Norovirus Genogroup II Epidemics and the Potential Effect of Climate Change on Norovirus Transmission in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Detection of Norovirus
2.3. Climate Data Sources
2.4. Statistical Analysis
3. Results
3.1. Surveillance of Norovirus Genogroup II
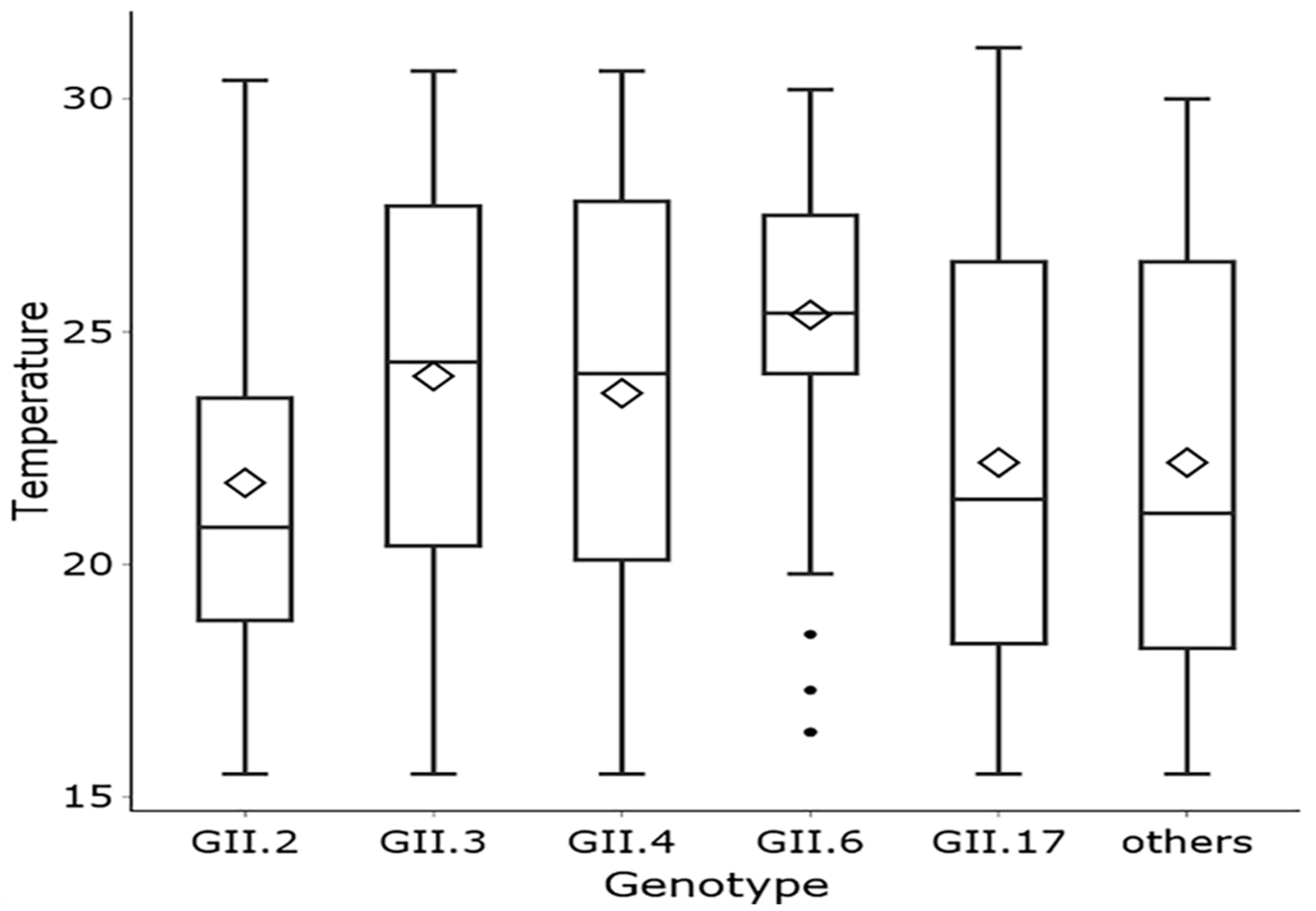
3.2. Climate Factors and Geographic Distribution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopman, B.A.; Reacher, M.H.; Vipond, I.B.; Hill, D.; Perry, C.; Halladay, T.; Brown, D.W.; Edmunds, W.J.; Sarangi, J. Epidemiology and Cost of Nosocomial Gastroenteritis, Avon, England, 2002–2003. Emerg. Infect. Dis. 2004, 10, 1827–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, M.-B.; Chen, C.-H.; Chen, S.-C.; Chou, Y.-C.; Yu, C.-P. Epidemiological and molecular analysis of human norovirus infections in Taiwan during 2011 and 2012. BMC Infect. Dis. 2013, 13, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-Y.; Chen, W.-C.; Chen, P.-C.; Hsu, S.-W.; Lo, Y.-C. An outbreak of norovirus gastroenteritis associated with asymptomatic food handlers in Kinmen, Taiwan. BMC Public Health 2016, 16, 372. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Feng, Y.; Chao, H.-C.; Lai, M.-W.; Huang, W.-L.; Lin, C.-Y.; Tsai, C.-N.; Chen, C.-L.; Chiu, C.-H. Emergence in Taiwan of novel norovirus GII.4 variants causing acute gastroenteritis and intestinal haemorrhage in children. J. Med. Microbiol. 2015, 64, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-L.; Chen, S.-Y.; Tsai, C.-N.; Chao, H.-C.; Lai, M.-W.; Chang, Y.-J.; Chen, C.-L.; Chiu, C.-H. Complicated norovirus infection and assessment of severity by a modified Vesikari disease score system in hospitalized children. BMC Pediatr. 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, R.; Shih, S.-M.; Yen, C.; Huang, Y.-C.; Parashar, U.D.; Lopman, B.A.; Wu, F.-T.; Hsiung, C.A.; Hall, A.J. Burden of Severe Norovirus Disease in Taiwan, 2003–2013. Clin. Infect. Dis. 2018, 67, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, C.; Dekas, K.; Charvalos, E. Climate changes, environment and infection: Facts, scenarios and growing awareness from the public health community within Europe. Anaerobe 2011, 17, 337–340. [Google Scholar] [CrossRef]
- Mounts, A.W.; Ando, T.; Koopmans, M.; Bresee, J.S.; Noel, J.; Glass, R.I. Cold Weather Seasonality of Gastroenteritis Associated with Norwalk-like Viruses. J. Infect. Dis. 2000, 181, S284–S287. [Google Scholar] [CrossRef]
- Patel, M.M.; Pitzer, V.; Alonso, W.; Vera, D.; Lopman, B.; Tate, J.; Viboud, C.; Parashar, U.D. Global Seasonality of Rotavirus Disease. Pediatr. Infect. Dis. J. 2013, 32, e134–e147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagai, J.S.; Sarkar, R.; Castronovo, D.; Kattula, D.; McEntee, J.; Ward, H.; Kang, G.; Naumova, E.N. Seasonality of Rotavirus in South Asia: A Meta-Analysis Approach Assessing Associations with Temperature, Precipitation, and Vegetation Index. PLoS ONE 2012, 7, e38168. [Google Scholar] [CrossRef]
- Greer, A.; Ng, V.; Fisman, D. Climate change and infectious disease in North America: The road ahead. Can. Med. Assoc. J. 2008, 178, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Rohayem, J. Norovirus seasonality and the potential impact of climate change. Clin. Microbiol. Infect. 2009, 15, 524–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.M.; Benjamin, A.L.; Levy, K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS ONE 2013, 8, e75922. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; McGeehin, M.A.; Bernard, S.M.; Ebi, K.L.; Epstein, P.R.; Grambsch, A.; Gubler, D.J.; Reither, P.; Romieu, I.; Rose, J.B.; et al. The potential health impacts of climate variability and change for the United States: Executive summary of the report of the health sector of the U.S. National Assessment. Environ. Health Perspect. 2000, 108, 367–376. [Google Scholar] [CrossRef]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.-W.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef]
- LeBaron, C.W.; Furutan, N.P.; Lew, J.F.; Allen, J.R.; Gouvea, V.; Moe, C.; Monroe, S. Viral agents of gastroenteritis. Public health importance and outbreak management. MMWR Recomm. Rep. 1990, 39, 1–24. [Google Scholar]
- Lew, J.F.; LeBaron, C.W.; Glass, R.I.; Török, T.; Griffin, P.M.; Wells, J.G.; Juranek, D.D.; Wahlquist, S.P. Recommendations for collection of laboratory specimens associated with outbreaks of gastroenteritis. MMWR Recomm. Rep. 1990, 39, 1–13. [Google Scholar]
- Zeng, S.-Q.; Halkosalo, A.; Salminen, M.; Szakal, E.; Puustinen, L.; Vesikari, T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- Liu, J.; Gratz, J.; Amour, C.; Kibiki, G.; Becker, S.; Janaki, L.; Verweij, J.J.; Taniuchi, M.; Sobuz, S.U.; Haque, R.; et al. A Laboratory-Developed TaqMan Array Card for Simultaneous Detection of 19 Enteropathogens. J. Clin. Microbiol. 2013, 51, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S.; Kageyama, T.; Fukushi, S.; Hoshino, F.B.; Shinohara, M.; Uchida, K.; Natori, K.; Takeda, N.; Katayama, K. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 2002, 100, 107–114. [Google Scholar] [CrossRef]
- Kroneman, A.; Vennema, H.; Deforche, K.; Avoort, H.; Peñaranda, S.; Oberste, M.; Vinje, J.; Koopmans, M. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; De Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.L.; Zickl, R. Winter Vomiting Disease. J. Infect. Dis. 1969, 119, 668–673. [Google Scholar] [CrossRef]
- Greer, A.L.; Drews, S.J.; Fisman, D.N. Why “Winter” Vomiting Disease? Seasonality, Hydrology, and Norovirus Epidemiology in Toronto, Canada. EcoHealth 2009, 6, 192–199. [Google Scholar] [CrossRef]
- Oswald, S.A.; Arnold, J.M. Direct impacts of climatic warming on heat stress in endothermic species: Seabirds as bioindicators of changing thermoregulatory constraints. Integr. Zool. 2012, 7, 121–136. [Google Scholar] [CrossRef]
- Wang, P.; Goggins, W.B.; Chan, E.Y. A time-series study of the association of rainfall, relative humidity and ambient temperature with hospitalizations for rotavirus and norovirus infection among children in Hong Kong. Sci. Total Environ. 2018, 643, 414–422. [Google Scholar] [CrossRef]
- Marshall, J.A.; Bruggink, L.D. The Dynamics of Norovirus Outbreak Epidemics: Recent Insights. Int. J. Environ. Res. Public Health 2011, 8, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
- Bruggink, L.D.; Marshall, J.A. The Incidence of Norovirus-Associated Gastroenteritis Outbreaks in Victoria, Australia (2002–2007) and Their Relationship with Rainfall. Int. J. Environ. Res. Public Health 2010, 7, 2822–2827. [Google Scholar] [CrossRef] [Green Version]
- Eregno, F.E.; Tryland, I.; Tjomsland, T.; Myrmel, M.; Robertson, L.J.; Heistad, A. Quantitative microbial risk assessment combined with hydrodynamic modelling to estimate the public health risk associated with bathing after rainfall events. Sci. Total Environ. 2016, 548–549, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Squires, R.B.; Karangwa, C.K.; Johnson, J.A.; Lepore, C.J.; Sosnovtsev, S.V.; Green, K.Y. Static and Evolving Norovirus Genotypes: Implications for Epidemiology and Immunity. PLOS Pathog. 2017, 13, e1006136. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.-W.; Lee, N.; Hung, T.-N.; Kwok, K.; Cheung, K.; Tin, E.K.Y.; Lai, R.W.M.; Nelson, E.A.S.; Leung, T.F.; Chan, P.K.S. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat. Commun. 2015, 6, 10061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Qian, F.; Xu, J.; Chan, M.; Shen, Z.; Zai, S.; Shan, M.; Cai, J.; Zhang, W.; He, J.; et al. A novel norovirus GII.17 lineage contributed to adult gastroenteritis in Shanghai, China, during the winter of 2014–2015. Emerg. Microbes Infect. 2015, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.; D’Souza, D.H.; Davidson, P.M. Thermal Inactivation Kinetics of Human Norovirus Surrogates and Hepatitis A Virus in Turkey Deli Meat. Appl. Environ. Microbiol. 2015, 81, 4850–4859. [Google Scholar] [CrossRef] [Green Version]
- Lopman, B.; Armstrong, B.; Atchison, C.; Gray, J.J. Host, Weather and Virological Factors Drive Norovirus Epidemiology: Time-Series Analysis of Laboratory Surveillance Data in England and Wales. PLoS ONE 2009, 4, e6671. [Google Scholar] [CrossRef]
- Chan, M.C.-W.; Roy, S.; Bonifacio, J.; Zhang, L.-Y.; Chhabra, P.; Chan, J.C.; Celma, C.; Igoy, M.A.; Lau, S.-L.; Mohammad, K.N.; et al. Detection of Norovirus Variant GII.4 Hong Kong in Asia and Europe, 2017−2019. Emerg. Infect. Dis. 2021, 27, 289–293. [Google Scholar] [CrossRef]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Hassard, F.; Sharp, J.H.; Taft, H.; Levay, L.; Harris, J.; McDonald, J.E.; Tuson, K.; Wilson, J.; Jones, D.; Malham, S.K. Critical Review on the Public Health Impact of Norovirus Contamination in Shellfish and the Environment: A UK Perspective. Food Environ. Virol. 2017, 9, 123–141. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, P.A. Precipitation Extremes under Climate Change. Curr. Clim. Chang. Rep. 2015, 1, 49–59. [Google Scholar] [CrossRef] [Green Version]

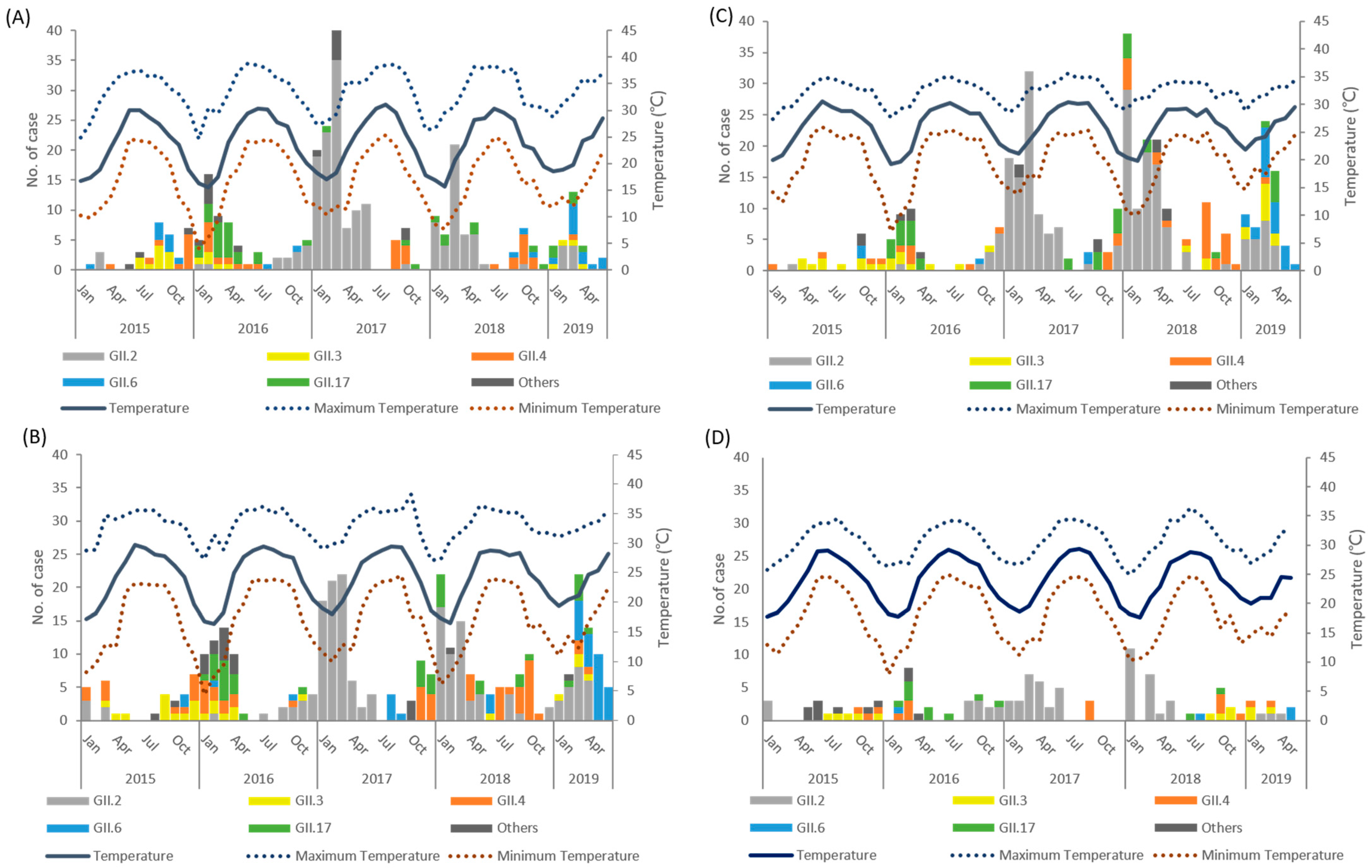
| Genotype | GII.2 (n = 643) | GII.3 (n = 86) | GII.4 (n = 164) | GII.6 (n = 111) | GII.17 (n = 132) | Others (n = 65) | p Value |
|---|---|---|---|---|---|---|---|
| Age (median, SD) | 12.9 (15.0) | 7.8 (11.3) | 11.5 (30.1) | 16.3 (22.6) | 31.1 (24.0) | 24.0 (17.5) | <0.0001 a |
| Age group | <0.0001 b | ||||||
| <2 y | 4 (0.6) | 7 (8.1) | 20 (12.2) | 1 (0.9) | 1 (0.8) | 4 (6.2) | |
| 2–5 y | 50 (7.8) | 12 (14.0) | 36 (22.0) | 13 (11.7) | 4 (3.0) | 1 (1.5) | |
| 5–12 y | 249 (38.7) | 43 (50.0) | 28 (17.1) | 35 (31.5) | 17 (12.9) | 15 (23.1) | |
| 12–18 y | 88 (13.7) | 9 (10.5) | 11 (6.7) | 10 (9.0) | 14 (10.6) | 1 (1.5) | |
| 18–64 y | 242 (37.6) | 15 (17.4) | 38 (23.2) | 44 (39.6) | 78 (59.1) | 43 (66.2) | |
| 65+ y | 10 (1.6) | 0 (0) | 31 (18.9) | 8 (7.2) | 18 (13.6) | 1 (1.5) | |
| Gender | 0.3989 b | ||||||
| Male | 366 (56.9) | 47 (54.7) | 88 (53.7) | 57 (51.4) | 64 (48.5) | 31 (47.7) | |
| Female | 277 (43.1) | 39 (45.3) | 76 (46.3) | 54 (48.6) | 68 (51.5) | 34 (52.3) | |
| Season | <0.0001 b | ||||||
| Spring | 303 (47.1) | 27 (31.4) | 30 (18.3) | 60 (54.1) | 58 (43.9) | 28 (43.1) | |
| Summer | 38 (5.9) | 11 (12.8) | 12 (7.3) | 23 (20.7) | 11 (8.3) | 7 (10.8) | |
| Autumn | 31 (4.8) | 26 (30.2) | 74 (45.1) | 20 (18.0) | 17 (12.9) | 11 (16.9) | |
| Winter | 271 (42.2) | 22 (25.6) | 48 (29.3) | 8 (7.2) | 46 (34.9) | 19 (29.2) | |
| Area | 0.0439 b | ||||||
| North | 176 (27.4) | 19 (22.1) | 42 (25.8) | 30 (27.0) | 40 (30.5) | 20 (30.8) | |
| West | 177 (27.5) | 24 (27.9) | 60 (36.8) | 39 (35.1) | 42 (32.1) | 20 (30.8) | |
| South | 225 (35.0) | 31 (36.1) | 45 (27.6) | 39 (35.1) | 37 (28.2) | 14 (21.5) | |
| East | 65 (10.1) | 12 (14.0) | 16 (9.8) | 3 (2.7) | 12 (9.2) | 11 (16.9) | |
| Identity | <0.0001 b | ||||||
| common patient | 145 (22.6) | 14 (16.3) | 29 (17.7) | 29 (26.1) | 56 (42.4) | 28 (43.1) | |
| Student | 420 (65.3) | 65 (75.6) | 82 (50.0) | 59 (53.2) | 35 (26.5) | 27 (41.5) | |
| Resident of populous institution | 20 (3.1) | 3 (3.5) | 29 (17.7) | 7 (6.3) | 19 (14.4) | 0 (0) | |
| Chef/Kitchen worker | 17 (2.6) | 0 (0) | 6 (3.7) | 7 (6.3) | 5 (3.8) | 5 (7.7) | |
| Nurse | 9 (1.4) | 2 (2.3) | 9 (5.5) | 4 (11.1) | 9 (6.8) | 3 (4.6) | |
| Prisoner/Military | 28 (4.4) | 0 (0) | 1 (0.6) | 1 (0.9) | 7 (5.3) | 0 (0) | |
| Others | 4 (0.6) | 2 (2.3) | 8 (4.9) | 4 (11.1) | 1 (0.8) | 2 (3.1) |
| Multivariate Linear Regression Model | |||
|---|---|---|---|
| Variate | Estimate parameter | p-value | R2 |
| Tempareture | −0.8773 | <0.0001 | 0.1712 |
| Rainfall | 0.0014 | 0.6303 | |
| Sunsine | 0.0328 | 0.0022 | |
| Norovirus Genotype | GII.2 | GII.3 | GII.4 | GII.6 | GII.17 | Others | p Value |
|---|---|---|---|---|---|---|---|
| Average Temperature (°C) | <0.0001 | ||||||
| 15–20 | 210 (32.7) | 19 (22.6) | 36 (22.8) | 9 (8.6) a | 49 (38.6) | 24 (40.0) | |
| 20–25 | 300 (46.7) | 26 (31.0) | 61 (38.6) | 36 (34.3) | 43 (33.9) | 11 (18.3) | |
| 25–30 | 132 (20.6) | 39 (46.4) | 61 (38.6) | 60 (57.1) b | 35 (27.6) | 25 (41.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.-C.; Hu, S.-C.; Liao, L.-M.; Chen, Y.-H.; Lin, J.-H. Norovirus Genogroup II Epidemics and the Potential Effect of Climate Change on Norovirus Transmission in Taiwan. Viruses 2022, 14, 641. https://doi.org/10.3390/v14030641
Chiu S-C, Hu S-C, Liao L-M, Chen Y-H, Lin J-H. Norovirus Genogroup II Epidemics and the Potential Effect of Climate Change on Norovirus Transmission in Taiwan. Viruses. 2022; 14(3):641. https://doi.org/10.3390/v14030641
Chicago/Turabian StyleChiu, Shu-Chun, Szu-Chieh Hu, Ling-Min Liao, Yu-Hua Chen, and Jih-Hui Lin. 2022. "Norovirus Genogroup II Epidemics and the Potential Effect of Climate Change on Norovirus Transmission in Taiwan" Viruses 14, no. 3: 641. https://doi.org/10.3390/v14030641
APA StyleChiu, S.-C., Hu, S.-C., Liao, L.-M., Chen, Y.-H., & Lin, J.-H. (2022). Norovirus Genogroup II Epidemics and the Potential Effect of Climate Change on Norovirus Transmission in Taiwan. Viruses, 14(3), 641. https://doi.org/10.3390/v14030641







