An Inducible Microbacterium Prophage vB_MoxS-R1 Represents a Novel Lineage of Siphovirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Host Bacterial Cultivation and Prophage Induction
2.2. Phage Amplification and Purification
2.3. Transmission Electron Microscopy (TEM) Observation
2.4. Host Range Determination
2.5. Phage DNA Extraction and Genome Sequencing
2.6. Genome Annotation
2.7. Phage Genome Network Analyses and Phylogenetic Analyses
3. Results and Discussion
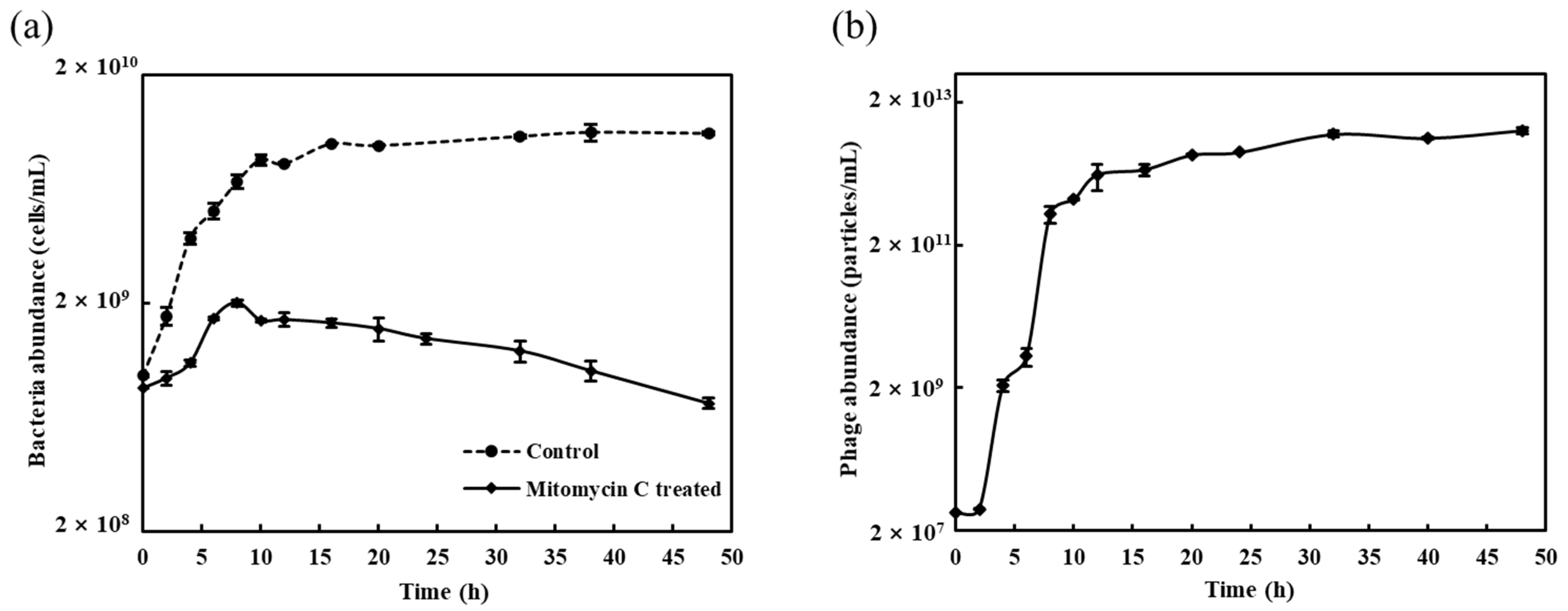
3.1. Induction and Basic Biological Features of Phage vB_MoxS-R1
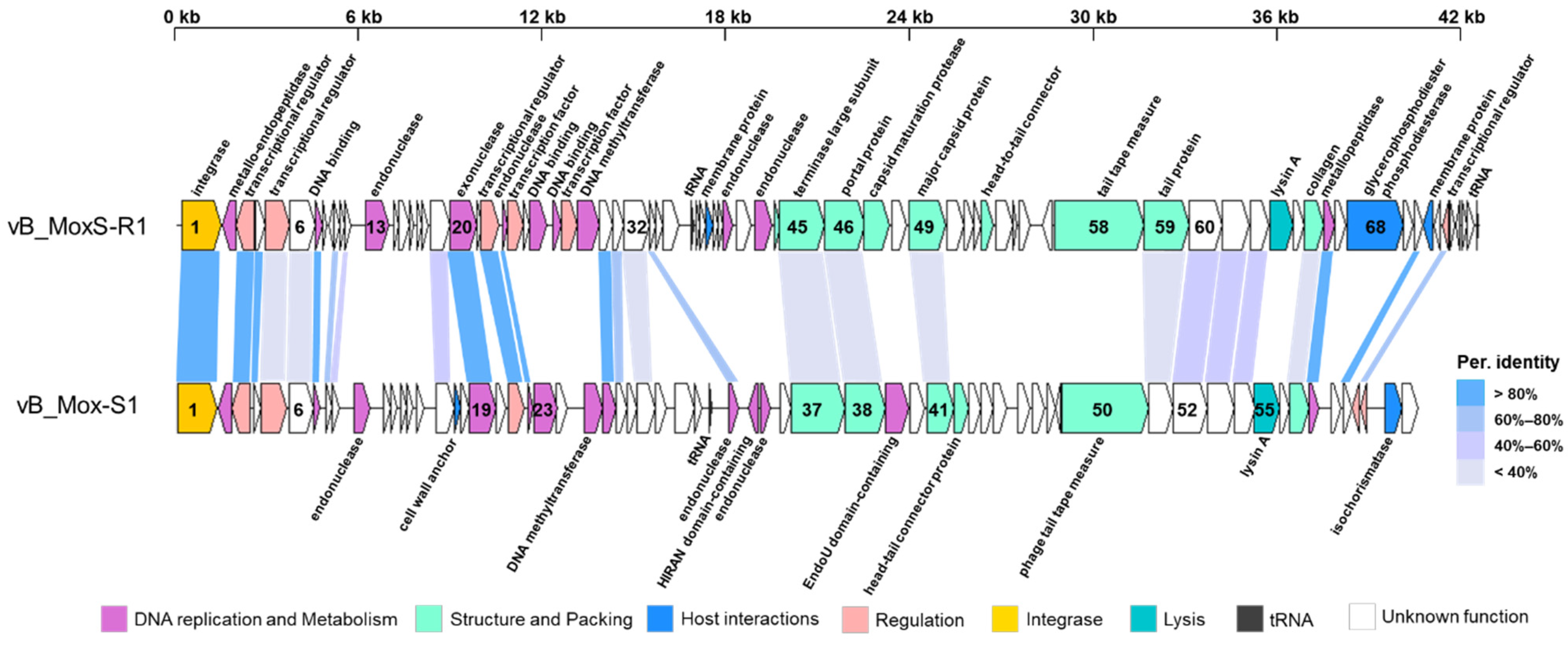
3.2. Genomic Properties and Structure of Phage vB_MoxS-R1
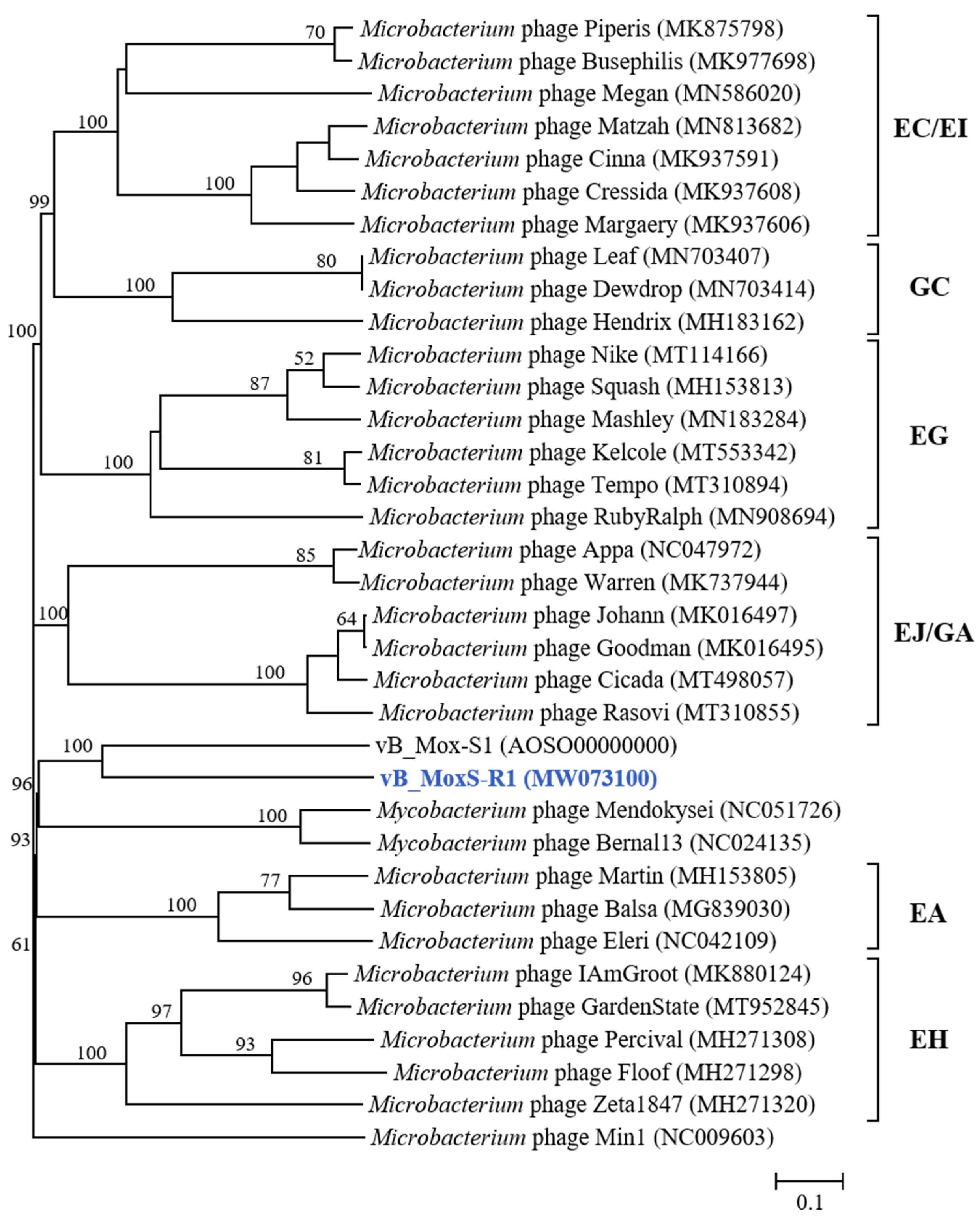
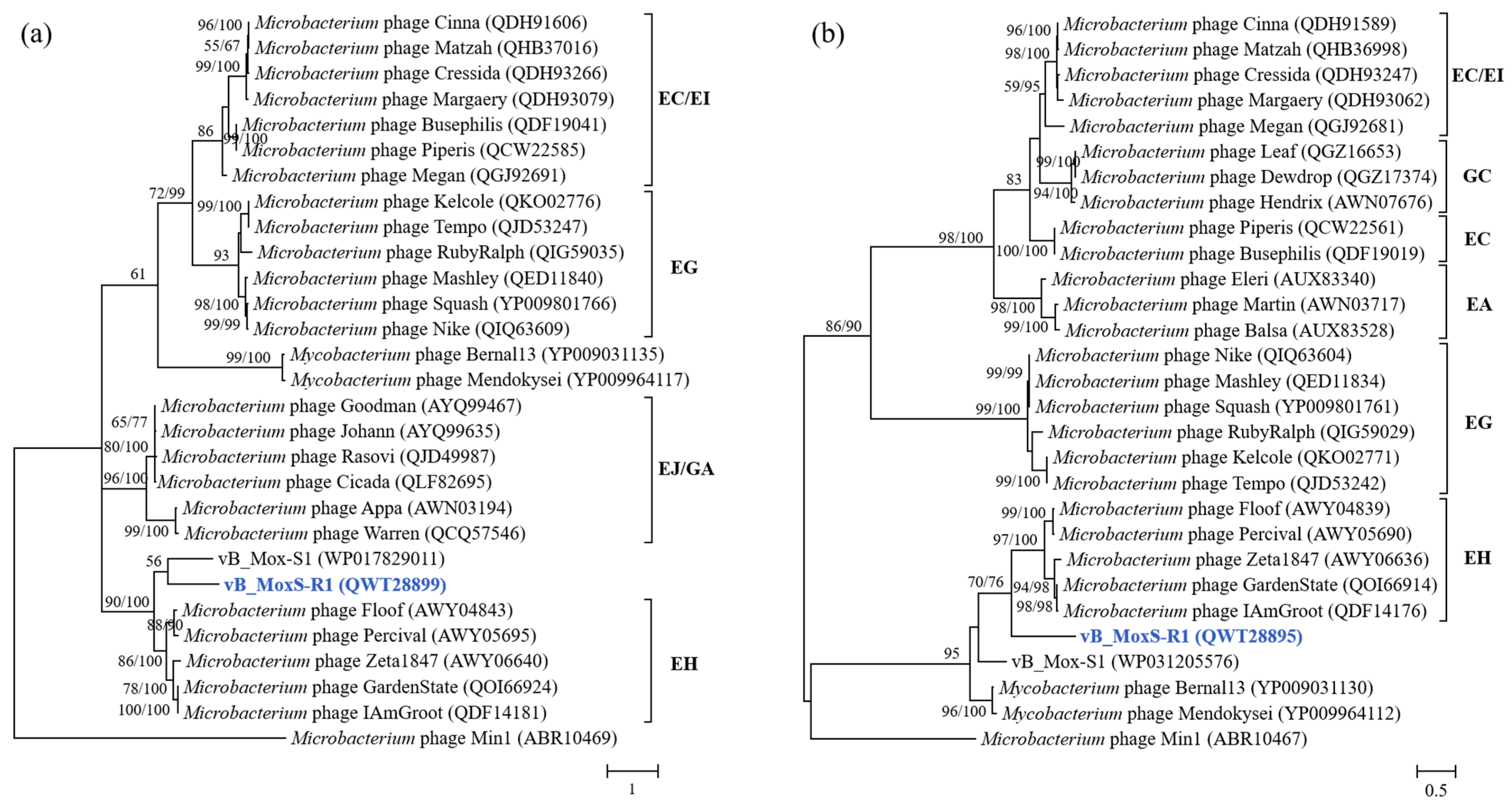
3.3. vB_MoxS-R1 Represents a New Phage Genus
3.4. DNA Repair and Modification
3.5. Diverse Transcriptional Regulators
3.6. Auxiliary Metabolic Genes
3.7. Putative Frameshift in the Terminase Large Subunit
3.8. Integrase and Lysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergh, O.; Rsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.M.; Fuhrman, J.A. Viral mortality of marine bacteria and cyanobacteria. Nature 1990, 343, 60–62. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl. Environ. Microbiol. 1982, 43, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinbauer, M.G.; Rassoulzadegan, F. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 2010, 6, 1–11. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; de Vargas, C.; Gasol, J.M.; et al. Patterns and ecological drivers of ocean viral communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [Green Version]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef]
- Kang, I.; Oh, H.M.; Kang, D.; Cho, J.C. Genome of a SAR116 bacteriophage shows the prevalence of this phage type in the oceans. Proc. Natl. Acad. Sci. USA 2013, 110, 12343–12348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Temperton, B.; Thrash, J.C.; Schwalbach, M.S.; Giovannoni, S.J. Abundant SAR11 viruses in the ocean. Nature 2013, 494, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, K.; Stewart, J.; Belas, R. Induction of multiple prophages from a marine bacterium: A genomic approach. Appl. Environ. Microbiol. 2006, 72, 4995–5001. [Google Scholar] [CrossRef] [Green Version]
- Davies, E.V.; Craig, W.; Fothergill, J.L.; James, C.E. The role of temperate bacteriophages in bacterial infection. Fems Microbiol. Lett. 2016, 363, fnw015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feiner, R.; Argov, T.; Rabinovich, L.; Sigal, N.; Borovok, I.; Herskovits, A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015, 13, 641–650. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. Isme J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Menouni, R.; Hutinet, G.; Petit, M.A.; Ansaldi, M. Bacterial genome remodeling through bacteriophage recombination. Fems Microbiol. Lett. 2015, 362, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.M.; Thormann, K.; Frunzke, J.; Margolin, W. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J. Bacteriol. 2015, 197, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, J.H. Prophages in marine bacteria: Dangerous molecular time bombs or the key to survival in the seas? Isme J. 2008, 2, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, K.; Ackermann, H.W.; Halden, R.U.; Jiao, N.; Chen, F. Searching for a “hidden” prophage in a marine bacterium. Appl. Environ. Microbiol. 2010, 76, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, C.B.; Rohwer, F.L. Piggyback-the-Winner in host-associated microbial communities. NPJ Biofilms Microbiomes 2016, 2, 16010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, B.; Silveira, C.B.; Bailey, B.A.; Barott, K.; Cantu, V.A.; Cobián-Güemes, A.G.; Coutinho, F.H.; Dinsdale, E.A.; Felts, B.; Furby, K.A.; et al. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Bae, J.W. Lysogeny is prevalent and widely distributed in the murine gut microbiota. Isme J. 2018, 12, 1127–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, H.W.; Dubow, M.S. General Properties of Bacteriophages; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Imis, V.; Leitet, C.; Riemann, L.; Hagström, Å. Plasmids and prophages in Baltic Sea bacterioplankton isolates. J. Mar. Biol. Assoc. UK 2006, 86, 567–575. [Google Scholar] [CrossRef]
- Jiang, S.C.; Paul, J.H. Significance of lysogeny in the marine environment: Studies with isolates and a model of lysogenic phage production. Microb. Ecol. 1998, 35, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.C.; Paul, J.H. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar. Ecol. Prog. Ser. 1994, 104, 163–172. [Google Scholar] [CrossRef]
- Stopar, D.; Erne, A.; Igman, M.; Polj Ak-Prijatelj, M.; Turk, V. Viral abundance and a high proportion of lysogens suggest that viruses are important members of the microbial community in the Gulf of Trieste. Microb. Ecol. 2004, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.F.; Brüssow, H. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 2002, 10, 521–529. [Google Scholar] [CrossRef]
- Canchaya, C.; Fournous, G.; Brüssow, H. The impact of prophages on bacterial chromosomes. Mol. Microbiol. 2010, 53, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, A.; Worsley, S.F.; Hutchings, M.I.; Van Wezel, G.P. Chemical ecology of antibiotic production by actinomycetes. Fems Microbiol. Rev. 2017, 41, 392–416. [Google Scholar] [CrossRef]
- Van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of Actinobacteria: New concepts for natural product discovery. Nat. Rev. Microbiol. 2020, 18, 546–558. [Google Scholar] [CrossRef]
- Kroiss, J.; Kaltenpoth, M.; Schneider, B.; Schwinger, M.G.; Hertweck, C.; Maddula, R.K.; Strohm, E.; Svatos, A. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 2010, 6, 261–263. [Google Scholar] [CrossRef]
- Berlemont, R.; Martiny, A.C. Phylogenetic distribution of potential cellulases in bacteria. Appl. Environ. Microbiol. 2013, 79, 1545–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, T. Aquatic actinomycetes: A critical survey of the occurrence, growth and role of actinomycetes in aquatic habitats. J. Appl. Bacteriol. 2010, 50, 397–423. [Google Scholar] [CrossRef] [PubMed]
- De, B.W.; Folman, L.B.; Summerbell, R.C.; Lynne, B. Living in a fungal world: Impact of fungi on soil bacterial niche development. Fems Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [Green Version]
- Erikson, D. Studies on some lake-mud strains of Micromonospora. J. Bacteriol. 1941, 41, 277–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewin, G.R.; Carlos, C.; Chevrette, M.G.; Horn, H.A.; Mcdonald, B.R.; Stankey, R.J.; Fox, B.G.; Currie, C.R. Evolution and ecology of Actinobacteria and their bioenergy applications. Annu. Rev. Microbiol. 2016, 70, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Tveit, A.T.; Urich, T.; Svenning, M.M. Metatranscriptomic analysis of arctic peat soil microbiota. Appl. Environ. Microbiol. 2014, 80, 5761–5772. [Google Scholar] [CrossRef] [Green Version]
- Book, A.J.; Lewin, G.R.; Mcdonald, B.R.; Takasuka, T.E.; Currie, C.R. Evolution of high cellulolytic activity in symbiotic Streptomyces through selection of expanded gene content and coordinated gene expression. PLoS Biol. 2016, 14, e1002475. [Google Scholar] [CrossRef] [PubMed]
- Kaltenpoth, M. Actinobacteria as mutualists: General healthcare for insects? Trends Microbiol. 2009, 17, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Turroni, F.; Duranti, S.; Lugli, G.A.; Mancabelli, L.; Ferrario, C.; Sinderen, D.V.; Ventura, M. Genomics of the genus Bifidobacterium reveals species-specific adaptation to the glycan-rich gut environment. Appl. Environ. Microbiol. 2015, 82, 980–991. [Google Scholar] [CrossRef] [Green Version]
- Pokusaeva, K.; Fitzgerald, G.F.; Sinderen, D.V. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Action, C.T. High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. Isme J. 2008, 3, 314–325. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, Y.; Xie, R.; Lang, A.S.; Liu, Y.; Lu, J.; Zhang, X.; Sun, J.; Suttle, C.A.; Jiao, N. Dynamics of heterotrophic bacterial assemblages within Synechococcus cultures. Appl. Environ. Microbiol. 2018, 84, e01517-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Woodhouse, J.N.; Te, S.H.; Yew-Hoong, G.K.; He, Y.; Xu, C.; Chen, L. Seasonal variation in the bacterial community composition of a large estuarine reservoir and response to cyanobacterial proliferation. Chemosphere 2018, 202, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Brennan, N.M.; Brown, R.; Goodfellow, M.; Ward, A.C.; Fox, P.F. Microbacterium gubbeenense sp. nov., from the surface of a smear-ripened cheese. Int. J. Syst. Evol. Microbiol. 2001, 51, 1969–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gneiding, K.; Frodl, R.; Funke, G. Identities of Microbacterium spp. encountered in human clinical specimens. J. Clin. Microbiol. 2008, 46, 3646–3652. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Hatano, K. Union of the genera Microbacterium Orla-Jensen and Aureobacterium Collins et al. in a redefined genus. Int. J. Syst. Bacteriol. 1998, 3, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Gonalves, J.M.S.; Reis, M.; Costa, R. Draft genome sequence of Microbacterium sp. strain alg239_v18, an actinobacterium retrieved from the marine sponge Spongia sp. Genome Announc. 2017, 5, e01457-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Lee, K.C.; Oh, H.M.; Lee, J.S. Microbacterium aquimaris sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 2008, 58 Pt 7, 1616–1620. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhou, P.; Cheng, H.; Wang, C.S.; Xu, X.W. Draft genome sequence of Microbacterium profundi shh49t, an actinobacterium isolated from deep-sea sediment of a polymetallic nodule environment. Genome Announc. 2015, 3, e615–e642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs-Sera, D.; Abad, L.A.; Alvey, R.M.; Anders, K.R.; Hatfull, G.F. Genomic diversity of bacteriophages infecting Microbacterium spp. PLoS ONE 2020, 15, e234636. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A.; Hatfull, G.F. PhagesDB: The actinobacteriophage database. Bioinformatics 2017, 33, 784–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimkina, T.; Venien-Bryan, C.; Hodgkin, J. Isolation, characterization and complete nucleotide sequence of a novel temperate bacteriophage Min1, isolated from the nematode pathogen Microbacterium nematophilum. Res. Microbiol. 2007, 158, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, B.; Liu, J.; Wang, R. Genome sequence of Microbacterium sp. strain R1, isolated from a Synechococcus culture. Microbiol. Resour. Announc. 2021, 10, e0054221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiao, N.; Chen, F.; Collier, J. Novel psychrotolerant picocyanobacteria isolated from Chesapeake Bay in the winter. J. Phycol. 2015, 51, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.V.; Krieger, S.; Stackebrandt, E.; Beatty, J.T. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, R.; Jiao, N. Complete genome sequence of Paracoccus marcusii phage vB_PmaS-R3 isolated from the South China Sea. Stand. Genom. Sci. 2015, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Ciglenecki, U.J.; Grom, J.; Toplak, I.; Jemersić, L.; Barlic-Maganja, D. Real-time RT-PCR assay for rapid and specific detection of classical swine fever virus: Comparison of SYBR Green and TaqMan MGB detection methods using novel MGB probes. J. Virol. Methods 2008, 147, 257–264. [Google Scholar] [CrossRef]
- Peng, X.; Nguyen, A.; Ghosh, D. Quantification of M13 and T7 bacteriophages by TaqMan and SYBR green qPCR. J. Virol. Methods 2018, 252, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.; Yiu, S.M.; Chin, F. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Aron, M.B.; Anderson, J.B.; Derbyshire, M.K.; Carol, D.W.S.; Gonzales, N.R.; Marc, G.; Hao, L.; He, S.; Hurwitz, D.I.; Jackson, J.D. CDD: A conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007, 35, D237–D240. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA genes in genomic sequences. Methods Mol. Biol. 2019, 1962, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Enright, A.J.; Dongen, S.V.; Ouzounis, C.A. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002, 30, 1575–1584. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Chu, X.; Zhang, H.; Luo, H.; Qin, F.; Zhai, Z.; Yang, M.; Sun, J.; Zhao, Y. Diverse, abundant, and novel viruses infecting the marine roseobacter RCA lineage. mSystems 2019, 4, e00494-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.B.; Bolduc, B.; Zablocki, O.; Kuhn, J.H.; Roux, S.; Adriaenssens, E.M.; Brister, J.R.; Kropinski, A.M.; Krupovic, M.; Lavigne, R. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat. Biotechnol. 2019, 37, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, F.; Chen, F.; Chu, X.; Luo, H.; Zhang, R.; Du, S.; Tian, Z.; Zhao, Y. Culturing novel and abundant pelagiphages in the ocean. Environ. Microbiol. 2021, 23, 1145–1161. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lai, J.; Chen, X.; Wang, L.; Yang, Y.; Wei, S.; Jiao, N.; Zhang, R. A novel phage infecting Alteromonas represents a distinct group of siphophages infecting diverse aquatic copiotrophs. mSphere 2021, 6, e0045421. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-A novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Sabehi, G.; Shaulov, L.; Silver, D.H.; Yanai, I.; Harel, A.; Lindell, D. A novel lineage of myoviruses infecting cyanobacteria is widespread in the oceans. Proc. Natl. Acad. Sci. USA 2012, 109, 2037–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhang, R.; Wang, N.; Cai, L.; Tong, Y.; Sun, Q.; Chen, F.; Jiao, N. Novel phage-host interactions and evolution as revealed by a cyanomyovirus isolated from an estuarine environment. Environ. Microbiol. 2018, 20, 2974–2989. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Yan, W.; Wang, A.; Ma, R.; Chen, X.; Lin, T.H.; Chen, Y.L.; Wei, S.; Jin, T.; Jiao, N.; et al. A novel broad host range phage infecting Alteromonas. Viruses 2021, 13, 987. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr. Microbiol. 2010, 61, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhang, R.; Xu, Y.; White, R.A.; Wang, Y.; Luo, T.; Jiao, N. A marine inducible prophage vB_CibM-P1 isolated from the aerobic anoxygenic phototrophic bacterium Citromicrobium bathyomarinum JL354. Sci. Rep. 2014, 4, 7118. [Google Scholar] [CrossRef] [Green Version]
- Hendry, G.S.; Fitz-James, P.C. Characteristics of phi T, the temperate bacteriophage carried by Bacillus megaterium 899a. J. Virol. 1974, 13, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.H.; Hammond, B.F.; Lai, C.H. Characterization of an inducible bacteriophage from a leukotoxic strain of Actinobacillus actinomycetemcomitans. Infect. Immun. 1982, 35, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Adriaenssens, E.M.; Edwards, R.; Nash, J.H.E.; Mahadevan, P.; Seto, D.; Ackermann, H.W.; Lavigne, R.; Kropinski, A.M. Integration of genomic and proteomic analyses in the classification of the Siphoviridae family. Virology 2015, 477, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Adriaenssens, E.; Brister, J.R. How to name and classify your phage: An informal guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, A.M.; Mohundro, M.M.; Mian, I.S.; Battista, J.R. The IrrE protein of Deinococcus radiodurans R1 is a novel regulator of recA expression. J. Bacteriol. 2002, 184, 6216–6224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narumi, I.; Satoh, K.; Cui, S.; Funayama, T.; Watanabe, H. PprA: A novel protein from Deinococcus radiodurans that stimulates DNA ligation. Mol. Microbiol. 2010, 54, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Analysis of Deinococcus radiodurans’s transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 2004, 168, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Vujicić-Zagar, A.; Dulermo, R.; Gorrec, M.L.; Vannier, F.; Servant, P.; Sommer, S.; Groot, A.D.; Serre, L. Crystal structure of the IrrE protein, a central regulator of DNA damage repair in deinococcaceae. J. Mol. Biol. 2009, 386, 704–716. [Google Scholar] [CrossRef]
- Bauermeister, A.; Bentchikou, E.; Moeller, R.; Rettberg, P. Roles of PprA, IrrE, and RecA in the resistance of Deinococcus radiodurans to germicidal and environmentally relevant UV radiation. Arch. Microbiol. 2009, 191, 913–918. [Google Scholar] [CrossRef]
- Goranov, A.I.; Kuester-Schoeck, E.; Wang, J.D.; Grossman, A.D. Characterization of the global transcriptional responses to different types of DNA damage and disruption of replication in Bacillus subtilis. J. Bacteriol. 2006, 188, 5595–5605. [Google Scholar] [CrossRef] [Green Version]
- Mcgrew, D.A.; Knight, K.L. Molecular design and functional organization of the RecA protein. Crit. Rev. BioChem. Mol. Biol. 2003, 38, 385–432. [Google Scholar] [CrossRef]
- Auchtung, J.M.; Lee, C.A.; Garrison, K.L.; Grossman, A.D. Identification and characterization of the immunity repressor (ImmR) that controls the mobile genetic element ICEBs1 of Bacillus subtilis. Mol. Microbiol. 2010, 64, 1515–1528. [Google Scholar] [CrossRef] [Green Version]
- Bose, B.; Grossman, A.D. Regulation of horizontal gene transfer in Bacillus subtilis by activation of a conserved site-specific protease. J. Bacteriol. 2011, 193, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Kota, S.; Misra, H.S. PprA: A protein implicated in radioresistance of Deinococcus radiodurans stimulates catalase activity in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.S.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef]
- Jeltsch, A. Beyond watson and crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem 2002, 3, 274–293. [Google Scholar] [CrossRef]
- Harrison, C.J.; Bohm, A.A.; Nelson, H.C.M. Crystal structure of DNA binding domain of the heat shock transcription factor. Science 1994, 263, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Miriam, G.; Adele, W.; Elin, K.; Bj Rn, A.; Ingar, L. Biochemical characterization of ParI, an orphan C5-DNA methyltransferase from Psychrobacter arcticus 273-4. Protein Expr. Purif. 2018, 150, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Tanneeru, K.; Guruprasad, L. Structure and dynamics of H. pylori 98-10 C5-cytosine specific DNA methyltransferase in complex with S-adenosyl-L-methionine and DNA. Mol. Biosyst. 2016, 12, 3111–3123. [Google Scholar] [CrossRef]
- Wilson, G.G. Organization of restriction-modification systems. Nucleic Acids Res. 1991, 19, 2539–2566. [Google Scholar] [CrossRef] [Green Version]
- Razin, A. DNA Methylase unduced by bacteriophage X174. Proc. Natl. Acad. Sci. USA 1973, 70, 3773–3775. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, D.; Huang, Y.; Zhu, X.; Rui, M.; Wan, T.; Zheng, X.; Shen, Y.; Chen, X.; Ma, K.; et al. Structural and functional characterization of deep-sea thermophilic bacteriophage GVE2 HNH endonuclease. Sci. Rep. 2017, 7, 42542. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Gupta, Y.K. Natural zinc ribbon HNH endonucleases and engineered zinc finger nicking endonuclease. Nucleic Acids Res. 2013, 41, 378–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kala, S.; Cumby, N.; Sadowski, P.D.; Hyder, B.Z.; Kanelis, V.; Davidson, A.R.; Maxwell, K.L. HNH proteins are a widespread component of phage DNA packaging machines. Proc. Natl. Acad. Sci. USA 2014, 111, 6022–6027. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Sadiq, F.A.; Han, X.; Zhao, J.; Chen, W. Identification, characterization, and phylogenetic analysis of eight new inducible prophages in Lactobacillus. Virus Res. 2020, 286, 198003. [Google Scholar] [CrossRef] [PubMed]
- Casadesus, J. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 2006, 70, 830–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Nadar, V.S.; Rosen, B.P. A novel MAs(III)-selective ArsR transcriptional repressor. Mol. Microbiol. 2017, 106, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Dong, J. The role of arsenic-thiol interactions in metalloregulation of the ars operon. J. Biol. Chem 1996, 271, 9291. [Google Scholar] [CrossRef] [Green Version]
- Rastorguev, S.M.; Zavilgelsky, G.B.; Tchurikov, N.A. IncI1 plasmid R64 encodes the ArsR protein that alleviates type I restriction. FEBS Lett. 1998, 426, 21–23. [Google Scholar] [CrossRef] [Green Version]
- Rawle, R.; Saley, T.C.; Kang, Y.S.; Wang, Q.; Walk, S.; Bothner, B.; McDermott, T.R. Introducing the ArsR-regulated arsenic stimulon. Front. Microbiol. 2021, 12, 630562. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, R.; Nikoleit, K.; Götz, F. Binding of ArsR, the repressor of the Staphylococcus xylosus (pSX267) arsenic resistance operon to a sequence with dyad symmetry within the ars promoter. Mol. Gen. Genet 1994, 242, 566–572. [Google Scholar] [CrossRef]
- Wu, J.R.; Rosen, B.P. The ArsR protein is a trans-acting regulatory protein. Mol. Microbiol. 2010, 5, 1331–1336. [Google Scholar] [CrossRef]
- Carlin, A.; Shi, W.; Dey, S.; Rosen, B.P. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J. Bacteriol. 1995, 177, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Rosinski, J.A.; Atchley, W.R. Molecular evolution of helix-turn-helix proteins. J. Mol. Evol. 1999, 49, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, Q.; Wei, R.; Li, R.; Zhao, D.; Ge, M.; Yao, Q.; Yu, X. The XRE family transcriptional regulator SrtR in Streptococcus suis is involved in oxidant tolerance and virulence. Front. Cell. Infect. Microbiol. 2018, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Mccallum, N.; Berger-Bächi, B.; Senn, M.M. Regulation of antibiotic resistance in Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Panyakampol, J.; Cheevadhanarak, S.; Sutheeworapong, S.; Chaijaruwanich, J.; Senachak, J.; Siangdung, W.; Jeamton, W.; Tanticharoen, M.; Paithoonrangsarid, K. Physiological and transcriptional responses to high temperature in Arthrospira (Spirulina) platensis C1. Plant Cell Physiol. 2015, 56, 481–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertonati, C.; Punta, M.; Fischer, M.; Yachdav, G.; Rost, B. Structural genomics reveals EVE as a new ASCH/PUA-related domain. Proteins 2010, 75, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Iyer, L.; Burroughs, A.; Aravind, L. The ASCH superfamily: Novel domains with a fold related to the PUA domain and a potential role in RNA metabolism. Bioinformatics 2006, 22, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhou, W.; Sheery, N.; Peters, C.; Li, M.; Wang, X.; Huang, J. Characterization of the Arabidopsis glycerophosphodiester phosphodiesterase (GDPD) family reveals a role of the plastid-localized AtGDPD1 in maintaining cellular phosphate homeostasis under phosphate starvation. Plant J. 2011, 66, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Phosphate depletion in the western North Atlantic Ocean. Science 2000, 289, 759–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheatley, R.M.; Poole, P.S. Mechanisms of bacterial attachment to roots. Fems Microbiol. Rev. 2018, 42, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, N.; Roggensack, S.E.; Rodrigue, S.; Thompson, J.W.; Biller, S.J.; Coe, A.; Ding, H.; Marttinen, P.; Malmstrom, R.R.; Stocker, R.; et al. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science 2014, 344, 416–420. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hendrix, R.W.; Duda, R.L. Conserved translational frameshift in dsDNA bacteriophage tail assembly genes. Mol. Cell 2004, 16, 11–21. [Google Scholar] [CrossRef]
- Gesteland, R.F.; Atkins, J.F. Recoding: Dynamic reprogramming of translation. Annu. Rev. Biochem. 1996, 65, 741–768. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.R.; Gilcrease, E.B.; Winn-Stapley, D.A.; Schicklmaier, P.; Schmieger, H.; Pedulla, M.L.; Ford, M.E.; Houtz, J.M.; Hatfull, G.F.; Hendrix, R.W. The generalized transducing Salmonella bacteriophage ES18: Complete genome sequence and DNA packaging strategy. J. Bacteriol. 2005, 187, 1091–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandler, M.; Fayet, O. Translational frameshifting in the control of transposition in bacteria. Mol. Microbiol. 1993, 7, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.E.; Hendrix, R.W.; Casjens, S.R. A programmed translational frameshift is required for the synthesis of a bacteriophage lambda tail assembly protein. J. Mol. Biol. 1993, 234, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Grindley, N.D.; Whiteson, K.L.; Rice, P.A. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006, 75, 567–605. [Google Scholar] [CrossRef] [Green Version]
- Nash, A.H. Integration and Excision of Bacteriophage λ: The mechanism of conservative site specific recombination. Annu. Rev. Genet. 1981, 15, 143–167. [Google Scholar] [CrossRef]
- Williams, K.P. Integration sites for genetic elements in prokaryotic tRNA and tmRNA genes: Sublocation preference of integrase subfamilies. Nucleic Acids Res. 2002, 30, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, I.; Wang, I.N.; Roof, W.D. Phages will out: Strategies of host cell lysis. Trends Microbiol. 2000, 8, 120–128. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, S.; São-José, C. More than a hole: The holin lethal function may be required to fully sensitize bacteria to the lytic action of canonical endolysins. Mol. Microbiol. 2016, 102, 92–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Huang, S.; Voget, S.; Simon, M.; Chen, F. A novel roseobacter phage possesses features of podoviruses, siphoviruses, prophages and gene transfer agents. Sci. Rep. 2016, 6, 30372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, L.; Ma, R.; Xu, Y.; Tong, Y.; Huang, Y.; Jiao, N.; Zhang, R. A novel roseosiphophage isolated from the oligotrophic South China Sea. Viruses 2017, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, K.; Jiao, N.; Chen, F. Genome sequences of siphoviruses infecting marine Synechococcus unveil a diverse cyanophage group and extensive phage-host genetic exchanges. Environ. Microbiol. 2012, 14, 540–558. [Google Scholar] [CrossRef] [PubMed]



| Tested Strains | Bacteria Taxa | Infection a |
|---|---|---|
| R1 | Actinobacteria; Micrococcales; Microbacterium oxydans | √ (lysogenic) |
| CBW1101-8 | Actinobacteria; Micrococcales; Microbacterium oxydans | √ (lysogenic) |
| CBW1101-9 | Actinobacteria; Micrococcales; Microbacterium oxydans | √ (lysogenic) |
| CBW1107-2 | Alphaproteobacteria; Rhizobiales; Mesorhizobium sediminum | × |
| CBW1107-5 | Alphaproteobacteria; Rhizobiales; Nitratireductor aquimarinus | × |
| CBW1107-11 | Alphaproteobacteria; Sphingomonadales; Sphingomonas xenophagum | × |
| CBW1107-12 | Flavobacteria; Flavobacteriales; Muricauda alvinocaridis | × |
| CBW1107-13 | Flavobacteria; Flavobacteriales; Arenibacter troitsensis | × |
| CBW1107-3 | Gammaproteobacteria; Alteromonadales; Marinobacter salsuginis | × |
| CBW1107-7 | Gammaproteobacteria; Alteromonadales; Marinobacter salsuginis | × |
| CBW1107-4 | Gammaproteobacteria; Oceanospirillales; Halomonas hydrothermalis | × |
| CBW1107-6 | Gammaproteobacteria; Oceanospirillales; Halomonas venusta | × |
| CBW1107-10 | Gammaproteobacteria; Oceanospirillales; Halomonas piezotolerans | × |
| Phage | Family | Genus | ORF Homolog No. | ORF aa Identity (%) | Homologous ORF in vB_MoxS-R1 |
|---|---|---|---|---|---|
| vB_Mox-S1 | - | - | 24 | 26.4–98.8 | see in Figure 3 |
| Microbacterium phage Nike | Siphoviridae | Squashvirus | 4 | 33.6–54.9 | ORF32, ORF38, ORF63, ORF68 |
| Microbacterium phage Squash | Siphoviridae | Squashvirus | 4 | 33.6–54.9 | ORF32, ORF38, ORF63, ORF68 |
| Microbacterium phage IAmGroot | Siphoviridae | unknown | 4 | 21.8–32.7 | ORF41, ORF45, ORF49, ORF58 |
| Microbacterium phage GardenState | Siphoviridae | unknown | 4 | 21.8–32.7 | ORF41, ORF45, ORF49, ORF58 |
| Microbacterium phage Percival | Siphoviridae | unknown | 3 | 29.6–32.4 | ORF45, ORF49, ORF58 |
| Microbacterium phage Floof | Siphoviridae | unknown | 3 | 29.3–32.6 | ORF45, ORF49, ORF58 |
| Microbacterium phage Zeta1847 | Siphoviridae | Zetavirus | 2 | 32.1–32.4 | ORF45, ORF49 |
| Mycobacterium phage Bernal13 | Siphoviridae | Bernalvirus | 4 | 24.9–43.4 | ORF1, ORF20, ORF45, ORF58 |
| Mycobacterium phage Mendokysei | Siphoviridae | Bernalvirus | 3 | 25.3–31.6 | ORF1, ORF20, ORF45 |
| ORF No. | Family | Regulated Cellular Process | Reference |
|---|---|---|---|
| 3 | Unknown | Unidentified | - |
| 5 | ArsR family | Arsenical resistance | [105,106,107,108,109,110,111] |
| 22 | XRE family | Stress response and virulence | [114,115,116] |
| 24 | ASC-1-like subfamily | Regulation on transcription coactivation and RNA-processing | [117,118] |
| 28 | Unknown | Unidentified | - |
| 73 | XRE family | Stress response and virulence | [114,115,116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Liu, B.; Xu, Y.; Zhang, Z.; Man, H.; Liu, J.; Chen, F. An Inducible Microbacterium Prophage vB_MoxS-R1 Represents a Novel Lineage of Siphovirus. Viruses 2022, 14, 731. https://doi.org/10.3390/v14040731
Zheng H, Liu B, Xu Y, Zhang Z, Man H, Liu J, Chen F. An Inducible Microbacterium Prophage vB_MoxS-R1 Represents a Novel Lineage of Siphovirus. Viruses. 2022; 14(4):731. https://doi.org/10.3390/v14040731
Chicago/Turabian StyleZheng, Hongrui, Binbin Liu, Yongle Xu, Zefeng Zhang, Hongcong Man, Jihua Liu, and Feng Chen. 2022. "An Inducible Microbacterium Prophage vB_MoxS-R1 Represents a Novel Lineage of Siphovirus" Viruses 14, no. 4: 731. https://doi.org/10.3390/v14040731
APA StyleZheng, H., Liu, B., Xu, Y., Zhang, Z., Man, H., Liu, J., & Chen, F. (2022). An Inducible Microbacterium Prophage vB_MoxS-R1 Represents a Novel Lineage of Siphovirus. Viruses, 14(4), 731. https://doi.org/10.3390/v14040731






