Four Weeks Treatment with Glecaprevir/Pibrentasvir + Ribavirin—A Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
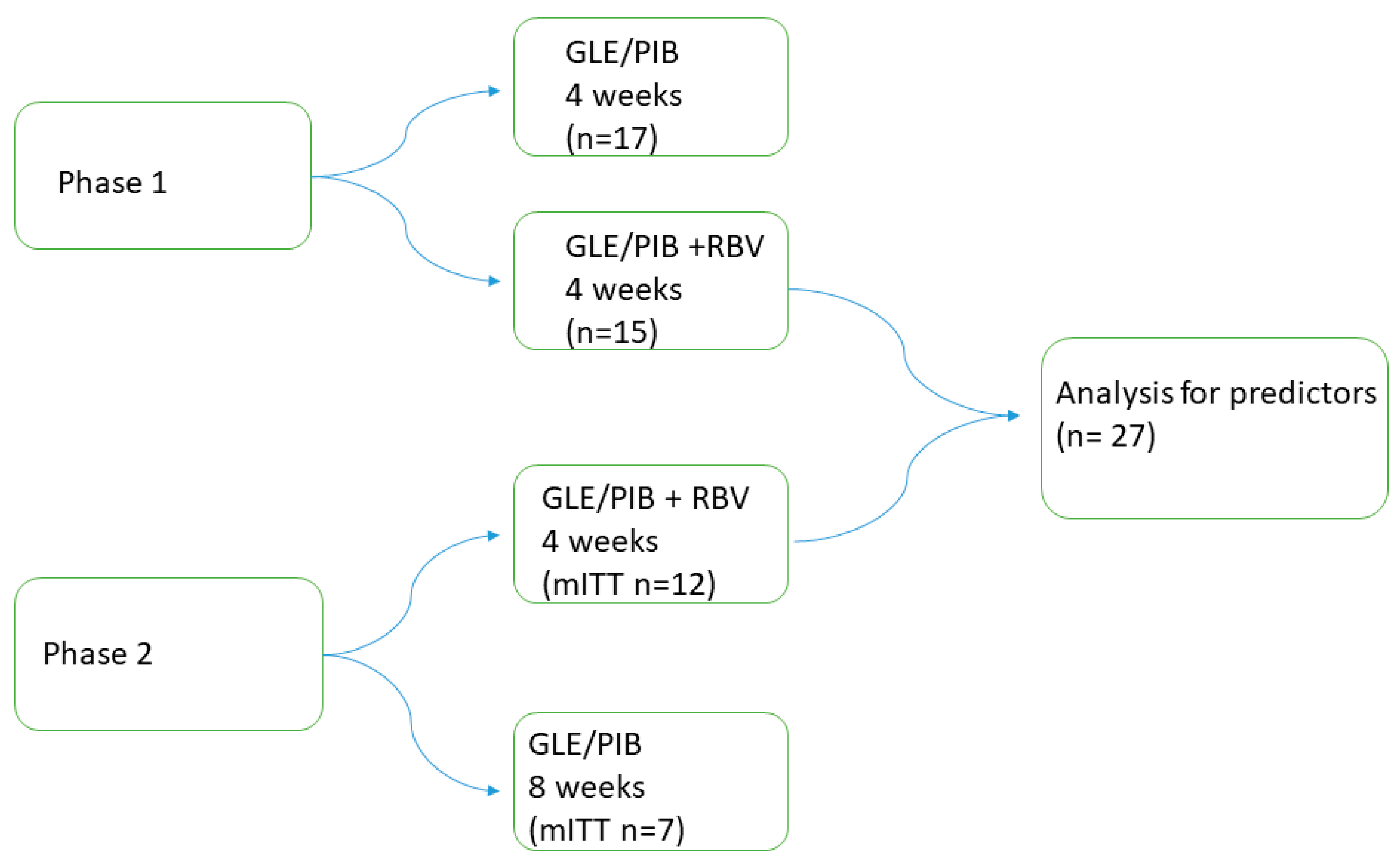
2.1. Study Design
2.2. Patients
2.3. 4RIBC Phase 1
2.4. Data Collection
2.5. Laboratory Methods
2.6. Safety and Compliance
2.7. Virological Relapse
2.8. Statistic and Sample Size
2.9. Ethical
3. Results
3.1. Baseline Characteristics
3.2. Virological Response
3.3. Safety and Compliance
3.4. Predictors for Achieving SVR12
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R.; et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef]
- Hajarizadeh, B.; Grebely, J.; Martinello, M.; Matthews, G.V.; Lloyd, A.R.; Dore, G.J. Hepatitis C treatment as prevention: Evidence, feasibility, and challenges. Lancet Gastroenterol. Hepatol. 2016, 1, 317–327. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=4A31033A10540FAFD7C0418369431855?sequence=1 (accessed on 14 October 2021).
- Gamkrelidze, I.; Pawlotsky, J.M.; Lazarus, J.V.; Feld, J.J.; Zeuzem, S.; Bao, Y.; Gabriela Pires Dos Santos, A.; Sanchez Gonzalez, Y.; Razavi, H. Progress towards hepatitis C virus elimination in high-income countries: An updated analysis. Liver Int. 2021, 41, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Razavi, H.; Sanchez Gonzalez, Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Doyle, J.S.; Wilson, D.P.; Wade, A.; Howell, J.; Pedrana, A.; Thompson, A.; Hellard, M.E. Reaching hepatitis C virus elimination targets requires health system interventions to enhance the care cascade. Int. J. Drug Policy 2017, 47, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Clinton Health Access Initiative. HCV Market Intelligence Report 2021 and Preliminary Market Insights; Clinton Health Access Initiative: Boston, MA, USA, 2021. [Google Scholar]
- World Health Organization. Accelerating Access to Hepatitis C Diagnostics and Treatment; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; Colledge, S.; Walker, J.G.; Leung, J.; Grebely, J.; Larney, S.; Martin, N.K.; et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, E.B.; Hajarizadeh, B.; Amin, J.; Litwin, A.H.; Gane, E.; Cooper, C.; Lacombe, K.; Hellard, M.; Read, P.; Powis, J.; et al. Adherence to Once-daily and Twice-daily Direct-acting Antiviral Therapy for Hepatitis C Infection Among People With Recent Injection Drug Use or Current Opioid Agonist Therapy. Clin. Infect. Dis. 2020, 71, e115–e124. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.; Townsend, K.; Gordon, L.A.; Sidharthan, S.; Silk, R.; Nelson, A.; Gross, C.; Calderón, M.; Proschan, M.; Osinusi, A.; et al. High adherence to all-oral directly acting antiviral HCV therapy among an inner-city patient population in a phase 2a study. Hepatol. Int. 2016, 10, 310–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, G.S.; Pett, S.; McCabe, L.; Jones, C.; Gilson, R.; Verma, S.; Ryder, S.D.; Collier, J.D.; Barclay, S.T.; Ala, A.; et al. Strategic treatment optimization for HCV (STOPHCV1): A randomised controlled trial of ultrashort duration therapy for chronic hepatitis C. Wellcome Open Res. 2021, 6, 93. [Google Scholar] [CrossRef]
- Gane, E.J.; Schwabe, C.; Hyland, R.H.; Yang, Y.; Svarovskaia, E.; Stamm, L.M.; Brainard, D.M.; McHutchison, J.G.; Stedman, C.A. Efficacy of the Combination of Sofosbuvir, Velpatasvir, and the NS3/4A Protease Inhibitor GS-9857 in Treatment-Naive or Previously Treated Patients With Hepatitis C Virus Genotype 1 or 3 Infections. Gastroenterology 2016, 151, 448–456.e441. [Google Scholar] [CrossRef] [Green Version]
- Kohli, A.; Kattakuzhy, S.; Sidharthan, S.; Nelson, A.; McLaughlin, M.; Seamon, C.; Wilson, E.; Meissner, E.G.; Sims, Z.; Silk, R.; et al. Four-Week Direct-Acting Antiviral Regimens in Noncirrhotic Patients With Hepatitis C Virus Genotype 1 Infection: An Open-Label, Nonrandomized Trial. Ann. Intern. Med. 2015, 163, 899–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, G.; Benhamou, Y.; Chen, G.; Li, J.; Shao, Q.; Ji, D.; Li, F.; Li, B.; Liu, J.; Hou, J.; et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: A phase 2, open-label, proof-of-concept study. Lancet Gastroenterol. Hepatol. 2016, 1, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Lawitz, E.; Poordad, F.; Gutierrez, J.A.; Wells, J.T.; Landaverde, C.E.; Evans, B.; Howe, A.; Huang, H.C.; Li, J.J.; Hwang, P.; et al. Short-duration treatment with elbasvir/grazoprevir and sofosbuvir for hepatitis C: A randomized trial. Hepatology 2017, 65, 439–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovrehus, A.L.H.; Krarup, H.; Birkemose, I.; Holm, D.K.; Mossner, B.; Ernst, A.; Christensen, P.B. Four weeks of ledipasvir/sofosbuvir and ribavirin with or without pegylated interferon for chronic hepatitis C in non-cirrhotic people who inject drugs. A randomized trial. J. Hepatol. 2018, 68, 840–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulkowski, M.S.; Flamm, S.; Kayali, Z.; Lawitz, E.J.; Kwo, P.; McPhee, F.; Torbeyns, A.; Hughes, E.A.; Swenson, E.S.; Yin, P.D.; et al. Short-duration treatment for chronic hepatitis C virus with daclatasvir, asunaprevir, beclabuvir and sofosbuvir (FOURward study). Liver Int. 2017, 37, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.W.; Christensen, P.B.; Fahnøe, U.; Pedersen, M.S.; Bukh, J.; Øvrehus, A. Inferior cure rate in pilot study of 4-week glecaprevir/pibrentasvir treatment with or without ribavirin of chronic hepatitis C. Liver Int. 2021, 41, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications-detail-redirect/9789241550345 (accessed on 18 October 2021).
- OPEN. Open Patient Data Explorative Network (OPEN). Available online: https://www.sdu.dk/da/ki/open (accessed on 10 September 2021).
- Mössner, B.K.; Staugaard, B.; Jensen, J.; Lillevang, S.T.; Christensen, P.B.; Holm, D.K. Dried blood spots, valid screening for viral hepatitis and human immunodeficiency virus in real-life. World J. Gastroenterol. 2016, 22, 7604–7612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latt, N.L.; Yanny, B.T.; Gharibian, D.; Gevorkyan, R.; Sahota, A.K. Eight-week ledipasvir/sofosbuvir in non-cirrhotic, treatment-naïve hepatitis C genotype-1 patients with hepatitis C virus-RNA <6 million: Single center, real world effectiveness and safety. World J. Gastroenterol. 2017, 23, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Matthews, G.V.; Bhagani, S.; Van der Valk, M.; Rockstroh, J.; Feld, J.J.; Rauch, A.; Thurnheer, C.; Bruneau, J.; Kim, A.; Hellard, M.; et al. Sofosbuvir/velpatasvir for 12 vs. 6 weeks for the treatment of recently acquired hepatitis C infection. J. Hepatol. 2021, 75, 829–839. [Google Scholar] [CrossRef]
- Etzion, O.; Dahari, H.; Yardeni, D.; Issachar, A.; Nevo-Shor, A.; Cohen-Naftaly, M.; Ashur, Y.; Uprichard, S.L.; Arbib, O.S.; Munteanu, D.; et al. Response guided therapy for reducing duration of direct acting antivirals in chronic hepatitis C infected patients: A Pilot study. Sci. Rep. 2020, 10, 17820. [Google Scholar] [CrossRef] [PubMed]
- Ahlén, G.; Frelin, L.; Brenndörfer, E.D.; Brass, A.; Weiland, O.; Chen, M.; Sällberg, M. Containing “The Great Houdini” of viruses: Combining direct acting antivirals with the host immune response for the treatment of chronic hepatitis C. Drug Resist. Updates 2013, 16, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelson, A.S.; Guedj, J. Modelling hepatitis C therapy--predicting effects of treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Ishida, H.; Lenz, O.; Lin, T.I.; Nyanguile, O.; Simmen, K.; Pyles, R.B.; Bourne, N.; Yi, M.; Li, K.; et al. Antiviral suppression vs restoration of RIG-I signaling by hepatitis C protease and polymerase inhibitors. Gastroenterology 2008, 135, 1710–1718.e1712. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Carrión, J.A.; Curry, M.; Turnes, J.; Cornberg, M.; Negro, F.; Brown, A.; Persico, M.; Wick, N.; Porcalla, A.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J. Hepatol. 2020, 72, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiffman, M.L. What future for ribavirin? Liver Int. 2009, 29 (Suppl. 1), 68–73. [Google Scholar] [CrossRef]
- Feld, J.J.; Jacobson, I.M.; Sulkowski, M.S.; Poordad, F.; Tatsch, F.; Pawlotsky, J.M. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017, 37, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.S.; Swan, T. A path to eradication of hepatitis C in low- and middle-income countries. Antivir. Res. 2015, 119, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Waked, I.; Esmat, G.; Elsharkawy, A.; El-Serafy, M.; Abdel-Razek, W.; Ghalab, R.; Elshishiney, G.; Salah, A.; Abdel Megid, S.; Kabil, K.; et al. Screening and Treatment Program to Eliminate Hepatitis C in Egypt. N. Engl. J. Med. 2020, 382, 1166–1174. [Google Scholar] [CrossRef]
- Bukh, J. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J. Hepatol. 2016, 65, s2–s21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, K.; Davis, C.; Cannon, M.; Montague, S.; Filipe, A.; Tong, L.; Simmonds, P.; Smith, D.; Thomson, E.C.; Dusheiko, G.; et al. Suboptimal SVR rates in African patients with atypical genotype 1 subtypes: Implications for global elimination of hepatitis C. J. Hepatol. 2019, 71, 1099–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourati, S.; Rodriguez, C.; Hézode, C.; Soulier, A.; Ruiz, I.; Poiteau, L.; Chevaliez, S.; Pawlotsky, J.M. Frequent Antiviral Treatment Failures in Patients Infected With Hepatitis C Virus Genotype 4, Subtype 4r. Hepatology 2019, 69, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, M.S.; Fahnøe, U.; Madsen, L.W.; Christensen, P.B.; Øvrehus, A.; Bukh, J. Characterization of a Novel Hepatitis C Virus Genotype 1 Subtype from a Patient Failing 4 Weeks of Glecaprevir-Pibrentasvir Treatment. Microbiol. Resour. Announc. 2021, 10, e0075521. [Google Scholar] [CrossRef] [PubMed]
- Zamor, P.J.; Brown, A.; Dylla, D.E.; Dillon, J.F.; Luetkemeyer, A.F.; Feld, J.J.; Mutimer, D.; Ghalib, R.; Crown, E.; Lovell, S.S.; et al. High Sustained Virologic Response Rates of Glecaprevir/Pibrentasvir in Patients With Dosing Interruption or Suboptimal Adherence. Am. J. Gastroenterol. 2021, 116, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Hajarizadeh, B.; Grebely, J.; Byrne, M.; Marks, P.; Amin, J.; McManus, H.; Butler, T.; Cunningham, E.B.; Vickerman, P.; Martin, N.K.; et al. Evaluation of hepatitis C treatment-as-prevention within Australian prisons (SToP-C): A prospective cohort study. Lancet Gastroenterol. Hepatol. 2021, 6, 533–546. [Google Scholar] [CrossRef]
- Martinello, M.; Orkin, C.; Cooke, G.; Bhagani, S.; Gane, E.; Kulasegaram, R.; Shaw, D.; Tu, E.; Petoumenos, K.; Marks, P.; et al. Short-Duration Pan-Genotypic Therapy With Glecaprevir/Pibrentasvir for 6 Weeks Among People With Recent Hepatitis C Viral Infection. Hepatology 2020, 72, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.M.; Barnaba, B.; Yu, S.; Brown, D.M.; Chattergoon, M.A.; Bair, N.; Naqvi, F.F.; Sulkowski, M.; Segev, D.L.; Desai, N.M. Four-Week Direct-Acting Antiviral Prophylaxis for Kidney Transplantation From Hepatitis C-Viremic Donors to Hepatitis C-Negative Recipients: An Open-Label Nonrandomized Study. Ann. Intern. Med. 2021, 174, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.E.; Singh, S.K.; Goldberg, H.J.; Mallidi, H.R.; Givertz, M.M.; Mehra, M.R.; Coppolino, A.; Kusztos, A.E.; Johnson, M.E.; Chen, K.; et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N. Engl. J. Med. 2019, 380, 1606–1617. [Google Scholar] [CrossRef]



| Study Population, n | 8 Weeks Glecaprevir/Pibrentasvir (n = 8) | 4 Weeks Glecaprevir/Pibrentasvir + Ribavirin (n = 13) |
|---|---|---|
| Age (years) median (IQR) | 36 (33.5–43.5) | 42 (39–45) |
| Male, (%) | 4 (50.0) | 12 (92.3) |
| BMI, median (IQR) | 23.0 (20.1–31.2) (n = 7) | 23.8 (21.8–25.9) (n = 11) |
| Current or past alcohol overuse | 5 (62.5) | 8 (61.5) |
| Current or past intravenous drug use | 8 (100) | 13 (100) |
| HCV Genotype | ||
| 1a | 2 | 6 |
| 2 | 1 | 1 |
| 3 | 5 | 6 |
| HCV RNA (log10 IU/mL) median (IQR) | 6.0 (4.4–6.9) | 6.3 (6.0–6.6) |
| INFL3 genotype | ||
| CC | 5 | 3 |
| Non CC | 3 | 9 |
| missing | 1 | |
| LSM in kPa, median (range) | 6.8 (4.1–7.7) | 6.6 (4.1–7.5) |
| Opioid agonist therapy | 7 (87.5) | 11 (84.6) |
| Methadone | 6 (75.0) | 9 (69.2) |
| Buprenorphine | 0 (0.0) | 2 (15.4) |
| Heroin (legal) | 5 (62.5) | 3 (23.1) |
| Other | 1 (12.5) | 0 (0.0) |
| Year since infected, median (range) | 20.5 (1–28) | 21.5 (10–32) (n = 12) |
| SVR (18) | Non SVR (9) | p-Value | |
|---|---|---|---|
| Age, Median (IQR) | 43 (39–46) | 42 (40–44) | 0.8195 |
| Sex (Male/female) | 13/5 | 7/2 | 1.000 |
| Genotype 3/non3 | 10/8 | 1/8 | 0.042 * |
| INFL3 CC/non CC | 5/13 | 1/7 n = 8 | 0.628 |
| Weight in kilograms (IQR) | 79.5 (72–89.5) | 73 (69.2–82) | 0.2365 |
| OAT (IQR) | 9 (50.0) | 5 (55.6) | 1.00 |
| BMI, median (IQR) | 26.4 (23.4–27.1) (n = 17) | 24.9 (21.8–26.7) (n = 8) | 0.4147 |
| Viral load (log10 IU/mL) at treatment initiation, median (IQR) | 5.8 (5.3–6.3) | 6.7 (6.4–7.2) | 0.0045 * |
| Current or past injection use (%) | 16 (88.9) | 8 (88.9) | 1.000 |
| Current or past alcohol use (%) | 10 (55.6) | 6 (66.7) | 0.692 |
| Years since infection, median (IQR) | 21 (15–27) (n = 17) | 23 (21–28) | 0.1309 |
| ALT (IU/L) at baseline median (IQR) | 74.5 (44–112) | 49 (44–60) | 0.1107 |
| Positive HCV RNA week 2 yes/no (%) | 6/10 (37.5) (n = 16) | 2/5 (28.6) (n = 7) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madsen, L.W.; Christensen, P.B.; Hansen, J.F.; Røge, B.T.; Holm, D.K.; Dröse, S.; Øvrehus, A. Four Weeks Treatment with Glecaprevir/Pibrentasvir + Ribavirin—A Randomized Controlled Clinical Trial. Viruses 2022, 14, 614. https://doi.org/10.3390/v14030614
Madsen LW, Christensen PB, Hansen JF, Røge BT, Holm DK, Dröse S, Øvrehus A. Four Weeks Treatment with Glecaprevir/Pibrentasvir + Ribavirin—A Randomized Controlled Clinical Trial. Viruses. 2022; 14(3):614. https://doi.org/10.3390/v14030614
Chicago/Turabian StyleMadsen, Lone W., Peer B. Christensen, Janne F. Hansen, Birgit T. Røge, Dorte K. Holm, Sandra Dröse, and Anne Øvrehus. 2022. "Four Weeks Treatment with Glecaprevir/Pibrentasvir + Ribavirin—A Randomized Controlled Clinical Trial" Viruses 14, no. 3: 614. https://doi.org/10.3390/v14030614
APA StyleMadsen, L. W., Christensen, P. B., Hansen, J. F., Røge, B. T., Holm, D. K., Dröse, S., & Øvrehus, A. (2022). Four Weeks Treatment with Glecaprevir/Pibrentasvir + Ribavirin—A Randomized Controlled Clinical Trial. Viruses, 14(3), 614. https://doi.org/10.3390/v14030614






