Updates on HTLV-1 Uveitis
Abstract
1. Introduction
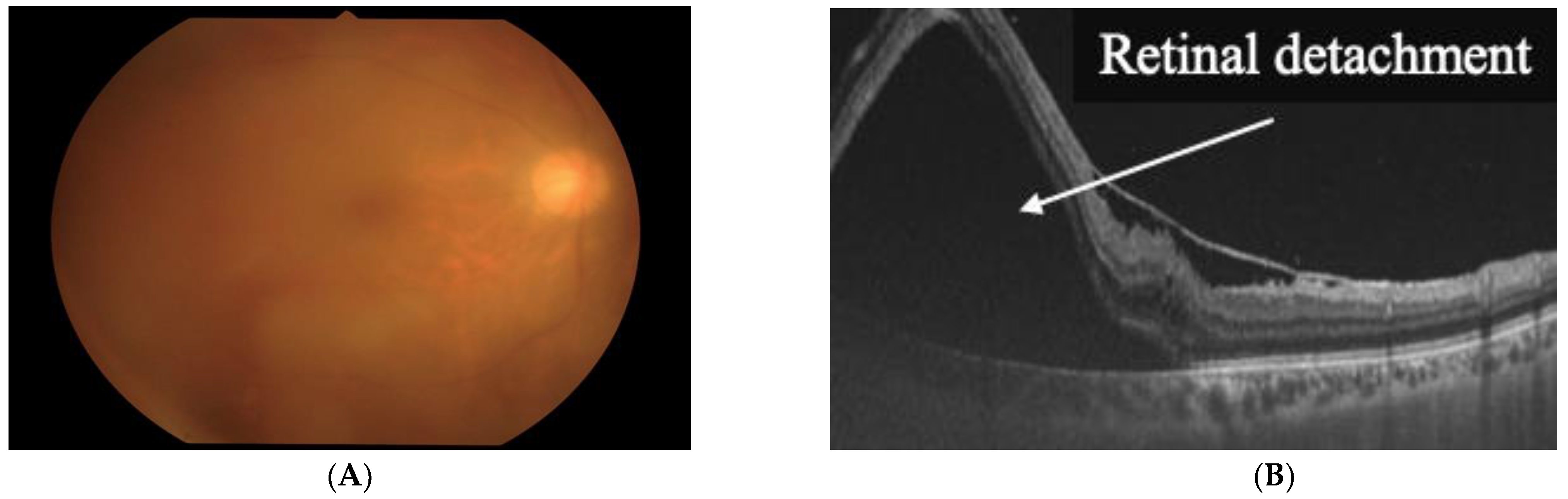
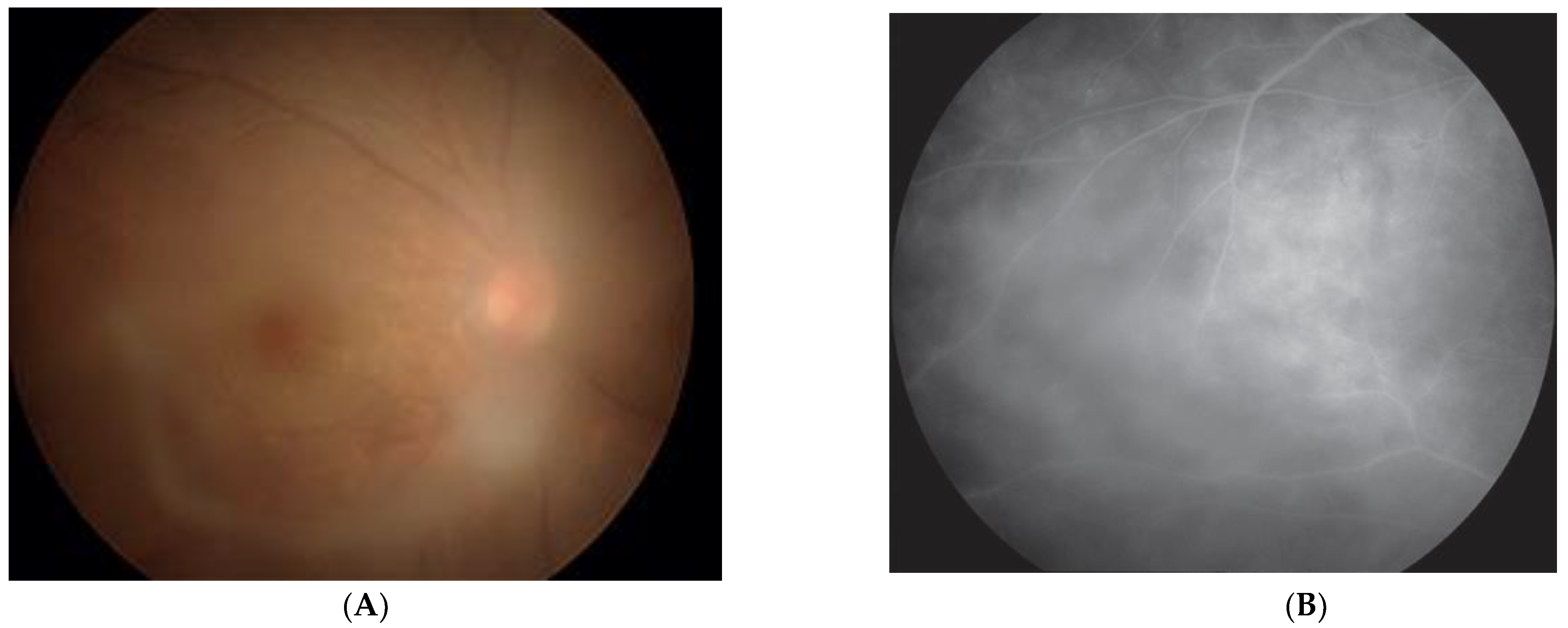
2. Clinical Features of HU
3. Tackling HTLV-1 in Ophthalmology: Nationwide Survey in Japan
4. Transmission Route for the Development of HU
5. Factors Associated with the Development of HU
6. Safety of Biologics in Terms of the Eye in HTLV-1 Infection
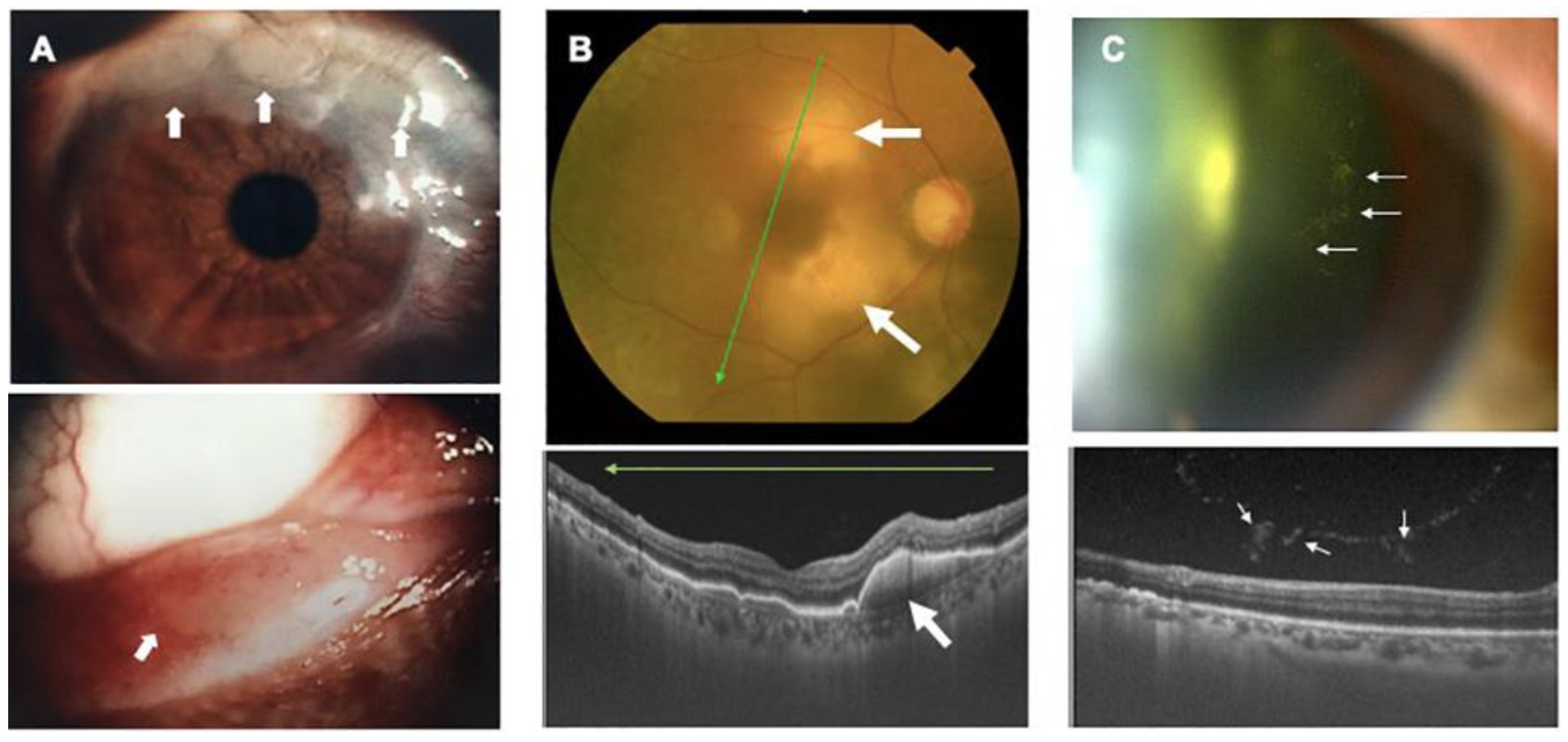
7. Ocular Manifestations of HTLV-1-Associated Diseases
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffin, J.M. The discovery of HTLV-1, the first pathogenic human retrovirus. Proc. Natl. Acad. Sci. USA 2015, 112, 15525–15529. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. HTLV-1-associated diseases. Int. J. Hematol. 1997, 66, 257–278. [Google Scholar] [CrossRef]
- WHO. Human T-Lymphotropic Virus Type 1: Technical Report. 2021. Available online: https://www.who.int/publications/i/item/9789240020221 (accessed on 25 February 2022).
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef]
- Yoshida, M.; Seiki, M.; Yamaguchi, K.; Takatsuki, K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 1984, 81, 2534–2537. [Google Scholar] [CrossRef]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de The, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986, 1, 1031–1032. [Google Scholar] [CrossRef]
- Ohba, N.; Matsumoto, M.; Sameshima, M.; Kabayama, Y.; Nakao, K.; Unoki, K.; Uehara, F.; Kawano, K.; Maruyama, I.; Osame, M. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn. J. Ophthalmol. 1989, 33, 1–12. [Google Scholar]
- Nakao, K.; Matsumoto, M.; Ohba, N. Seroprevalence of antibodies to HTLV-I in patients with ocular disorders. Br. J. Ophthalmol. 1991, 75, 76–78. [Google Scholar] [CrossRef]
- Mochizuki, M.; Watanabe, T.; Yamaguchi, K.; Takatsuki, K.; Yoshimura, K.; Shirao, M.; Nakashima, S.; Mori, S.; Araki, S.; Miyata, N. HTLV-I uveitis: A distinct clinical entity caused by HTLV-I. Jpn. J. Cancer Res. 1992, 83, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamaguchi, K.; Takatsuki, K.; Watanabe, T.; Mori, S.; Tajima, K. HTLV-I and uveitis. Lancet 1992, 339, 1110. [Google Scholar] [CrossRef]
- Iwanaga, M. Epidemiology of HTLV-1 Infection and ATL in Japan: An Update. Front. Microbiol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Ikeda, E.; Ono, A.; Hikita, N.; Arima, K.; Mochizuki, M.; Yamaguchi, K.; Tajima, K.; Kiyokawa, H. Estimated Prevalence Rate of HTLV-I Uveitis in Chikugo. J. Jpn. Ophthalmol. Soc. 1998, 102, 327–332. [Google Scholar]
- Ijichi, S.; Osame, M. Human T lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis (HAM/TSP): Recent perspectives. Intern. Med. 1995, 34, 713–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamoi, K.; Mochizuki, M. HTLV-1 uveitis. Front. Microbiol. 2012, 3, 270. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K.; Mochizuki, M. HTLV infection and the eye. Curr. Opin. Ophthalmol. 2012, 23, 557–561. [Google Scholar] [CrossRef]
- Kamoi, K. HTLV-1 in Ophthalmology. Front. Microbiol. 2020, 11, 388. [Google Scholar] [CrossRef]
- Kamoi, K.; Okayama, A.; Izumo, S.; Hamaguchi, I.; Uchimaru, K.; Tojo, A.; Watanabe, T.; Ohno-Matsui, K. Tackling HTLV-1 infection in ophthalmology: A nationwide survey of ophthalmic care in an endemic country, Japan. Br. J. Ophthalmol. 2020, 104, 1647–1651. [Google Scholar] [CrossRef]
- Beilke, M.A. Human T-cell lymphotropic virus type I-associated uveitis in an African American. Am. J. Ophthalmol. 1995, 119, 104–105. [Google Scholar] [CrossRef]
- Merle, H.; Smadja, D.; Le Hoang, P.; Bera, O.; Cabre, P.; Landau, M.; Vernant, J.C. Ocular manifestations in patients with HTLV-I associated infection--a clinical study of 93 cases. Jpn. J. Ophthalmol. 1996, 40, 260–270. [Google Scholar] [PubMed]
- Sandy, G.J.; Taylor, J.; Steiger, M.; Wearne, M.; Weber, J.N. HTLV-I associated uveitis: A report of two cases. Eye 1996, 10, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.H.; Segurado, A.A.; Hirata, C.E.; Sampaio, M.W.; Souza, E.C.; Nukui, Y.; Cliquet, M.; Saez-Alquezar, A.; Olivalves, E.; Mochizuki, M. Human T-cell lymphotropic virus type 1 infection and ocular manifestations in Sao Paulo, Brazil. Arch. Ophthalmol. 1999, 117, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Chew, R.; Henderson, T.; Aujla, J.; Whist, E.; Einsiedel, L. Turning a blind eye: HTLV-1-associated uveitis in Indigenous adults from Central Australia. Int. Ophthalmol. 2018, 38, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Kamoi, K.; Komizo, T.; Miyata, K.; Mochizuki, M. Human T Cell Leukemia Virus Type 1 and Eye Diseases. J. Ocul. Pharmacol. Ther. 2017, 33, 216–223. [Google Scholar] [CrossRef]
- Sonoda, K.H.; Hasegawa, E.; Namba, K.; Okada, A.A.; Ohguro, N.; Goto, H.; Group, J.U.S.W. Epidemiology of uveitis in Japan: A 2016 retrospective nationwide survey. Jpn. J. Ophthalmol. 2021, 65, 184–190. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sugita, S.; Kamoi, K. Immunological homeostasis of the eye. Prog. Retin. Eye Res. 2013, 33, 10–27. [Google Scholar] [CrossRef]
- Kamoi, K.; Mochizuki, M. HTLV-1 Uveitis. In Intraocular Inflammation; Zierhut, M., Pavesio, C., Ohno, S., Orefice, F., Rao, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1197–1201. [Google Scholar]
- Kamoi, K.; Mochizuki, M. Human T-Cell Leukemia Virus Type 1. In Emerging Infectious Uveitis; Chee, S.-P., Khairallah, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 143–148. [Google Scholar]
- Nakao, K.; Ohba, N. Clinical features of HTLV-I associated uveitis. Br. J. Ophthalmol. 1993, 77, 274–279. [Google Scholar] [CrossRef]
- Takahashi, T.; Takase, H.; Urano, T.; Sugita, S.; Miyata, K.; Miyata, N.; Mochizuki, M. Clinical features of human T-lymphotropic virus type 1 uveitis: A long-term follow-up. Ocul. Immunol. Inflamm. 2000, 8, 235–241. [Google Scholar] [CrossRef]
- Ohba, N.; Nakao, K.; Isashiki, Y.; Kaminagayoshi, T.; Sonoda, S.; Yashiki, S.; Osame, M. Clinical features of HTLV-I associated uveitis determined in multicenter collaborative study. Study Group for HTLV-I Associated Ocular Diseases. Jpn. J. Ophthalmol. 1994, 38, 168–174. [Google Scholar]
- Yamaguchi, K.; Mochizuki, M.; Watanabe, T.; Yoshimura, K.; Shirao, M.; Araki, S.; Miyata, N.; Mori, S.; Kiyokawa, T.; Takatsuki, K. Human T lymphotropic virus type 1 uveitis after Graves’ disease. Br. J. Ophthalmol. 1994, 78, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, M.; Shimizu, K.; Kawaguchi, T.; Miyata, K.; Mochizuki, M. A clinical survey of uveitis in HTLV-1 endemic region. Ocul. Immunol. Inflamm. 2009, 17, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Abematsu, N.; Sakamoto, T. Systemic diseases in patients with HTLV-1-associated uveitis. Br. J. Ophthalmol. 2018, 102, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Current status of HTLV-1 infection. Int. J. Hematol. 2011, 94, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Chiyoda, S.; Kusaba, E.; Kamihira, S. Trends in the seroprevalence of HTLV-1 in Japanese blood donors in Nagasaki Prefecture, 2000–2006. Int. J. Hematol. 2009, 90, 186–190. [Google Scholar] [CrossRef]
- Nishijima, T.; Shimada, S.; Noda, H.; Miyake, K. Towards the elimination of HTLV-1 infection in Japan. Lancet Infect. Dis. 2019, 19, 15–16. [Google Scholar] [CrossRef]
- Satake, M.; Iwanaga, M.; Sagara, Y.; Watanabe, T.; Okuma, K.; Hamaguchi, I. Incidence of human T-lymphotropic virus 1 infection in adolescent and adult blood donors in Japan: A nationwide retrospective cohort analysis. Lancet Infect. Dis. 2016, 16, 1246–1254. [Google Scholar] [CrossRef]
- Goncalves, D.U.; Proietti, F.A.; Ribas, J.G.; Araujo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef]
- Rosadas, C.; Taylor, G.P. Mother-to-Child HTLV-1 Transmission: Unmet Research Needs. Front. Microbiol. 2019, 10, 999. [Google Scholar] [CrossRef]
- Kamoi, K.; Uchimaru, K.; Tojo, A.; Watanabe, T.; Ohno-Matsui, K. HTLV-1 uveitis and Graves’ disease presenting with sudden onset of blurred vision. Lancet 2022, 399, 60. [Google Scholar] [CrossRef]
- Kamoi, K.; Horiguchi, N.; Kurozumi-Karube, H.; Hamaguchi, I.; Yamano, Y.; Uchimaru, K.; Tojo, A.; Watanabe, T.; Ohno-Matsui, K. Horizontal transmission of HTLV-1 causing uveitis. Lancet Infect. Dis. 2021, 21, 578. [Google Scholar] [CrossRef]
- Bangham, C.R.; Cook, L.B.; Melamed, A. HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin. Cancer Biol. 2014, 26, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Akbarin, M.M.; Rahimi, H.; Hassannia, T.; Shoja Razavi, G.; Sabet, F.; Shirdel, A. Comparison of HTLV-I Proviral Load in Adult T Cell Leukemia/Lymphoma (ATL), HTLV-I-Associated Myelopathy (HAM-TSP) and Healthy Carriers. Iran. J. Basic. Med. Sci. 2013, 16, 208–212. [Google Scholar] [PubMed]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: A nationwide prospective study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef]
- Bathon, J.M.; Martin, R.W.; Fleischmann, R.M.; Tesser, J.R.; Schiff, M.H.; Keystone, E.C.; Genovese, M.C.; Wasko, M.C.; Moreland, L.W.; Weaver, A.L.; et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N. Engl. J. Med. 2000, 343, 1586–1593. [Google Scholar] [CrossRef]
- Leonardi, C.L.; Powers, J.L.; Matheson, R.T.; Goffe, B.S.; Zitnik, R.; Wang, A.; Gottlieb, A.B.; Etanercept Psoriasis Study, G. Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 2003, 349, 2014–2022. [Google Scholar] [CrossRef]
- Braun, J.; Baraliakos, X.; Brandt, J.; Listing, J.; Zink, A.; Alten, R.; Burmester, G.; Gromnica-Ihle, E.; Kellner, H.; Schneider, M.; et al. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology 2005, 44, 670–676. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Van Assche, G.; Vermeire, S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 2004, 126, 1593–1610. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Mullard, A. Top product sales forecasts for 2018. Nat. Rev. Drug Discov. 2018, 17, 86. [Google Scholar] [CrossRef]
- Toussirot, E.; Aubin, F. Paradoxical reactions under TNF-alpha blocking agents and other biological agents given for chronic immune-mediated diseases: An analytical and comprehensive overview. RMD Open. 2016, 2, e000239. [Google Scholar] [CrossRef] [PubMed]
- Wendling, D.; Prati, C. Paradoxical effects of anti-TNF-alpha agents in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Umekita, K.; Okayama, A. HTLV-1 Infection and Rheumatic Diseases. Front. Microbiol. 2020, 11, 152. [Google Scholar] [CrossRef]
- Terada, Y.; Kamoi, K.; Ohno-Matsui, K.; Miyata, K.; Yamano, C.; Coler-Reilly, A.; Yamano, Y. Treatment of rheumatoid arthritis with biologics may exacerbate HTLV-1-associated conditions: A case report. Medicine 2017, 96, e6021. [Google Scholar] [CrossRef] [PubMed]
- Melsheimer, R.; Geldhof, A.; Apaolaza, I.; Schaible, T. Remicade((R)) (infliximab): 20 years of contributions to science and medicine. Biologics 2019, 13, 139–178. [Google Scholar] [PubMed]
- Uchida, M.; Kamoi, K.; Ando, N.; Wei, C.; Karube, H.; Ohno-Matsui, K. Safety of Infliximab for the Eye Under Human T-Cell Leukemia Virus Type 1 Infectious Conditions in vitro. Front. Microbiol. 2019, 10, 2148. [Google Scholar] [CrossRef]
- Kurozumi-Karube, H.; Kamoi, K.; Ando, N.; Uchida, M.; Hamaguchi, I.; Ohno-Matsui, K. In Vitro Evaluation of the Safety of Adalimumab for the Eye Under HTLV-1 Infection Status: A Preliminary Study. Front Microbiol. 2020, 11, 522579. [Google Scholar] [CrossRef]
- Kamoi, K.; Okayama, A.; Izumo, S.; Hamaguchi, I.; Uchimaru, K.; Tojo, A.; Ohno-Matsui, K. Adult T-Cell Leukemia/Lymphoma-Related Ocular Manifestations: Analysis of the First Large-Scale Nationwide Survey. Front. Microbiol. 2018, 9, 3240. [Google Scholar] [CrossRef]
- Kamoi, K.; Nagata, Y.; Mochizuki, M.; Kobayashi, D.; Ohno, N.; Uchimaru, K.; Tojo, A.; Ohno-Matsui, K. Formation of Segmental Rounded Nodules During Infiltration of Adult T-Cell Leukemia Cells Into the Ocular Mucous Membrane. Cornea 2016, 35, 137–139. [Google Scholar] [CrossRef]
- Kamoi, K.; Ohno-Matsui, K. Intraocular Infiltration. Am. J. Trop. Med. Hyg. 2020, 102, 7–8. [Google Scholar] [CrossRef]
- Sugita, S.; Shimizu, N.; Watanabe, K.; Mizukami, M.; Morio, T.; Sugamoto, Y.; Mochizuki, M. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br. J. Ophthalmol. 2008, 92, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Shimizu, N.; Watanabe, K.; Katayama, M.; Horie, S.; Ogawa, M.; Takase, H.; Sugamoto, Y.; Mochizuki, M. Diagnosis of bacterial endophthalmitis by broad-range quantitative PCR. Br. J. Ophthalmol. 2011, 95, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Kamoi, K.; Ogawa, M.; Watanabe, K.; Shimizu, N.; Mochizuki, M. Detection of Candida and Aspergillus species DNA using broad-range real-time PCR for fungal endophthalmitis. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Ogawa, M.; Shimizu, N.; Morio, T.; Ohguro, N.; Nakai, K.; Maruyama, K.; Nagata, K.; Takeda, A.; Usui, Y.; et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology 2013, 120, 1761–1768. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sugita, S.; Kamoi, K.; Takase, H. A new era of uveitis: Impact of polymerase chain reaction in intraocular inflammatory diseases. Jpn. J. Ophthalmol. 2017, 61, 1–20. [Google Scholar] [CrossRef]
- Suzumiya, J.; Marutsuka, K.; Nabeshima, K.; Nawa, Y.; Koono, M.; Tamura, K.; Kimura, N.; Hisano, S.; Tachibana, N.; Inoue, S.; et al. Autopsy findings in 47 cases of adult T-cell leukemia/lymphoma in Miyazaki prefecture, Japan. Leuk Lymphoma 1993, 11, 281–286. [Google Scholar] [CrossRef]
- Maeda, T.; Babazono, A.; Nishi, T.; Yasui, M.; Matsuda, S.; Fushimi, K.; Fujimori, K. The Impact of Opportunistic Infections on Clinical Outcome and Healthcare Resource Uses for Adult T Cell Leukaemia. PLoS ONE 2015, 10, e0135042. [Google Scholar] [CrossRef]
- Hirano, M.; Ohno, N.; Tanosaki, R.; Mochizuki, M.; Ohno-Matsui, K.; Uchimaru, K.; Tojo, A.; Kamoi, K. Adult T-cell leukemia cell-induced uveitis: Rapid increase in adult T-cell leukemia cells disrupts the blood-ocular barrier. Int. J. Hematol. 2017, 106, 842–846. [Google Scholar] [CrossRef]
- Taylor, G.P.; Matsuoka, M. Natural history of adult T-cell leukemia/lymphoma and approaches to therapy. Oncogene 2005, 24, 6047–6057. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Choi, I.; Chihara, D.; Seto, M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015, 106, 344–351. [Google Scholar] [CrossRef]
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Kamoi, K.; Kato, S.; Uchimaru, K.; Tojo, A.; Ohno-Matsui, K. Frosted branch angiitis after allogeneic haematopoietic stem cell transplantation in adult T-cell leukaemia-lymphoma. Lancet Haematol. 2020, 7, e772. [Google Scholar] [CrossRef]

| Timing of Testing for HTLV-1 Antibodies | Northern (n = 4) | Central/Metropolitan (n = 40) | Southern (n = 16) | Total | |||
|---|---|---|---|---|---|---|---|
| Hokkaido/Tohoku (n = 4) | Kanto (n = 22) | Chubu (n = 7) | Kinki (n = 11) | Chugoku/Shikoku (n = 7) | Kyushu (n = 9) | ||
| Carried out routinely in considering differential diagnoses for uveitis | 50.0% | 52.5% | 84.6% | 58.3% | |||
| 50.0% | 50.0% | 57.1% | 54.5% | 75.0% | 88.9% | ||
| When ocular manifestation such as HAU, ATL, or HAM is suspected | 50.0% | 47.5% | 25.0% | 41.7% | |||
| 50.0% | 50.0% | 42.9% | 45.5% | 42.9% | 11.1% | ||
| When requested by Patient | 0.0% | 7.5% | 0.0% | 5.0% | |||
| 0.0% | 4.5% | 0.0% | 18.2% | 0.0% | 0.0% | ||
| When administering Steroid | 25.0% | 2.5% | 0.0% | 3.3% | |||
| 25.0% | 0.0% | 0.0% | 9.1% | 0.0% | 0.0% | ||
| When administering Immunosuppressant | 25.0% | 2.5% | 0.0% | 3.3% | |||
| 25.0% | 0.0% | 0.0% | 9.1% | 0.0% | 0.0% | ||
| When administering biological product | 20.0% | 0.0% | 0.0% | 1.7% | |||
| 20.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | ||
| When performing Surgery | 0.0% | 5.0% | 0.0% | 3.3% | |||
| 0.0% | 0.0% | 14.3% | 9.1% | 0.0% | 0.0% | ||
| Others | 0.0% | 10.0% | 0.0% | 6.7% | |||
| 0.0% | 18.2% | 0.0% | 0.0% | 0.0% | 0.0% | ||
| Test for HTLV-1 Antibodies in Considering Differential Diagnoses for Uveitis | Northern (n = 5) | Central/Metropolitan (n = 47) | Southern (n = 16) | Total | |||
|---|---|---|---|---|---|---|---|
| Hokkaido/Tohoku (n = 5) | Kanto (n = 26) | Chubu (n = 9) | Kinki (n = 12) | Chugoku/ Shikoku (n = 7) | Kyushu (n = 9) | ||
| Yes | 80.0% | 82.9% | 100.0% | 86.8% | |||
| 80.0% | 80.1% | 77.8% | 66.7% | 100.0% | 100.0% | ||
| No/Un-identified | 20.0% | 17.1% | 0.0% | 13.2% | |||
| 20.0% | 19.9% | 22.2% | 33.3% | 0.0% | 0.0% | ||
| ATL-Related Ocular Manifestations | Number | % |
|---|---|---|
| Intraocular infiltration | 22 | 45.8 |
| Opportunistic infection | 19 | 39.6 |
| Cytomegalovirus | (19) | (100.0) |
| Herpesvirus | (2) | (10.5) |
| Toxoplasma | (1) | (5.3) |
| Dry eye | 3 | 6.3 |
| Scleritis | 2 | 4.2 |
| Uveitis | 1 | 2.1 |
| Anemic retinopathy | 1 | 2.1 |
| Total | 48 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoi, K.; Watanabe, T.; Uchimaru, K.; Okayama, A.; Kato, S.; Kawamata, T.; Kurozumi-Karube, H.; Horiguchi, N.; Zong, Y.; Yamano, Y.; et al. Updates on HTLV-1 Uveitis. Viruses 2022, 14, 794. https://doi.org/10.3390/v14040794
Kamoi K, Watanabe T, Uchimaru K, Okayama A, Kato S, Kawamata T, Kurozumi-Karube H, Horiguchi N, Zong Y, Yamano Y, et al. Updates on HTLV-1 Uveitis. Viruses. 2022; 14(4):794. https://doi.org/10.3390/v14040794
Chicago/Turabian StyleKamoi, Koju, Toshiki Watanabe, Kaoru Uchimaru, Akihiko Okayama, Seiko Kato, Toyotaka Kawamata, Hisako Kurozumi-Karube, Noe Horiguchi, Yuan Zong, Yoshihisa Yamano, and et al. 2022. "Updates on HTLV-1 Uveitis" Viruses 14, no. 4: 794. https://doi.org/10.3390/v14040794
APA StyleKamoi, K., Watanabe, T., Uchimaru, K., Okayama, A., Kato, S., Kawamata, T., Kurozumi-Karube, H., Horiguchi, N., Zong, Y., Yamano, Y., Hamaguchi, I., Nannya, Y., Tojo, A., & Ohno-Matsui, K. (2022). Updates on HTLV-1 Uveitis. Viruses, 14(4), 794. https://doi.org/10.3390/v14040794







