VE (95% CI) | |||
|---|---|---|---|---|---|
| 2014/2015 | |||||
| Influenza virus | 210 | 1.005 [0.538–1.878] | −1% [−88–46%] | ||
| A | 186 | 1.170 [0.571–2.399] | −17% [−140–43%] | ||
| A(H3N2) | 178 | 1.261 [0.609–2.610] | −26% [−161–39%] | ||
| 149 | 1.965 [0.733–5.270] | −96% [−427–27%] | ||
| 132 | 0.400 [0.131–1.222] | 60% [−22–87%] | ||
| A(H1N1)pdm09 | 118 | 1.339 [0.274–6.555] | −34% [−555–73%] | ||
| B | B/Yamagata-like | 134 | 0.509 [0.187–1.389] | 49% [−39–81%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaaijk, P.; Swaans, N.; Nicolaie, A.M.; Bruin, J.P.; van Boxtel, R.A.J.; de Lange, M.M.A.; Meijer, A.; Sanders, E.A.M.; van Houten, M.A.; Rots, N.Y.; et al. Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-like Illness in Older Adults. Viruses 2022, 14, 797. https://doi.org/10.3390/v14040797
Kaaijk P, Swaans N, Nicolaie AM, Bruin JP, van Boxtel RAJ, de Lange MMA, Meijer A, Sanders EAM, van Houten MA, Rots NY, et al. Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-like Illness in Older Adults. Viruses. 2022; 14(4):797. https://doi.org/10.3390/v14040797
Chicago/Turabian StyleKaaijk, Patricia, Niels Swaans, Alina M. Nicolaie, Jacob P. Bruin, Renée A. J. van Boxtel, Marit M. A. de Lange, Adam Meijer, Elisabeth A. M. Sanders, Marianne A. van Houten, Nynke Y. Rots, and et al. 2022. "Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-like Illness in Older Adults" Viruses 14, no. 4: 797. https://doi.org/10.3390/v14040797
APA StyleKaaijk, P., Swaans, N., Nicolaie, A. M., Bruin, J. P., van Boxtel, R. A. J., de Lange, M. M. A., Meijer, A., Sanders, E. A. M., van Houten, M. A., Rots, N. Y., Luytjes, W., & van Beek, J. (2022). Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-like Illness in Older Adults. Viruses, 14(4), 797. https://doi.org/10.3390/v14040797






