Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blood Samples
2.3. Generation of an In-House Human TNFR2 ELISA
2.4. ELISA
2.5. HTLV-1 PVL
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Establishment of a Quantitative Sandwich ELISA for Human sTNFR2
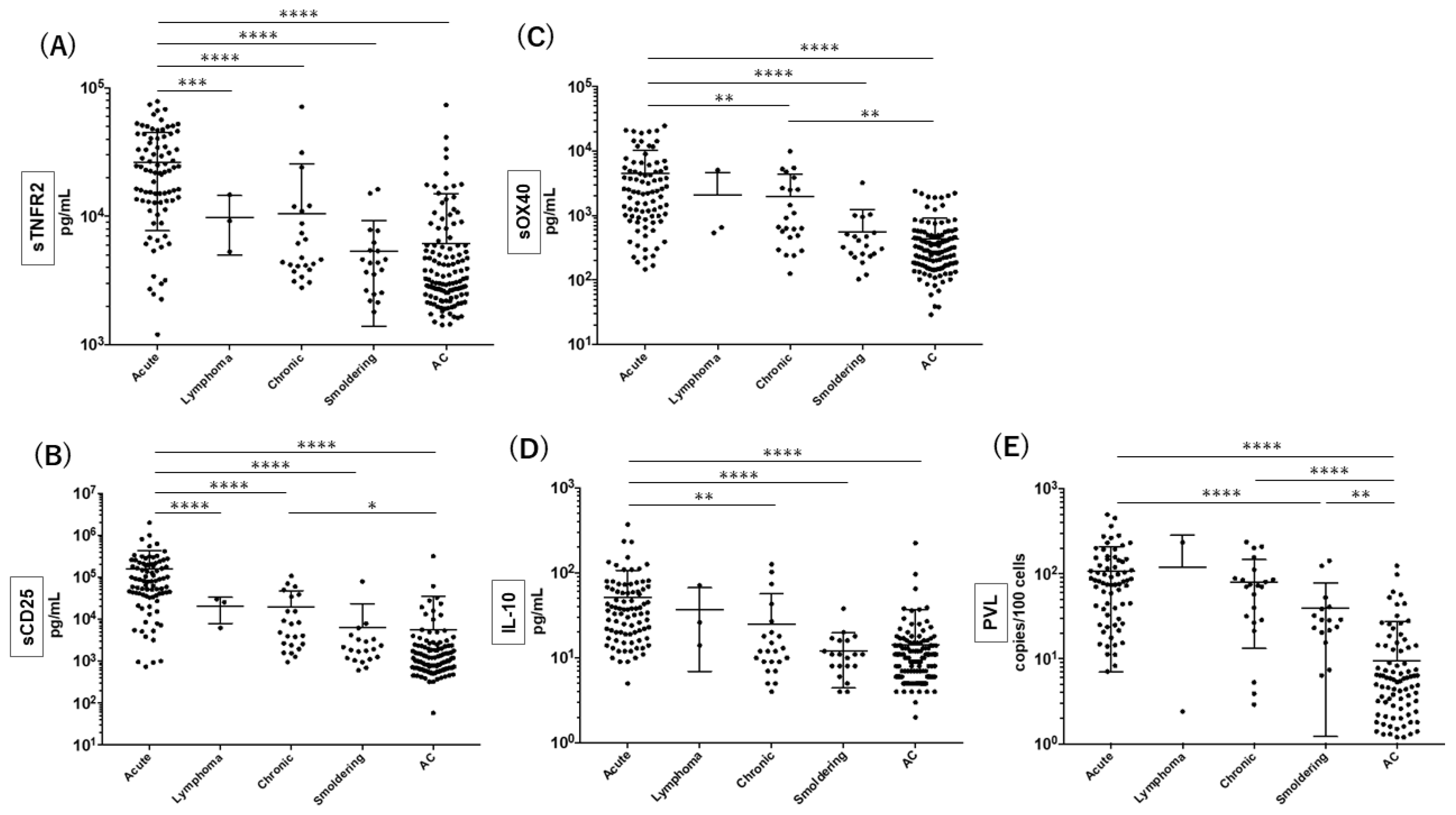
3.2. Quantitation of sTNFR2 Together with the Other Putative Biomarkers for Acute ATL
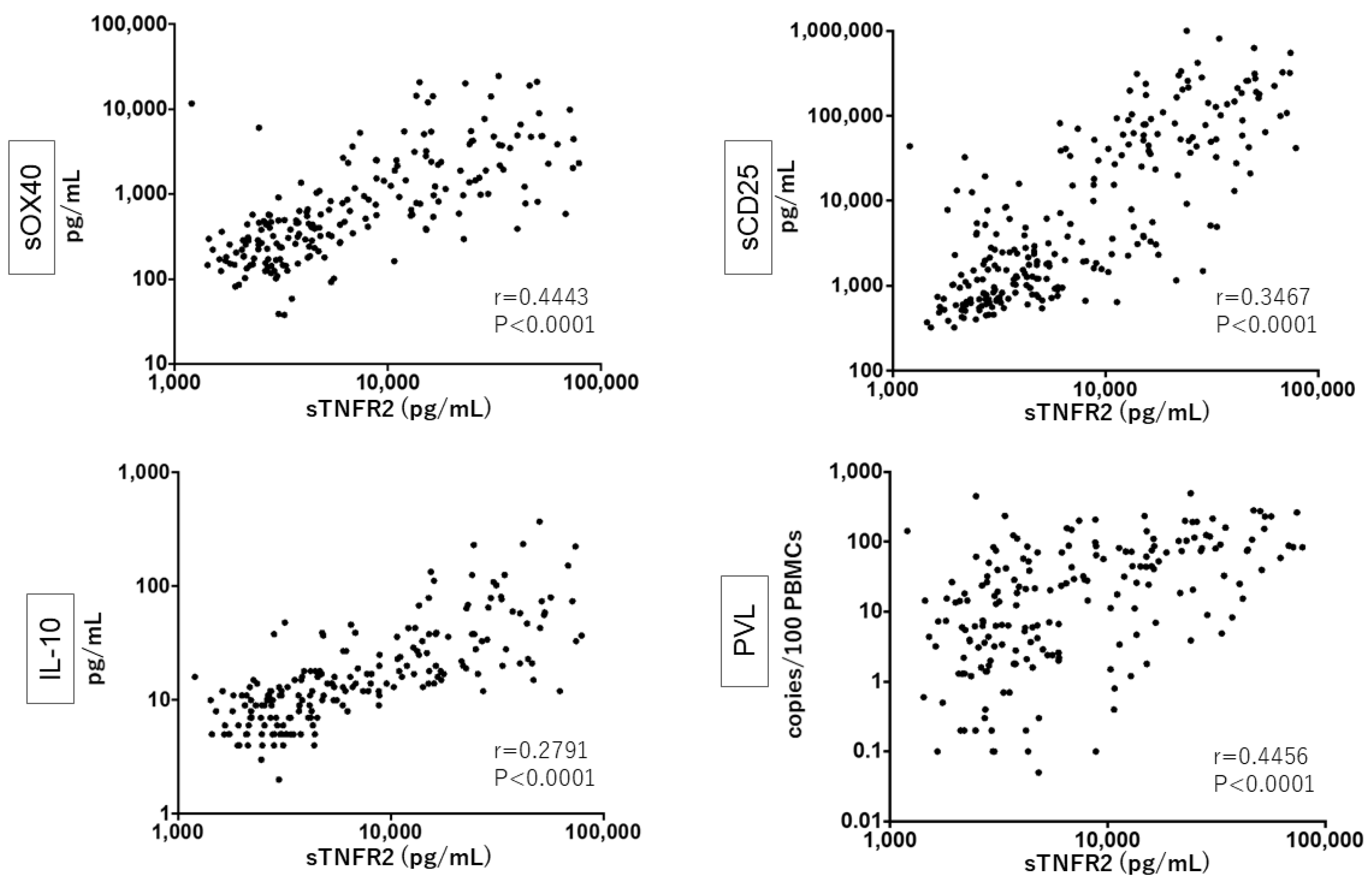
3.3. Close Relationship between sTNFR2, sCD25, sOX40, and IL-10 Levels and PVL
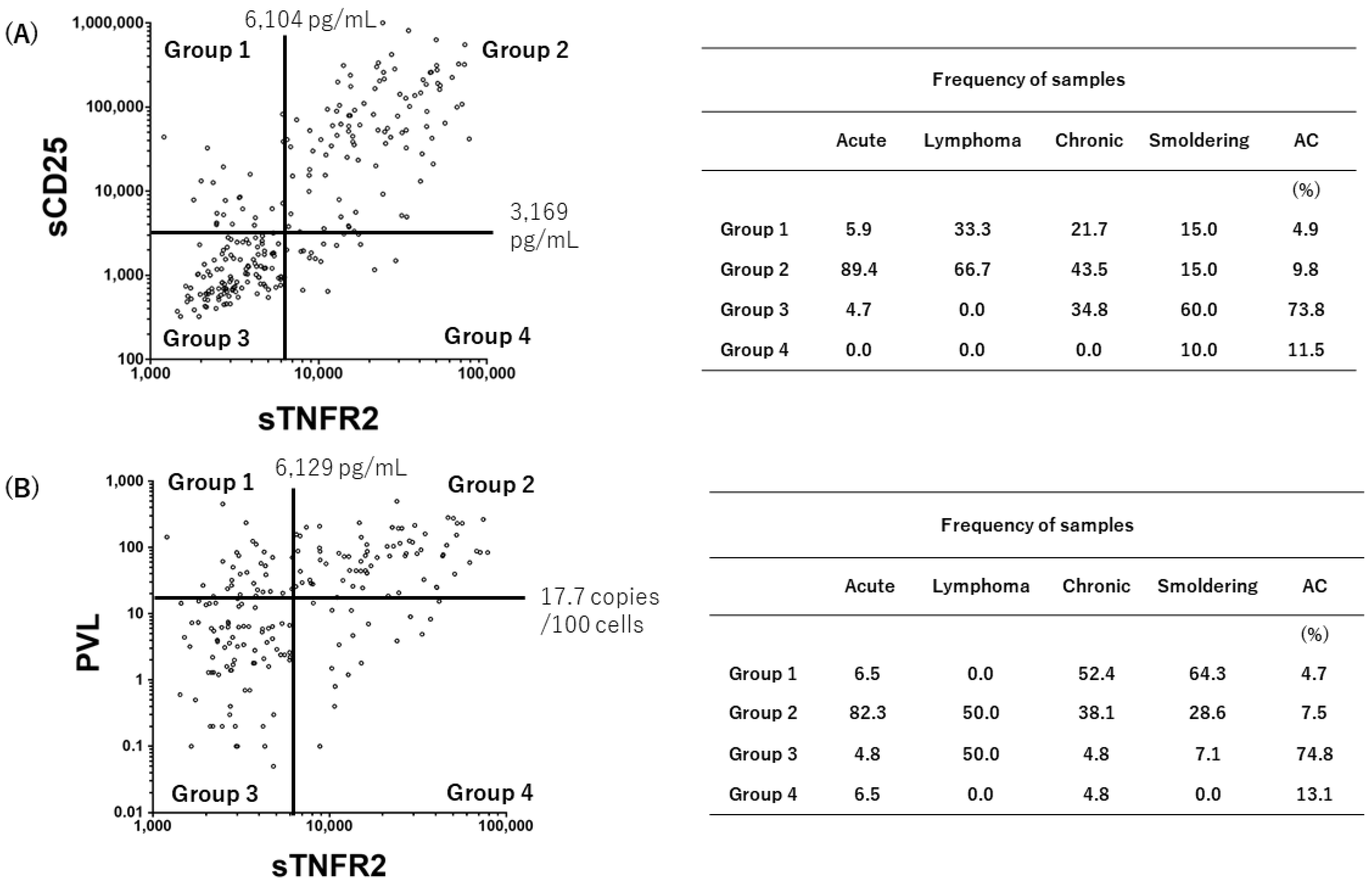
3.4. Grouping of the Status of HTLV-1-Infected Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchiyama, T.; Yodoi, J.; Sagawa, K.; Takatsuki, K.; Uchino, H. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood 1977, 50, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [Green Version]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 1981, 78, 6476–6480. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Seiki, M.; Yamaguchi, K.; Takatsuki, K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 1984, 81, 2534–2537. [Google Scholar] [CrossRef] [Green Version]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.R.; Arau’jo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B.F. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-cell leukemia: A review of epidemiological evidence. Front. Microbiol. 2012, 3, 322. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–1987). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Utsunomiya, A.; Fukuda, H.; Fukushima, T.; Takatsuki, Y.; Ikeda, S.; Matsuda, M.; Nagoshi, H.; Ueda, R.; Tamura, K.; et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan clinical oncology group study JCOG9801. J. Clin. Oncol. 2007, 25, 5458–5464. [Google Scholar] [CrossRef]
- Katsuya, H.; Ishitsuka, K.; Utsunomiya, A.; Hanada, S.; Eto, T.; Moriuchi, Y.; Saburi, Y.; Miyahara, M.; Sueoka, E.; Uike, N.; et al. Treatment and survival among 1594 patients with ATL. Blood 2015, 126, 2570–2577. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, Y.; Iwanaga, M.; Imaizumi, Y.; Tawara, M.; Joh, T.; Kohno, T.; Yamada, Y.; Kamihira, S.; Ikeda, S.; Miyazaki, Y.; et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood 2010, 115, 4337–4343. [Google Scholar] [CrossRef]
- Manns, A.; Miley, W.J.; Wilks, R.J.; Morgan, O.S.; Hanchard, B.; Wharfe, G.; Cranston, B.; Maloney, E.; Welles, S.L.; Blattner, W.A.; et al. Quantitative proviral DNA and antibody levels in the natural history of HTLV-I infection. J. Infect. Dis. 1999, 180, 1487–1493. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M.; Watanabe, T.; Utsunomiya, A.; Okayama, A.; Uchimaru, K.; Koh, K.R.; Ogata, M.; Kikuchi, H.; Sagara, Y.; Uozumi, K.; et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: A nationwide prospective study in Japan. Blood 2010, 116, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, N.; Lai, P.K.; Ip, S.H.; Kung, P.C.; Hinuma, Y.; Matsuoka, M.; Hattori, T.; Takatsuki, K.; Purtilo, D.T. Soluble interleukin 2 receptors in sera of Japanese patients with adult T cell leukemia mark activity of disease. Blood 1988, 71, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Takahashi, Y.; Tanaka, R.; Miyagi, T.; Saito, M.; Fukushima, T. Association of high levels of plasma OX40 with acute adult T-cell leukemia. Int. J. Hematol. 2019, 109, 319–327. [Google Scholar] [CrossRef]
- Inagaki, A.; Ishida, T.; Ishii, T.; Komatsu, H.; Iida, S.; Ding, J.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; Utsunomiya, A.; et al. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: High Interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int. J. Cancer 2006, 118, 3054–3061. [Google Scholar] [CrossRef]
- Imura, A.; Hori, T.; Imada, K.; Kawamata, S.; Tanaka, Y.; Uchiyama, T. OX40 Expressed on Fresh Leukemic Cells From Adult T-Cell Leukemia Patients Mediates Cell Adhesion to Vascular Endothelial Cells: Implication for the Possible Involvement of OX40 in Leukemic Cell Infiltration. Blood 1997, 89, 2951–2958. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, K.; Ishitsuka, K.; Chuman, Y.; Otsuka, M.; Kuwazuru, Y.; Iwahashi, M.; Utsunomiya, A.; Hanada, S.; Sakurami, T.; Arima, T. Tumor Necrosis Factor-β in the Serum of Adult T-cell Leukemia with Hypercalcemia. Blood 1991, 77, 2451–245520. [Google Scholar] [CrossRef] [Green Version]
- Cabal-Hierro, L.; Lazo, P.S. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- MacEwan, D.J. TNF ligands and receptors-a matter of life and death. Br. J. Pharmacol. 2002, 135, 855–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheller, J.; Chalaris, A.; Garbers, C.; Rose-John, S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends Immunol. Oxf. 2011, 32, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.L.H.; Yamashita, Y.; Miyara, M.; Imaizumi, N.; Kato, M.; Sakihama, S.; Hayashi, M.; Miyagi, T.; Karimata, K.; Uchihara, J.; et al. Proteomic profiling of HTLV-1 carriers and ATL patients reveals sTNFR2 as a novel diagnostic biomarker for acute ATL. Blood Adv. 2020, 4, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Kamihira, S.; Sugahara, K.; Tsuruda, K.; Minami, S.; Uemura, A.; Akamatsu, N.; Nagai, H.; Murata, K.; Hasegawa, H.; Hirakata, Y.; et al. Proviral status of HTLV-1 integrated into the host genomic DNA of adult T-cell leukemia cells. Clin. Lab. Haematol. 2005, 27, 235–241. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tozawa, H.; Koyanagi, Y.; Shida, H. Recognition of human T cell leukemia virus type I (HTLV-I) gag and pX gene products by MHC-restricted cytotoxic T lymphocytes induced in rats against syngeneic HTLV-I-infected cells. J. Immunol. 1990, 144, 4202–4211. [Google Scholar]
- Asao, H.; Fu, X.Y. Interferon-gamma has dual potentials in inhibiting or promoting cell proliferation. J. Biol. Chem. 2000, 275, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Takahashi, Y.; Kodama, A.; Saito, M.; Ansari, A.A.; Tanaka, Y. Suppression of CCR5-tropic HIV type 1 infection by OX40 stimulation via enhanced production of β-chemokines. AIDS Res. Hum. Retrovir. 2010, 26, 1147–1154. [Google Scholar] [CrossRef]
- Nagai, M.; Usuku, K.; Matsumoto, W.; Kodama, D.; Takenouchi, N.; Moritoyo, T.; Hashiguchi, S.; Ichinose, M.; Bangham, C.R.; Izumo, S.; et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: High proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 1998, 4, 586–593. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vanamee, É.S.; Faustman, D.L. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef]
- Paleolog, E.M.; Delasalle, S.A.; Buurman, W.A.; Feldmann, M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Blood 1994, 84, 2578–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Sakuma, Y.; Tada, M.; Sakuma, S.; Sudo, M.; Abe, H. p55 and p75 tumor necrosis factor receptor expression on human glioblastoma cells. Neurol. Med. Chir. 1995, 35, 567–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Sekioka, T.; Usui, M.; Imashuku, S. Opportunistic Infections in Patients with HTLV-1 Infection. Case Rep. Hematol. 2015, 2015, 943867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, N.; Nagahiro, Y.; Yoshida, S.; Tahara, Y.; Himeji, D.; Kuriyama, T.; Tochigi, T.; Nakaike, T.; Shimokawa, T.; Yamashita, K.; et al. Clinical features and treatment outcomes of opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia-lymphoma (ATL) at a single institution from 2006 to 2016. J. Clin. Exp. Hematop. 2019, 59, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Joyce, D.A.; Gibbons, D.P.; Green, P.; Steer, J.H.; Feldmann, M.; Brennan, F.M. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur. J. Immunol. 1994, 24, 2699–2705. [Google Scholar] [CrossRef]
- Coyne, C.P.; Willetto, C.; Fenwick, B.W. A mechanism of TNFR type II (75 kDa) “shedding” in macrophages. Shock 1999, 11, 19–28. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Tian, T.; Ma, D. Increased Regulatory T Cells in Peripheral Blood of Acute Myeloid Leukemia Patients Rely on Tumor Necrosis Factor (TNF)-α-TNF Receptor-2 Pathway. Front. Immunol. 2018, 9, 1274. [Google Scholar] [CrossRef] [Green Version]
- Torrey, H.; Khodadoust, M.; Tran, L.; Baum, D.; Defusco, A.; Kim, Y.H.; Faustman, D.L. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia 2019, 33, 1206–1218. [Google Scholar] [CrossRef] [Green Version]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 2017, 10, eaaf8608. [Google Scholar] [CrossRef]
- Torrey, H.; Kühtreiber, W.M.; Okubo, Y.; Tran, L.; Case, K.; Zheng, H.; Vanamee, E.; Faustman, D.L. A novel TNFR2 agonist antibody expands highly potent regulatory T cells. Sci. Signal. 2020, 13, eaba9600. [Google Scholar] [CrossRef]
- Tschachler, E.; Bohnlein, E.; Felzmann, S.; Reitz, M.S., Jr. Human T-Lymphotropic Virus Type I tax Regulates the Expression of the Human Lymphotoxin Gene. Blood 1993, 81, 95–100. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Ayres, T.M.; Wong, G.H.; Goeddel, D.V. A novel domain within the 55 kd TNF receptor signals cell death. Cell 1993, 74, 845–853. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, M.; Imaizumi, N.; Tanaka, R.; Mizuguchi, M.; Hayashi, M.; Miyagi, T.; Uchihara, J.; Ohshiro, K.; Todoroki, J.; Karube, K.; et al. Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia. Viruses 2022, 14, 751. https://doi.org/10.3390/v14040751
Kato M, Imaizumi N, Tanaka R, Mizuguchi M, Hayashi M, Miyagi T, Uchihara J, Ohshiro K, Todoroki J, Karube K, et al. Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia. Viruses. 2022; 14(4):751. https://doi.org/10.3390/v14040751
Chicago/Turabian StyleKato, Megumi, Naoki Imaizumi, Reiko Tanaka, Mariko Mizuguchi, Masaki Hayashi, Takashi Miyagi, Junnosuke Uchihara, Kazuiku Ohshiro, Junpei Todoroki, Kennosuke Karube, and et al. 2022. "Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia" Viruses 14, no. 4: 751. https://doi.org/10.3390/v14040751
APA StyleKato, M., Imaizumi, N., Tanaka, R., Mizuguchi, M., Hayashi, M., Miyagi, T., Uchihara, J., Ohshiro, K., Todoroki, J., Karube, K., Masuzaki, H., Tanaka, Y., & Fukushima, T. (2022). Elevation of the Plasma Levels of TNF Receptor 2 in Association with Those of CD25, OX40, and IL-10 and HTLV-1 Proviral Load in Acute Adult T-Cell Leukemia. Viruses, 14(4), 751. https://doi.org/10.3390/v14040751






