An Approach to Quantifying the Interaction between Behavioral and Transmission Clusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Swiss HIV Cohort Study
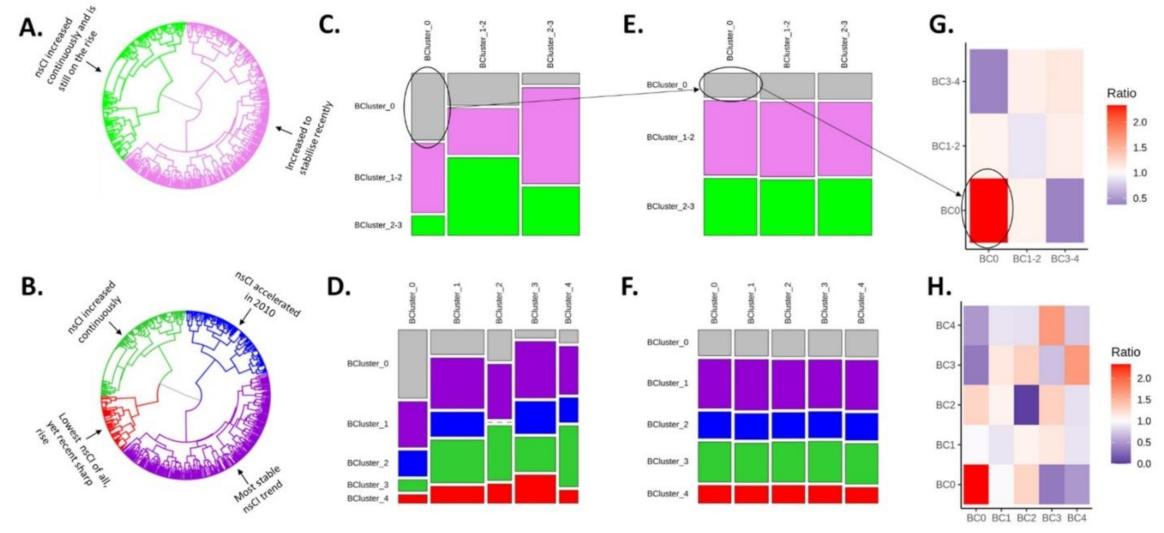
2.2. Intersections: Mapping Behavior on Transmission
2.3. Are MSM Who Share a Behavioral Cluster Likely to Also Share a Transmission Cluster?
2.4. Strength of Interaction between Behavioral Clusters
2.5. Potential Influence of Behavioral Patterns on the HCV Phylogeny (Interaction Ratios)
2.6. Sample Size and Power Considerations
2.7. Toy Monte Carlo Analysis
2.8. The Interaction Ratios
3. Results
3.1. Intersections: Mapping Behavior on Transmission
3.2. Are MSM Who Shared a Behavioral Cluster Likely to Also Share a Transmission Cluster?
3.3. Potential Influence of Behavioral Patterns on HCV Transmission Clustering
3.4. Sample Size and Power Considerations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampel, B.; Kusejko, K.; Kouyos, R.; Böni, J.; Flepp, M.; Stöckle, M.; Conen, A.; Béguelin, C.; Künzler-Heule, P.; Nicca, D.; et al. Chemsex drugs on the rise: A longitudinal analysis of the Swiss HIV Cohort Study from 2007 to 2017. HIV Med. 2019, 21, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kouyos, R.D.; Hasse, B.; Calmy, A.; Cavassini, M.; Furrer, H.; Stöckle, M.; Vernazza, P.L.; Bernasconi, E.; Weber, R.; Günthard, H.F.; et al. Increases in Condomless Sex in the Swiss HIV Cohort Study. Open Forum. Infect. Dis. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nostlinger, C.; Platteau, T.; Bogner, J.; Buyze, J.; Dec-Pietrowska, J.; Dias, S.; Newbury-Helps, J.; Kocsis, A.; Mueller, M.; Rojas, D.; et al. Implementation and Operational Research: Computer-Assisted Intervention for Safer Sex in HIV-Positive Men Having Sex with Men: Findings of a European Randomized Multi-Center Trial. J. Acquir. Immune Defic. Syndr. 2016, 71, e63–e72. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.S.; Carballo-Diéguez, A.; Coates, T.; Goodreau, S.; McGowan, I.; Sanders, E.J.; Smith, A.; Goswami, P.; Sanchez, J. Successes and challenges of HIV prevention in men who have sex with men. Lancet 2012, 380, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Nostlinger, C.; Niderost, S.; Platteau, T.; Müller, M.C.; Staneková, D.; Gredig, D.; Roulin, C.; Rickenbach, M.; Colebunders, R. Sexual Protection Behavior in HIV-Positive Gay Men: Testing a Modified Information-Motivation-Behavioral Skills Model. Arch. Sex. Behav. 2011, 40, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Vizcaya, L.; Kusejko, K.; Schmidt, A.J.; Carrillo-Montoya, G.; Nicca, D.; Wandeler, G.; Braun, D.L.; Fehr, J.; Darling, K.; Bernasconi, E.; et al. Clusters of sexual behaviour in HIV-positive men who have sex with men reveal highly dissimilar time trends. Clin. Infect. Dis. 2020, 70, 416–424. [Google Scholar]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Un-transmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef] [Green Version]
- Vernazza, P.; Hirschel, B.; Bernasconi, E.; Flepp, M. Les personnes séropositives ne souffrant d’aucune autre MST et suivant un traitement antirétroviral efficace ne transmettent pas le VIH par voie sexuelle. Bull. Des. Médecins Suisses Schweiz. Ische Ärztezeitung Boll. Dei Med. Svizz. 2008, 89, 165–169. [Google Scholar]
- Salazar-Vizcaya, L.; Kouyos, R.D.; Zahnd, C.; Wandeler, G.; Battegay, M.; Darling, K.E.A.; Bernasconi, E.; Calmy, A.; Vernazza, P.; Furrer, H. Hepatitis C virus transmission among human immunodeficiency vi-rus-infected men who have sex with men: Modeling the effect of behavioral and treatment interventions. Hepatology 2016, 64, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Swiss HIV Cohort Study. Available online: https://shcs.ch/ (accessed on 1 March 2022).
- van Sighem, A.; Vidondo, B.; Glass, T.R.; Bucher, H.C.; Vernazza, P.; Gebhardt, M.; Wolf, F.D.; Derendinger, S.; Jeannin, A.; Bezemer, D.; et al. Resurgence of HIV Infection among Men Who Have Sex with Men in Switzerland: Mathematical Modelling Study. PLoS ONE 2012, 7, e44819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, P.; Schmidt, A.J.; Cavassini, M.; Hansjakob, F.; Alexandra, C.; Manuel, B.; Enos, B.; Bruno, L.; Pietro, V. The HIV care cascade in Switzerland: Reaching the UNAIDS/WHO targets for patients diagnosed with HIV. AIDS 2015, 29, 2509–2515. [Google Scholar] [CrossRef] [Green Version]
- Andresen, S.; Balakrishna, S.; Nicca, D.; Günthard, H.F.; Schmidt, A.J.; Calmy, A.; Darling, K.E.A.; Stöckle, M.; Schmid, P.; Bernasconi, E.; et al. Behavioral Patterns to Identify Key Populations for Syphilis Prevention. In Proceedings of the 17th European AIDS Conference (EACS), Basel, Switzerland, 6–9 November 2019. [Google Scholar]
- Salazar-Vizcaya, L.; Kouyos, R.; Metzner, K.J.; Cortes, K.C.; Böni, J.; Shah, C.; Fehr, J.; Braun, D.L.; Bernasconi, E.; Mbunkah, H.A.; et al. Changing Trends in International Versus Domestic HCV Transmission in HIV-Positive Men Who Have Sex with Men: A Perspective for the Direct-Acting Antiviral Scale-Up Era. J. Infect. Dis. 2019, 220, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephane, C.; Claus, E.; Peter, D.; Jeffrey, G.; Stephan, W.; Aditya, A.; Clay, F.; Robert, V.; Helios, D.R. PWR: Basic Functions for Power Analysis. Available online: https://CRAN.R-project.org/package=pwr (accessed on 5 April 2022).
- Braun, D.L.; Hampel, B.; Martin, E.; Kouyos, R.; Kusejko, K.; Grube, C.; Flepp, M.; Stöckle, M.; Conen, A.; Béguelin, C.; et al. High Number of Potential Transmitters Revealed in a Population-based Systematic Hepatitis C Virus RNA Screening Among Human Immunodeficiency Virus-infected Men Who Have Sex with Men. Clin. Infect. Dis. 2018, 68, 561–568. [Google Scholar]
- Rauch, A.; Martin, M.; Weber, R.; Hirschel, B.; Tarr, P.E.; Bucher, H.C.; Vernazza, P.; Bernasconi, E.; Zinkernagel, A.; Evison, J.; et al. Unsafe Sex and Increased Incidence of Hepatitis C Virus Infection among HIV-Infected Men Who Have Sex with Men: The Swiss HIV Cohort Study. Clin. Infect. Dis. 2005, 41, 395–402. [Google Scholar] [CrossRef] [Green Version]
- van Santen, D.K.; van der Helm, J.J.; Del Amo, J.; Meyer, L.; Monforte, A.D.; Price, M.; Béguelin, C.A.; Zangerle, R.; Sannes, M.; Porter, K.; et al. Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990–2014. J. Hepatol. 2017, 67, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danta, M.; Rodger, A.J. Transmission of HCV in ssssHIV-positive populations. Curr. Opin. HIV AIDS 2011, 6, 451–458. [Google Scholar] [CrossRef]
- Boerekamps, A.; van den Berk, G.E.; Lauw, F.N.; Leyten, E.M.; Kasteren, M.E.v.; Eeden, A.v.; Posthouwer, D.; Claassen, M.A.; Dofferhoff, A.S.; Verhagen, D.W.M.; et al. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immu-nodeficiency Virus-Positive Men Who Have Sex with Men After Unrestricted Access to HCV Therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1360–1365. [Google Scholar] [CrossRef]
- Braun, D.L.; Hampel, B.; Kouyos, R.; Nguyen, H.; Shah, C.; Flepp, M.; Stöckle, M.; Conen, A.; Béguelin, C.; Künzler-Heule, P.; et al. High Cure Rates with Grazoprevir-Elbasvir with or without Ribavirin Guided by Genotypic Resistance Testing Among Human Immunodeficiency Virus/Hepatitis C Virus–coinfected Men Who Have Sex with Men. Clin. Infect. Dis. 2019, 68, 569–576. [Google Scholar] [CrossRef]
- Bachmann, N.; Kusejko, K.; Nguyen, H.; Chaudron, S.E.; Kadelka, C.; Turk, T.; Böni, J.; Perreau, M.; Klimkait, T.; Yerly, S.; et al. Phylogenetic Cluster Analysis Identifies Virological and Behavioral Drivers of HIV Transmission in MSM. Clin. Infect. Dis. 2021, 72, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Kusejko, K.; Marzel, A.; Hampel, B.; Bachmann, N.; Nguyen, H.; Fehr, J.; Braun, D.; Battegay, M.; Bernasconi, E.; Calmy, A.; et al. Quantifying the drivers of HIV transmission and prevention in men who have sex with men: A population model-based analysis in Switzerland. HIV Med. 2018, 19, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Moodie, E.E.M.; Cox, J.; Lambert, G.; Otis, J.; Roger, M.; Brenner, B. New Challenges in HIV Research: Combining Phylogenetic Cluster Size and Epidemiological Data. Epidemiol. Methods 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Lewis, F.; Hughes, G.J.; Rambaut, A.; Pozniak, A.; Brown, A.J.L. Episodic Sexual Transmission of HIV Revealed by Molecular Phylodynamics. PLoS Med. 2008, 5, e50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhong, L.; Romero-Severson, E.; Alam, S.J.; Henry, C.J.; Volz, E.M.; Koopman, J.S. Episodic HIV Risk Behavior Can Greatly Amplify HIV Prevalence and the Fraction of Transmissions from Acute HIV Infection. Stat. Commun. Infect. Dis. 2012, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirkhanian, Y.A. Social Networks, Sexual Networks and HIV Risk in Men Who Have Sex with Men. Curr. HIV/AIDS Rep. 2014, 11, 81–92. [Google Scholar] [CrossRef]
- Basten, M.; Heijne, J.C.M.; Geskus, R.; Den Daas, C.; Kretzschmar, M.; Matser, A. Sexual risk behavior trajectories among MSM at risk for HIV in Amsterdam, The Netherlands. AIDS 2018, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Vizcaya, L.; Kusejko, K.; Günthard, H.F.; Böni, J.; Metzner, K.J.; Braun, D.L.; Nicca, D.; Bernasconi, E.; Calmy, A.; Darling, K.E.A.; et al. An Approach to Quantifying the Interaction between Behavioral and Transmission Clusters. Viruses 2022, 14, 784. https://doi.org/10.3390/v14040784
Salazar-Vizcaya L, Kusejko K, Günthard HF, Böni J, Metzner KJ, Braun DL, Nicca D, Bernasconi E, Calmy A, Darling KEA, et al. An Approach to Quantifying the Interaction between Behavioral and Transmission Clusters. Viruses. 2022; 14(4):784. https://doi.org/10.3390/v14040784
Chicago/Turabian StyleSalazar-Vizcaya, Luisa, Katharina Kusejko, Huldrych F. Günthard, Jürg Böni, Karin J. Metzner, Dominique L. Braun, Dunja Nicca, Enos Bernasconi, Alexandra Calmy, Katharine E. A. Darling, and et al. 2022. "An Approach to Quantifying the Interaction between Behavioral and Transmission Clusters" Viruses 14, no. 4: 784. https://doi.org/10.3390/v14040784
APA StyleSalazar-Vizcaya, L., Kusejko, K., Günthard, H. F., Böni, J., Metzner, K. J., Braun, D. L., Nicca, D., Bernasconi, E., Calmy, A., Darling, K. E. A., Wandeler, G., Kouyos, R. D., Rauch, A., & the Swiss HIV Cohort Study. (2022). An Approach to Quantifying the Interaction between Behavioral and Transmission Clusters. Viruses, 14(4), 784. https://doi.org/10.3390/v14040784





