A Recombinant Turkey Herpesvirus Expressing the F Protein of Newcastle Disease Virus Genotype XII Generated by NHEJ-CRISPR/Cas9 and Cre-LoxP Systems Confers Protection against Genotype XII Challenge in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chickens
2.2. Cell Culture and Virus
2.3. Design of sgRNAs and Construction of the Donor Plasmid
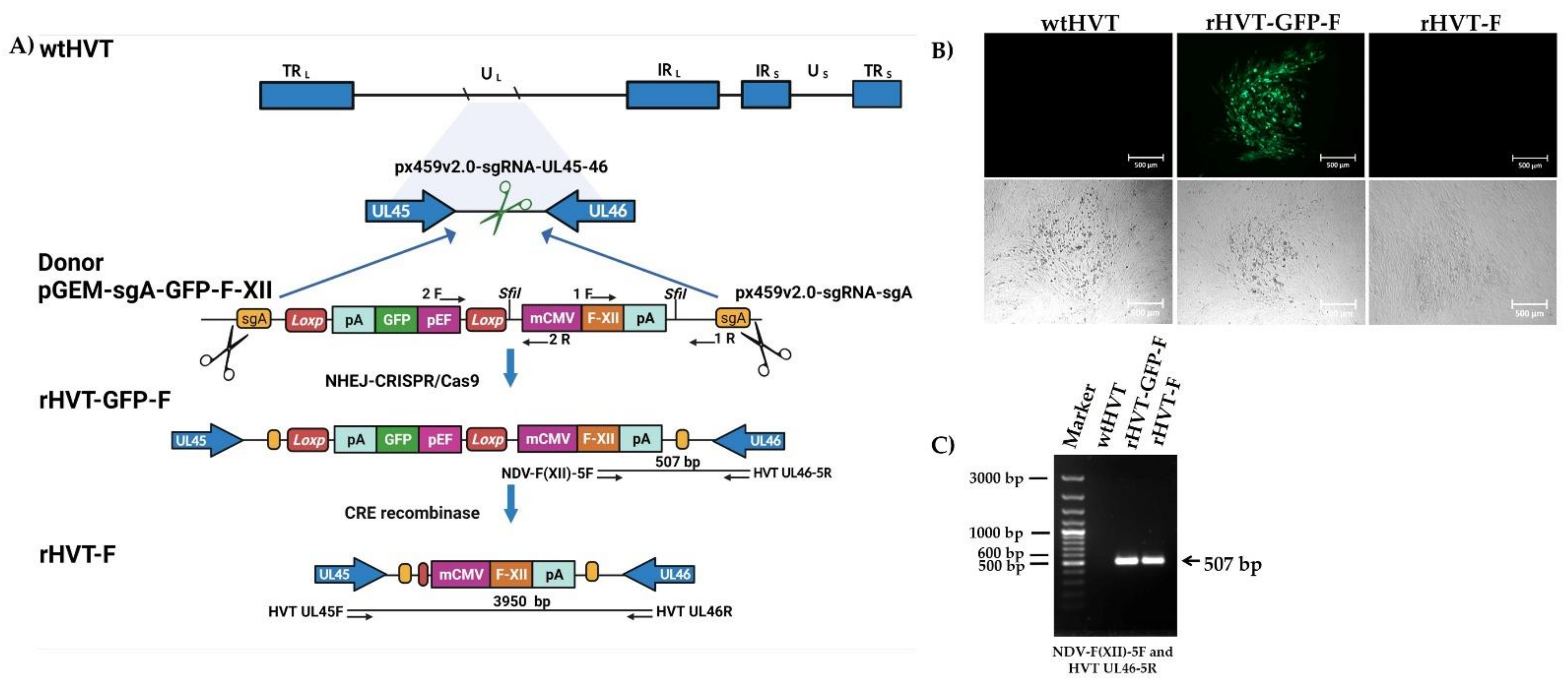
2.4. Generation of the Recombinant HVT Containing the F Gene from NDV of Genotype XII
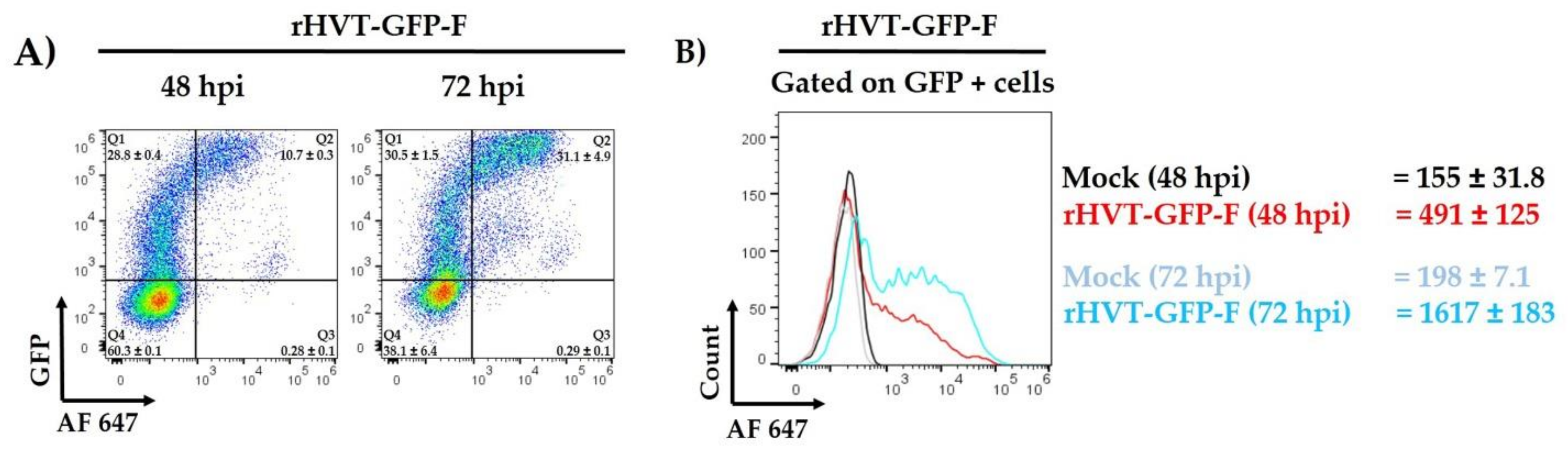
2.5. Flow Cytometry Analysis
2.6. Excision of the GFP Reporter Cassette Via the Cre-LoxP System
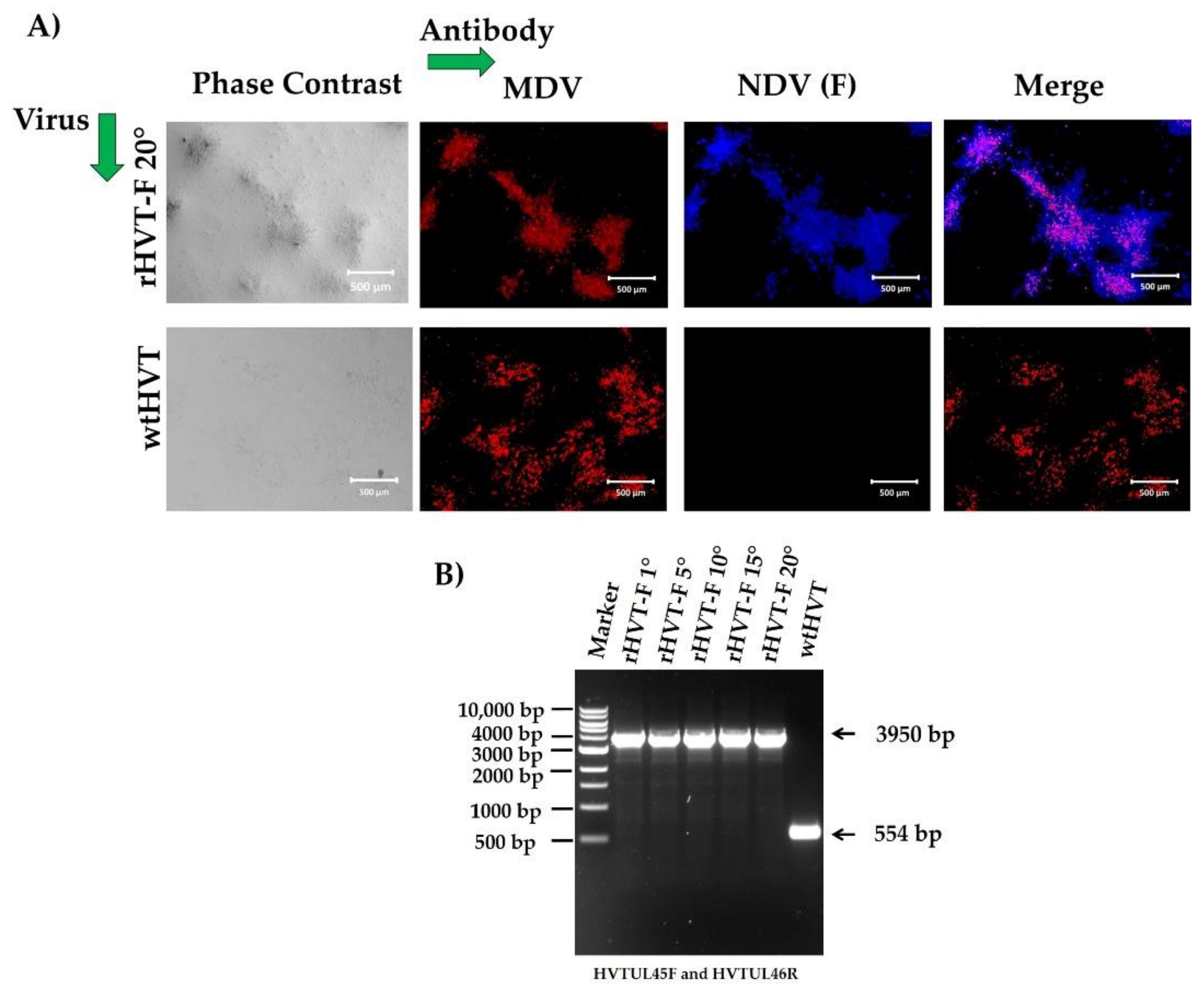
2.7. Indirect Immunofluorescence Assay (IFA)
2.8. Western Blot Analysis
2.9. In Vitro Growth Properties and Plaque Assay
2.10. Genetic Stability of the rHVT-F Virus
2.11. Vaccination in SPF Chickens and Efficacy of the rHVT-F Vaccine against NDV Genotype XII Challenge in SPF Chickens
2.12. ELISA and HI Test
2.13. Evaluation of Challenge Virus Shedding
2.14. Replication and Stability of rHVT-F Virus In Vivo
2.15. Statistical Analysis
3. Results
3.1. Construction and Rescue of the rHVT-F Virus
3.2. Fusion Protein Expression on the Cell Surface by Flow Cytometry Analyses
3.3. Characterization of the rHVT-F Virus
3.4. Genetic Stability of the rHVT-F Virus
3.5. Humoral Immune Response to Vaccination in SPF Chickens
3.6. Efficacy against Genotype XII NDV Challenge
3.7. Detection of the Replication by rHVT-F Virus Isolation from Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cattoli, G.; Susta, L.; Terregino, C.; Brown, C. Newcastle disease: A review of field recognition and current methods of laboratory detection. J. Vet. Diagn. Investig. 2011, 23, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle disease vaccines—A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, G.; Gonze, M.; Carlier, M.C.; Petit, P.; Burny, A.; Long, L. Protective Effects of Hn and F Glycoprotein-Specific Monoclonal Antibodies On Experimental Newcastle Disease. Avian Pathol. 1986, 15, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Diel, D.G.; Da Silva, L.H.A.; Liu, H.; Wang, Z.; Miller, P.J.; Afonso, C.L. Genetic diversity of avian paramyxovirus type 1: Proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 2012, 12, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef] [PubMed]
- Diel, D.G.; Susta, L.; Garcia, S.C.; Killian, M.L.; Brown, C.C.; Miller, P.J.; Afonso, C.L. Complete Genome and Clinicopathological Characterization of a Virulent Newcastle Disease Virus Isolate from South America. J. Clin. Microbiol. 2012, 50, 378–387. [Google Scholar] [CrossRef][Green Version]
- Chumbe, A.; Izquierdo-Lara, R.; Tataje-Lavanda, L.; Figueroa, A.; Segovia, K.; Gonzalez, R.; Cribillero, G.; Montalvan, A.; Fernández-Díaz, M.; Icochea, E. Characterization and Sequencing of a Genotype XII Newcastle Disease Virus Isolated from a Peacock (Pavo cristatus) in Peru. Genome Announc. 2015, 3, e00792-15. [Google Scholar] [CrossRef]
- Chumbe, A.; Izquierdo-Lara, R.; Tataje, L.; Gonzalez, R.; Cribillero, G.; González, A.E.; Fernández-Díaz, M.; Icochea, E. Pathotyping and Phylogenetic Characterization of Newcastle Disease Viruses Isolated in Peru: Defining Two Novel Subgenotypes Within Genotype XII. Avian Dis. 2017, 61, 16–24. [Google Scholar] [CrossRef]
- Morgan, R.W.; Gelb, J.; Schreurs, C.S.; Lütticken, D.; Rosenberger, J.K.; Sondermeijer, P.J.A. Protection of Chickens from Newcastle and Marek’s Diseases with a Recombinant Herpesvirus of Turkeys Vaccine Expressing the Newcastle Disease Virus Fusion Protein. Avian Dis. 1992, 36, 858–870. [Google Scholar] [CrossRef]
- Morgan, R.W.; Gelb, J.; Pope, C.R.; Sondermeijer, P.J.A. Efficacy in Chickens of a Herpesvirus of Turkeys Recombinant Vaccine Containing the Fusion Gene of Newcastle Disease Virus: Onset of Protection and Effect of Maternal Antibodies. Avian Dis. 1993, 37, 1032–1040. [Google Scholar] [CrossRef]
- Heckert, R.A.; Riva, J.; Cook, S.; McMillen, J.; Schwartz, R.D. Onset of Protective Immunity in Chicks after Vaccination with a Recombinant Herpesvirus of Turkeys Vaccine Expressing Newcastle Disease Virus Fusion and Hemagglutinin-Neuraminidase Antigens. Avian Dis. 1996, 40, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.; Tatár-Kis, T.; Mató, T.; Felföldi, B.; Kovács, E.; Gardin, Y. Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers. Vet. Immunol. Immunopathol. 2014, 158, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.L.; Tulman, E.R.; Lu, Z.; Zsak, L.; Rock, D.L.; Kutish, G.F. The Genome of Turkey Herpesvirus. J. Virol. 2001, 75, 971–978. [Google Scholar] [CrossRef]
- Okazaki, W.; Purchase, H.G.; Burmester, B.R. Protection against Marek’s Disease by Vaccination with a Herpesvirus of Turkeys. Avian Dis. 1970, 14, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Rauw, F.; Gardin, Y.; Palya, V.; Anbari, S.; Lemaire, S.; Boschmans, M.; Van den Berg, T.; Lambrecht, B. Improved vaccination against Newcastle disease by an in ovo recombinant HVT-ND combined with an adjuvanted live vaccine at day-old. Vaccine 2010, 28, 823–833. [Google Scholar] [CrossRef]
- Esaki, M.; Godoy, A.; Rosenberger, J.K.; Rosenberger, S.C.; Gardin, Y.; Yasuda, A.; Dorsey, K.M. Protection and Antibody Response Caused by Turkey Herpesvirus Vector Newcastle Disease Vaccine. Avian Dis. 2013, 57, 750–755. [Google Scholar] [CrossRef]
- Palya, V.; Tatár-Kis, T.; Arafa, A.S.A.; Felföldi, B.; Mató, T.; Setta, A. Efficacy of a Turkey Herpesvirus Vectored Newcastle Disease Vaccine against Genotype VII.1.1 Virus: Challenge Route Affects Shedding Pattern. Vaccines 2021, 9, 37. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Saito, S.; Saeki, S.; Sato, T.; Tanimura, N.; Isobe, T.; Mase, M.; Imada, T.; Yuasa, N.; Yamaguchi, S. Complete, Long-Lasting Protection against Lethal Infectious Bursal Disease Virus Challenge by a Single Vaccination with an Avian Herpesvirus Vector Expressing VP2 Antigens. J. Virol. 2002, 76, 5637–5645. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Dorsey, K.; Chrzastek, K.; Moraes, M.; Jackwood, M.; Hilt, D.; Gardin, Y. Vaccine Protection of Turkeys Against H5N1 Highly Pathogenic Avian Influenza Virus with a Recombinant Turkey Herpesvirus Expressing the Hemagglutinin Gene of Avian Influenza. Avian Dis. 2016, 60, 413–417. [Google Scholar] [CrossRef]
- Li, Y.; Reddy, K.; Reid, S.M.; Cox, W.J.; Brown, I.H.; Britton, P.; Nair, V.; Iqbal, M. Recombinant herpesvirus of turkeys as a vector-based vaccine against highly pathogenic H7N1 avian influenza and Marek’s disease. Vaccine 2011, 29, 8257–8266. [Google Scholar] [CrossRef]
- Esaki, M.; Noland, L.; Eddins, T.; Godoy, A.; Saeki, S.; Saitoh, S.; Yasuda, A.; Dorsey, K.M. Safety and Efficacy of a Turkey Herpesvirus Vector Laryngotracheitis Vaccine for Chickens. Avian Dis. 2013, 57, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, W.; Chu, J.; Huang, X.; Wu, Z.; Yan, M.; Zhang, Q.; Zhao, P.; Igietseme, J.U.; Black, C.M.; et al. Construction of Recombinant HVT Expressing PmpD, and Immunological Evaluation against Chlamydia psittaci and Marek’s Disease Virus. PLoS ONE 2015, 10, e0124992. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Sharma, J.M.; Ahmad, J.; Reddy, D.N.; McMillen, J.K.; Cook, S.M.; Wild, M.A.; Schwartz, R.D. Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek’s diseases in specific-pathogen-free chickens. Vaccine 1996, 14, 469–477. [Google Scholar] [CrossRef]
- Andoh, K.; Yamazaki, K.; Honda, Y.; Honda, T. Turkey herpesvirus with an insertion in the UL3-4 region displays an appropriate balance between growth activity and antibody-eliciting capacity and is suitable for the establishment of a recombinant vaccine. Arch. Virol. 2017, 162, 931–941. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Kojima, C.; Komori, Y.; Tanimura, N.; Mase, M.; Yamaguchi, S. Protection of Chickens against Very Virulent Infectious Bursal Disease Virus (IBDV) and Marek’s Disease Virus (MDV) with a Recombinant MDV Expressing IBDV VP2. Virology 1999, 257, 352–362. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Y.; Pedrera, M.; Chang, P.; Baigent, S.; Moffat, K.; Shen, Z.; Nair, V.; Yao, Y. A simple and rapid approach to develop recombinant avian herpesvirus vectored vaccines using CRISPR/Cas9 system. Vaccine 2018, 36, 716–722. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Y.; Pedrera, M.; Chang, P.; Baigent, S.; Moffat, K.; Shen, Z.; Nair, V.; Yao, Y. Generating Recombinant Avian Herpesvirus Vectors with CRISPR/Cas9 Gene Editing. J. Vis. Exp. 2019, 143, 58193. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Wang, M.; Tong, Q.; Sun, Y.; Pu, J.; Sun, H.; Liu, J. Recombinant turkey herpesvirus expressing H9 hemagglutinin providing protection against H9N2 avian influenza. Virology 2019, 529, 7–15. [Google Scholar] [CrossRef]
- Chang, P.; Ameen, F.; Sealy, J.E.; Sadeyen, J.-R.; Bhat, S.; Li, Y.; Iqbal, M. Application of HDR-CRISPR/Cas9 and Erythrocyte Binding for Rapid Generation of Recombinant Turkey Herpesvirus-Vectored Avian Influenza Virus Vaccines. Vaccines 2019, 7, 192. [Google Scholar] [CrossRef]
- Baigent, S.J.; Petherbridge, L.J.; Smith, L.P.; Zhao, Y.; Chesters, P.M.; Nair, V.K. Herpesvirus of turkey reconstituted from bacterial artificial chromosome clones induces protection against Marek’s disease. J. Gen. Virol. 2006, 87, 769–776. [Google Scholar] [CrossRef]
- Shang, W.; Wang, F.; Fan, G.; Wang, H. Key elements for designing and performing a CRISPR/Cas9-based genetic screen. J. Genet. Genom. 2017, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas immune system: Biology, mechanisms and applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tan, C.; Wang, F.; Wang, Y.; Zhou, R.; Cui, D.; You, W.; Zhao, H.; Ren, J.; Feng, B. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 2016, 44, e85. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.S.; Chan, C.P.; Wong, N.H.M.; Ho, C.H.; Ho, T.H.; Lei, T.; Deng, W.; Tsao, S.W.; Chen, H.; Kok, K.H.; et al. CRISPR/Cas9-mediated genome editing of Epstein–Barr virus in human cells. J. Gen. Virol. 2015, 96, 626–636. [Google Scholar] [CrossRef]
- Xu, A.; Qin, C.; Lang, Y.; Wang, M.; Lin, M.; Li, C.; Zhang, R.; Tang, J. A simple and rapid approach to manipulate pseudorabies virus genome by CRISPR/Cas9 system. Biotechnol. Lett. 2015, 37, 1265–1272. [Google Scholar] [CrossRef]
- Tang, Y.D.; Liu, J.T.; Fang, Q.Q.; Wang, T.Y.; Sun, M.X.; An, T.Q.; Tian, Z.J.; Cai, X.H. Recombinant Pseudorabies Virus (PRV) Expressing Firefly Luciferase Effectively Screened for CRISPR/Cas9 Single Guide RNAs and Antiviral Compounds. Viruses 2016, 8, 90. [Google Scholar] [CrossRef]
- Tang, Y.D.; Liu, J.T.; Wang, T.Y.; An, T.Q.; Sun, M.X.; Wang, S.J.; Fang, Q.Q.; Hou, L.L.; Tian, Z.J.; Cai, X.H. Live attenuated pseudorabies virus developed using the CRISPR/Cas9 system. Virus Res. 2016, 225, 33–39. [Google Scholar] [CrossRef]
- Liang, X.; Sun, L.; Yu, T.; Pan, Y.; Wang, D.; Hu, X.; Fu, Z.; He, Q.; Cao, G. A CRISPR/Cas9 and Cre/Lox system-based express vaccine development strategy against re-emerging Pseudorabies virus. Sci. Rep. 2016, 6, 19176. [Google Scholar] [CrossRef]
- Tang, Y.D.; Guo, J.C.; Wang, T.Y.; Zhao, K.; Liu, J.T.; Gao, J.C.; Tian, Z.J.; An, T.Q.; Cai, X.H. CRISPR/Cas9-mediated 2-sgRNA cleavage facilitates pseudorabies virus editing. Faseb J. 2018, 32, 4293–4301. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.Q.; Zheng, H.H.; Yang, Y.R.; Liu, F.; Zheng, L.L.; Jin, Y.; Chen, H.Y. Construction and immunogenicity of a gE/gI/TK-deleted PRV based on porcine pseudorabies virus variant. Mol. Cell. Probes 2020, 53, 101605. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, J.; Gao, Y.; Cui, H.; Liu, C.; Qi, X.; Zhang, Y.; Wang, Y.; Wang, X. The Natural Large Genomic Deletion Is Unrelated to the Increased Virulence of the Novel Genotype Fowl Adenovirus 4 Recently Emerged in China. Viruses 2018, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, W.; Wang, J.; Al Yaghchi, C.; Ahmed, J.; Chard, L.; Lemoine, N.R.; Wang, Y. Efficiently Editing the Vaccinia Virus Genome by Using the CRISPR-Cas9 System. J. Virol. 2015, 89, 5176–5179. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bassett, A.; Nair, V. Targeted editing of avian herpesvirus vaccine vector using CRISPR/Cas9 nuclease. Int. J. Vaccines Technol. 2016, 1, 1–7. [Google Scholar]
- Zou, Z.; Huang, K.; Wei, Y.; Chen, H.; Liu, Z.; Jin, M. Construction of a highly efficient CRISPR/Cas9-mediated duck enteritis virus-based vaccine against H5N1 avian influenza virus and duck Tembusu virus infection. Sci. Rep. 2017, 7, 1478. [Google Scholar] [CrossRef]
- Chang, P.; Yao, Y.; Tang, N.; Sadeyen, J.R.; Sealy, J.; Clements, A.; Bhat, S.; Munir, M.; Bryant, J.E.; Iqbal, M. The Application of NHEJ-CRISPR/Cas9 and Cre-Lox System in the Generation of Bivalent Duck Enteritis Virus Vaccine against Avian Influenza Virus. Viruses 2018, 10, 81. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, N.; Sadigh, Y.; Baigent, S.; Shen, Z.; Nair, V.; Yao, Y. Application of CRISPR/Cas9 Gene Editing System on MDV-1 Genome for the Study of Gene Function. Viruses 2018, 10, 279. [Google Scholar] [CrossRef]
- Atasoy, M.O.; Rohaim, M.A.; Munir, M. Simultaneous Deletion of Virulence Factors and Insertion of Antigens into the Infectious Laryngotracheitis Virus Using NHEJ-CRISPR/Cas9 and Cre–Lox System for Construction of a Stable Vaccine Vector. Vaccines 2019, 7, 207. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Y.; Sadigh, Y.; Moffat, K.; Shen, Z.; Nair, V.; Yao, Y. Generation of A Triple Insert Live Avian Herpesvirus Vectored Vaccine Using CRISPR/Cas9-Based Gene Editing. Vaccines 2020, 8, 97. [Google Scholar] [CrossRef]
- Alfonso, C.L.; Miller, P.J.; Grund, C.; Kock, G.; Peeters, B.; Selleck, P.W.; Srinivas, G. Newcastle Disease. In Manual of Diagnostic Test and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018; pp. 964–983. [Google Scholar]
- Sondermeijer, P.J.A.; Claessens, J.A.J.; Jenniskens, P.E.; Adrian Mockett, A.P.; Thijssen, R.A.J.; Willemse, M.J.; Morgan, R.W. Avian herpesvirus as a live viral vector for the expression of heterologous antigens. Vaccine 1993, 11, 349–358. [Google Scholar] [CrossRef]
- McPherson, M.C.; Cheng, H.H.; Delany, M.E. Marek’s disease herpesvirus vaccines integrate into chicken host chromosomes yet lack a virus-host phenotype associated with oncogenic transformation. Vaccine 2016, 34, 5554–5561. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; De Almeida, R.S.; Gil, P.; Majó, N.; Nofrarías, M.; Briand, F.X.; Jestin, V.; Albina, E. Can genotype mismatch really affect the level of protection conferred by Newcastle disease vaccines against heterologous virulent strains? Vaccine 2018, 36, 3917–3925. [Google Scholar] [CrossRef]
- Dortmans, J.C.F.M.; Peeters, B.P.H.; Koch, G. Newcastle disease virus outbreaks: Vaccine mismatch or inadequate application? Vet. Microbiol. 2012, 160, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.; King, D.J.; Afonso, C.L.; Suarez, D.L. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine 2007, 25, 7238–7246. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.; Estevez, C.; Yu, Q.; Suarez, D.L.; King, D.J. Comparison of Viral Shedding Following Vaccination with Inactivated and Live Newcastle Disease Vaccines Formulated with Wild-Type and Recombinant Viruses. Avian Dis. Dig. 2009, 53, 39–49. [Google Scholar] [CrossRef]
- Kaspers, B.; Schat, K.A.; Göbel, T.; Vervelde, L. Avian Immunology, 3rd ed.; Lavoisier: Cachan, France, 2021. [Google Scholar]
- Rauw, F.; Van Borm, S.; Welby, S.; Ngabirano, E.; Gardin, Y.; Palya, V.; Lambrecht, B. Quantification of rHVT-F genome load in feather follicles by specific real-time qPCR as an indicator of NDV-specific humoral immunity induced by day-old vaccination in SPF chickens. Avian Pathol. 2015, 44, 154–161. [Google Scholar] [CrossRef]
- Palya, V.; Kiss, I.; Tatár-Kis, T.; Mató, T.; Felföldi, B.; Gardin, Y. Advancement in Vaccination Against Newcastle Disease: Recombinant HVT NDV Provides High Clinical Protection and Reduces Challenge Virus Shedding with the Absence of Vaccine Reactions. Avian Dis. 2012, 56, 282–287. [Google Scholar] [CrossRef]
- Meulemans, G.; Letellier, C.; Gonze, M.; Carlier, M.C.; Burny, A. Newcastle disease virus f glycoprotein expressed from a recombinant vaccinia virus vector protects chickens against live-virus challenge. Avian Pathol. 1988, 17, 821–827. [Google Scholar] [CrossRef]
- Mao, Z.; Bozzella, M.; Seluanov, A.; Gorbunova, V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair 2008, 7, 1765–1771. [Google Scholar] [CrossRef]

| Primers and sgRNAs | Sequences (5′→3′) |
|---|---|
| NDV-F(XII)-5F | TGGGAACAATACCCTCGATCA |
| HVT UL46-5R | GTTTCGGAATCTGGCAGGGT |
| HVT UL45F | TCGCAAACGCCAAAGTTCTG |
| HVT UL46R | CGAGCAATGACCCTCCAGTT |
| 1F | CGTTGTAAAACGACGGCCAG |
| 1R | TGGCTTGGGAACAATACCCT |
| 2F | ATTGAGTCACCACCCCTATGC |
| 2R | CCCAACTTCTCGGGGACTGT |
| sgRNA-UL45-46-F | CACCgTAGACATTATAAACATAATA |
| sgRNA-UL45-46-R | AAACTATTATGTTTATAATGTCTAc |
| Group | Number of Viral Shedding Chickens (Positive/Total) | |||||
|---|---|---|---|---|---|---|
| 3 dpc (a) | 5 dpc | 8 dpc | ||||
| Oral | Cloacal | Oral | Cloacal | Oral | Cloacal | |
| Vaccinated | 2/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| Control | 7/7 | 5/7 | 6/6 | 6/6 | NS (b) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón, K.; Rojas-Neyra, A.; Carbajal-Lévano, B.; Luján-Valenzuela, L.; Ticona, J.; Isasi-Rivas, G.; Montalvan, A.; Criollo-Orozco, M.; Huaccachi-Gonzáles, E.; Tataje-Lavanda, L.; et al. A Recombinant Turkey Herpesvirus Expressing the F Protein of Newcastle Disease Virus Genotype XII Generated by NHEJ-CRISPR/Cas9 and Cre-LoxP Systems Confers Protection against Genotype XII Challenge in Chickens. Viruses 2022, 14, 793. https://doi.org/10.3390/v14040793
Calderón K, Rojas-Neyra A, Carbajal-Lévano B, Luján-Valenzuela L, Ticona J, Isasi-Rivas G, Montalvan A, Criollo-Orozco M, Huaccachi-Gonzáles E, Tataje-Lavanda L, et al. A Recombinant Turkey Herpesvirus Expressing the F Protein of Newcastle Disease Virus Genotype XII Generated by NHEJ-CRISPR/Cas9 and Cre-LoxP Systems Confers Protection against Genotype XII Challenge in Chickens. Viruses. 2022; 14(4):793. https://doi.org/10.3390/v14040793
Chicago/Turabian StyleCalderón, Katherine, Aldo Rojas-Neyra, Brigith Carbajal-Lévano, Luis Luján-Valenzuela, Julio Ticona, Gisela Isasi-Rivas, Angela Montalvan, Manuel Criollo-Orozco, Edison Huaccachi-Gonzáles, Luis Tataje-Lavanda, and et al. 2022. "A Recombinant Turkey Herpesvirus Expressing the F Protein of Newcastle Disease Virus Genotype XII Generated by NHEJ-CRISPR/Cas9 and Cre-LoxP Systems Confers Protection against Genotype XII Challenge in Chickens" Viruses 14, no. 4: 793. https://doi.org/10.3390/v14040793
APA StyleCalderón, K., Rojas-Neyra, A., Carbajal-Lévano, B., Luján-Valenzuela, L., Ticona, J., Isasi-Rivas, G., Montalvan, A., Criollo-Orozco, M., Huaccachi-Gonzáles, E., Tataje-Lavanda, L., Alvarez, K. L. F., Fernández-Sánchez, M., Fernández-Díaz, M., Tang, N., Yao, Y., & Nair, V. (2022). A Recombinant Turkey Herpesvirus Expressing the F Protein of Newcastle Disease Virus Genotype XII Generated by NHEJ-CRISPR/Cas9 and Cre-LoxP Systems Confers Protection against Genotype XII Challenge in Chickens. Viruses, 14(4), 793. https://doi.org/10.3390/v14040793









