Viral Shrimp Diseases Listed by the OIE: A Review
Abstract
1. Introduction
2. DNA Viral Diseases
2.1. White Spot Syndrome Disease (WSSD)
2.2. Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV)
3. RNA Viral Diseases
3.1. Infectious Myonecrosis Virus (IMNV)
3.2. Yellow Head Virus Genotype 1 (YHV Genotype 1)
3.3. Taura Syndrome Virus (TSV)
3.4. White Tail Disease (WTD)
| Virus Type | Pathogen | Taxonomy | Morphology | Reference | ||
|---|---|---|---|---|---|---|
| DNA virus | ds DNA | WSSV | Family | Nimaviridae |
| [9,13,16,18,20,22,52] |
| Genus | Whispovirus | |||||
| ss DNA | IHHNV (Decapod penstylhamaparvovirus 1) | Family | Parvoviridae |
| [57,59,60,65,66,85,161,162] | |
| subfamily | Hamaparvovirinae | |||||
| Genus | Penstylhamaparvovirus | |||||
| RNA virus | ds RNA | IMNV (PsIMNV) | Family | Totiviridae |
| [73,77,79,83,85] |
| Genus | Similar Giardiavirus | |||||
| ss RNA | YHV | Order | Nidovirales |
| [68,95,104] | |
| Family | Roniviridae | |||||
| Genus | Okavirus | |||||
| TSV | Order | Picornavirales |
| [2,62,95,116,117] | ||
| Family | Dicistroviridae | |||||
| Genus | Aparavirus | |||||
| WTD (MrNV) | Famliy | Nodaviridae |
| [56,140,141,142,144,145,146,149,150,157,163] | ||
| Genus | Gammanodavirus | |||||
| WTD (XSV) | Unassigned |
| ||||
| Virus Type | Pathogen | ORF | Characteristics | Reference | |
|---|---|---|---|---|---|
| DNA virus | ds DNA | WSSV | ORF75 |
| [9,13,20,24,164,165,166,167,168,169,170,171,172] |
| ORF94 |
| ||||
| ORF125 |
| ||||
| ORF14/15 |
| ||||
| ORF23/24 |
| ||||
| ORF109 |
| ||||
| ORF182 |
| ||||
| ORF153 |
| ||||
| ORF-wsv002 |
| ||||
| ORF-wsv421 |
| ||||
| ORF-wsv308 |
| ||||
| ss DNA | IHHNV (Decapod penstylhamaparvovirus 1) | ORF1 |
| [57,58,59,85,173,174] | |
| ORF2 |
| ||||
| ORF3 |
| ||||
| RNA virus | ds RNA | IMNV (PsIMNV) | ORF1 (59 ORF) |
| [62,73,74,76,83,86] |
| ORF2 (39 ORF) |
| ||||
| ss RNA | YHV | ORF1a |
| [1,92,100,104,107,111,114,163,175,176] | |
| ORF1a/ORF1b |
| ||||
| ORF1b |
| ||||
| ORF2 |
| ||||
| ORF3 |
| ||||
| ORF4 |
| ||||
| GAV | ORF1a |
| [1,92,100,107,114,177] | ||
| ORF1b |
| ||||
| ORF1a/ORF1b |
| ||||
| ORF2 |
| ||||
| ORF3 |
| ||||
| ORF4 |
| ||||
| TSV | ORF1 |
| [95,128,130,178] | ||
| ORF2 |
| ||||
| WTD (MrNV) | ORF1 (RNA-1) |
| [56,140,150,153,156,157] | ||
| ORF2 (RNA-2) |
| ||||
| WTD (XSV) | XSV genome |
| |||
| Type | Pathogen | Origin | Host Species | Isolation | ORF Region | GenBank No. | Year | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| DNA virus | ds DNA | WSSV | Mexico | Penaeus vannamei | Mx-F | Hypothetical protein (ORF13 and ORF16) gene; Nonfunctional hypothetical protein gene | HQ257380 | 2001 | [179] |
| Mx-H | HQ257381 | 2004 | |||||||
| Mx-C | Nonfunctional hypothetical protein genes | HQ257382 | 2005 | ||||||
| Mx-G | HQ257383 | 2004 | |||||||
| Mx-L1 | HQ257384 | 2001 | |||||||
| WSSV-MX08 | Complete genome | KU216744 | 2008 | [33] | |||||
| Penaeus vannamei | LG | Partial genome | MG432482 | 2012 | [180] | ||||
| JP | MG432479 | 2011 | |||||||
| AC1 | MG432474 | 2011 | |||||||
| DV1 | MG432477 | 2011 | |||||||
| LC1 | MG432481 | 2011 | |||||||
| LC10 | MG432480 | 2011 | |||||||
| ACF2 | MG432475 | 2012 | |||||||
| ACF4 | MG432476 | 2012 | |||||||
| GVE05 | MG432478 | 2005 | |||||||
| India | Penaeus monodon | ANI | wsv285 gene | KX980155 | 2016 | [181] | |||
| WSSV-IN-07-I | Unknown gene | EF468499 | 2007 | [182] | |||||
| WSSV-IN-06-I | EF468498 | 2006 | |||||||
| WSSV-IN-05-I | EU327499 | 2005 | |||||||
| WSSV-IN-05-II | ORF23/ORF24 region genomic sequence | EU327500 | 2005 | ||||||
| Penaeus vannamei | IN_AP4RU | Complete genome | MG702567 | 2013 | [38] | ||||
| Iran | Penaeus vannamei | IRWSSVKH2 | Hypothetical protein 75 gene | KF157839 | 2012 | [183] | |||
| IRWSSVKH4 | ORF75 gene | KC906268 | 2011 | ||||||
| IRWSSVKH5 | KF157833 | 2012 | |||||||
| IRWSSVKH3 | KF157832 | 2012 | |||||||
| IRWSSVSIS3 | KP455493 | 2014 | |||||||
| IRWSSVSIS2 | KF956791 | 2013 | |||||||
| Penaeus indicus; Penaeus vannamei | IWV-MS21 | ORF75 gene | KX694234 | 2013 | |||||
| IWV-MS24 | KX694236 | 2014 | |||||||
| IWV-MS25 | KX694237 | 2014 | |||||||
| IWV-MS26 | KX694238 | 2014 | |||||||
| IWV-MS19 | KX694242 | 2013 | |||||||
| IWV-MS18 | KX584741 | 2013 | |||||||
| China | Penaeus japonicus | WSSV-CN | Complete genome | AF332093 | 1996 | [30] | |||
| WSSV-CN01 | KT995472 | 1994 | [34] | ||||||
| Procambarus clarkii | WSSV-CN02 | Complete genome | KT995470 | 2010 | [34] | ||||
| WSSV-CN-Pc | KX686117 | 2015 | [36] | ||||||
| Penaeus vannamei | WSSV-CN03 | Complete genome | KT995471 | 2010 | [34] | ||||
| Marsupenaeus japonicus | WSSV-CN04 | Complete genome | KY827813 | 2012 | [35] | ||||
| Thailand | Penaeus monodon | WSSV-TH | Complete genome | AF369029 | 1996 | [29] | |||
| TH-96-II | Nonfunctional ORF14 gene; ORFI, ORFII, ORFIII, ORFIV, and ORFV genes; ORF15 and ORF16 gene | AY753327 | 2005 | [184] | |||||
| Taiwan | Penaeus monodon | WSSV-TW | Complete genome | AF440570 | 1994 | [31] | |||
| South Korea | Penaeus vannamei | WSSV-KR | Complete genome | JX515788 | 2011 | [32,34] | |||
| Australia | Penaeus monodon | WSSV-AU | Complete genome | MF768985 | 2016 | [37] | |||
| USA | Penaeus vannamei | CN_95_DFPE | Complete genome | MN840357 | 2017 | [41] | |||
| Ecuador | Penaeus vannamei | WSSV-EC-15098 | Complete genome | MH090824 | 2015 | [39] | |||
| Brazil | Penaeus vannamei | WSSV-chimera | Complete genome | MG264599 | 2015 | [40] | |||
| FSL39 | Partial genome | MF784752 | |||||||
| ss DNA | IHHNV (Type I) | Australia | Penaeus monodon | Australian | Non-structural protein gene Non-structural protein 1 gene Capsid protein genes | GQ475529 | 2008 | [60] | |
| IHHNV (Type II) | Thailand | Penaeus monodon | - | Non-structural protein 2 gene Non-structural protein 1 gene Capsid protein genes | AY362547 | 2003 | [173] | ||
| IHHNV_TH | AY102034 | 2000 | [185] | ||||||
| Taiwan | Penaeus monodon | Taiwan B | Non-structural protein 2 gene Non-structural protein 1 gene; Capsid protein genes | AY355307 | 2003 | [186] | |||
| Vietnam | Penaeus monodon | IHHNV-VN | Non-structural protein 2 gene Non-structural protein 1 gene Capsid protein genes | JN616415 | 2009 | [60] | |||
| ST | KC513422 | 2011 | |||||||
| Penaeus monodon; Penaeus vannamei | KK-Lv-VIET1 | Non-structural protein 1 gene | MN481525 | 2019 | [187] | ||||
| Penaeus stylirostris | VN2007 | Complete genome | KF031144 | 2007 | [57] | ||||
| India | Penaeus monodon | IN-07 | Complete genome | GQ411199 | 2007 | [60] | |||
| IHHNV | Capsid protein gene | FJ169961 | 2007 | [173] | |||||
| IHHNV (Type III) | Vietnam | Penaeus monodon | KG | Complete genome | JX840067 | 2012 | [57] | ||
| Taiwan | Penaeus monodon | Taiwan A | Non-structural protein 2 gene; Non-structural protein 1 gene; Capsid protein genes | AY355306 | 2003 | [186] | |||
| Taiwan C | AY355308 | 2003 | |||||||
| Ecuador | Penaeus vannamei | IHHNV | Non-structural protein 2 gene; Non-structural protein 1 gene; Capsid protein genes | AY362548 | 2003 | [186] | |||
| Brazil | Penaeus vannamei | IHHNV_BR | Partial genome | KJ862253 | 2013 | [60] | |||
| China | Penaeus penicillatus | IHHNV | Complete genome | KJ830753 | - | [60] | |||
| Penaeus monodon | Fujian | EF633688 | 2007 | [188] | |||||
| Ganyu | JX258653 | 2009 | [57] | ||||||
| Penaeus vannamei | CSH-1 | KF907320 | 2012 | ||||||
| Penaeus vannamei | Sheyang | KF214742 | 2011 | ||||||
| Hawaii | Penaeus stylirostris | Hawaii A | Complete genome | NC_002190 | 1990 | [60] | |||
| Hawaii B | AF218266 | 1990 | |||||||
| Malaysia | Macrobrachium rosenbergii | IHHNV | Non-structural protein genome | HM536212 | 2009 | [189] | |||
| Taiwan | Macrobrachium rosenbergii | AC-04-367 | Non-structural protein 1 gene | DQ057982 | - | ||||
| AC-05-005 | DQ057983 | - | |||||||
| Mexico | Penaeus stylirostris | IHHNV | Non-structural protein 2 gene; Non-structural protein 1 gene; Capsid protein genes | AF273215 | 2000 | [190] | |||
| South Korea | Penaeus vannamei | K1 | Structural protein gene | HQ699073 | 2010 | [191] | |||
| K2 | HQ699074 | 2010 | |||||||
| KLV-2010-01 | Complete genome | JN377975 | 2010 | [58] | |||||
| IHHNV(Type A) | Madagascar | Penaeus monodon | IHHNV | Non-structural protein 1 gene; Structural protein genes; Unnamed retrotransposon reverse transcriptase gene | DQ228358 | - | [191] | ||
| Australia | Penaeus monodon | Au2005 | Non-structural protein 2 gene; Non-structural protein 1-like gene; Viral capsid protein gene | EU675312 | - | [188] | |||
| IHHNV (Type B) | Tanzania Mozambique | Penaeus monodon | East Africa | Non-structural protein 1 gene; Structural protein genes | AY124937 | 2000 | [185] | ||
| RNA virus | ds RNA | IMNV (PsIMNV) | Indonesia | Penaeus vannamei | ID-EJ-12-1 | ORF1/ORF2 and ORF1 polyprotein genes | KJ636783 | 2012 | [40,77] |
| ID-EJ-12-1 | ORF1 polyprotein | AIC34743 | 2012 | ||||||
| ID-EJ-12-2 | ORF1/ORF2 | AIC34746 | 2012 | ||||||
| ID-EJ-12-3 | ORF1 polyprotein | AIC34749 | 2012 | ||||||
| ID-LP-12-2 | AIC34750 | 2012 | |||||||
| ID-BB-12 | AIC34752 | 2012 | |||||||
| ID-EJ-06 | Structural protein | ABN05324 | - | ||||||
| ID-LP-11 | Complete genome | KJ636782 | 2011 | ||||||
| ID-LP-11 | ORF1 polyprotein | AIC34741 | 2011 | ||||||
| ID-LP-12-1 | ORF1/ORF2 | AIC34748 | 2012 | ||||||
| IMNV | Complete genome | EF061744 | - | [74] | |||||
| Indonesia | KF836757 | 2013 | [192] | ||||||
| Brazil | Penaeus vannamei | BZ-03 | Structural protein | AAT67230 | - | [77] | |||
| ZS2011001 | Capsid protein | AGF33812 | 2004 | ||||||
| Brazil 01 | Structural protein | ADG37656 | 2007 | ||||||
| Brazil 02 | ADN43996 | 2007 | |||||||
| IMNV-BZ-11-UAZ219 | ORF1 polyprotein | AIC34754 | 2011 | ||||||
| IMNV | Complete genome | AY570982 | - | [74] | |||||
| ss RNA | YHV (genotype 1) | Thailand | Penaeus monodon | YHV1992 | Complete genome | FJ848673 | 1992 | [98,101] | |
| YHV1995 | Complete genome | FJ848674 | 1995 | ||||||
| Chachoengsao 1998 | Complete genome | EU487200 | 1988 | [98,108] | |||||
| YHA-98-Ref | pp1ab gene | EU785033 | 1998 | [98,114] | |||||
| Thailand: Cholburi | Envelope structural glycoprotein gene | EF156405 | 1999 | [108] | |||||
| YHV1999 | Complete genome | FJ848675 | [98,101] | ||||||
| YHV-PmA | 3C-like protease gene | EU977577 | - | [108] | |||||
| Replicase polyprotein 1ab gene | EU977578 | ||||||||
| RNA polymerase gene | EU977579 | ||||||||
| Helicase gene | EU977580 | ||||||||
| Nucleocapsid gene | EU977581 | ||||||||
| Glycoprotein 116 gene | EU977582 | ||||||||
| Glycoprotein 64 gene | EU977583 | ||||||||
| Genomic sequence | EU977584 | ||||||||
| THA-00-DRH | pp1ab gene | EU785032 | 2000 | [98,114] | |||||
| THA-01-D4 | EU785004 | 2001 | |||||||
| THA-01-D8 | EU785034 | 2001 | |||||||
| THA-01-D9 | EU785019 | 2001 | |||||||
| THA-01-D10 | EU784984 | 2001 | |||||||
| THA-02-D34 | EU785001 | 2002 | |||||||
| THA-03-D1 | EU784982 | 2003 | |||||||
| THA-03-D2 | EU784991 | 2003 | |||||||
| THA-03-D3 | EU784998 | 2003 | |||||||
| THA-03-DB1 | EU785023 | 2003 | |||||||
| THA-03-D29 | EU785035 | 2003 | |||||||
| THA-03-D30 | EU784999 | 2003 | |||||||
| THA-03-D33 | EU785000 | 2003 | |||||||
| Penaeus vannamei | YHV | ORF1b genes | FJ627274 | 2007 | [106] | ||||
| Mexico | Penaeus vannamei | YHV | 3C-like protease gene | DQ978355 | 2000 | [108] | |||
| ORF1a and ORF1b polyprotein gene | DQ978356 | ||||||||
| Nonfunctional ORF1b polyprotein gene | DQ978357 | ||||||||
| ORF1b polyprotein gene | DQ978358 | ||||||||
| Helicase gene | DQ978359 | ||||||||
| Nucleocapsid gene | DQ978360 | ||||||||
| Glycoprotein 116 gene | DQ978361 | ||||||||
| Glycoprotein 64 gene | DQ978362 | ||||||||
| ORF4-like gene | DQ978363 | ||||||||
| China | Fenneropenaeus chinensis | Hb2012 | Replicase polyprotein 1b mRNA | KF278563 | 2012 | [98] | |||
| GAV (genotype 2) | Australia | Penaeus monodon | GAV | Complete genome | AF227196 | - | [98,101,108] | ||
| NC_010306 | - | [101] | |||||||
| AUS-97-MCMS1 | pp1ab gene | EU784980 | 1997 | [98,114] | |||||
| AUS-97-MCMS2 | EU784989 | 1997 | |||||||
| AUS-97-MCMS3 | EU785038 | 1997 | |||||||
| AUS-00-H2 | EU785029 | 2000 | |||||||
| AUS-00-HL4 | EU785030 | 2000 | |||||||
| AUS-00-HL5 | EU785031 | 2000 | |||||||
| AUS-00-HL11 | EU785028 | 2000 | |||||||
| AUS-96-Ref | EU785026 | 1996 | |||||||
| Vietnam | Penaeus monodon | VNT-01-H65 | pp1ab gene | EU785039 | 2001 | [114] | |||
| VNT-01-H77 | EU785013 | 2001 | |||||||
| VNM-02-H6 | EU785009 | 2002 | |||||||
| VNM-02-H64 | EU785008 | 2002 | |||||||
| Thailand | Penaeus monodon | THA-03-HB3 | pp1ab gene | EU785024 | 2003 | [114] | |||
| THA-03-HG | EU785025 | 2003 | |||||||
| THA-03-HA | EU785021 | 2003 | |||||||
| THA-03-HN | EU785022 | 2003 | |||||||
| THA-04-H20 | EU784992 | 2004 | |||||||
| THA-04-HK | EU785027 | 2004 | |||||||
| YHV (genotype 3) | Vietnam | Penaeus monodon | VNM-02-H5 | pp1ab gene | EU785006 | 2002 | [98,114] | ||
| VNM-02-H258 | EU784994 | 2002 | |||||||
| VNM-02-H81 | EU785016 | 2002 | |||||||
| VNM-02-H70 | EU785012 | 2002 | |||||||
| VNM-01-H41 | EU785040 | 2001 | |||||||
| VNM-01-H42 | EU785041 | 2001 | |||||||
| VNM-02-H278 | EU784996 | 2002 | |||||||
| VNM-02-H264 | EU784995 | 2002 | |||||||
| VNM-02-H93 | EU785020 | 2002 | |||||||
| VNM-02-H93 | p20 gene; pp3 gene | EU785042 | 2002 | [114] | |||||
| Indonesia | Penaeus monodon | IDN-04-H7 | pp1ab gene | EU785011 | 2004 | [114] | |||
| IDN-04-H11 | EU784985 | 2004 | |||||||
| IDN-04-H10 | EU784983 | 2004 | |||||||
| IDN-04-H4 | EU785002 | 2004 | [98,114] | ||||||
| Malaysia | Penaeus monodon | MYS-03-H1 | pp1ab gene | EU784981 | 2003 | [114] | |||
| MYS-03-H2 | EU784990 | 2003 | |||||||
| MYS-03-H3 | EU784997 | 2003 | |||||||
| Mozambique | Penaeus monodon | MOZ-04-H1 | pp1ab gene | EU784986 | 2004 | ||||
| YHV (genotype 4) | Thailand | Penaeus monodon | YHV type 4 | ORF1b polyprotein gene | EU170438 | - | [98,193] | ||
| gp116 gene | EU123854 | ||||||||
| Indonesia | Penaeus monodon | IND-02-H9 | pp1ab gene | EU785017 | 2002 | [98,114] | |||
| IND-02-H5 | EU785005 | 2002 | |||||||
| IND-02-H7 | EU785010 | 2002 | |||||||
| India | Penaeus monodon | IND-02-H9 | p20 gene; pp3 gene | EU785043 | 2002 | [114] | |||
| YHV (genotype 5) | Thailand | Penaeus monodon | THA-03-SG21 | pp1ab gene | EU784993 | 2003 | |||
| YHV | ORF1b polyprotein gene | EU853170 | 2005 | [193] | |||||
| Malaysia | Penaeus monodon | MYS-03-H4 | pp1ab gene | EU785003 | 2003 | [114] | |||
| Philippines | Penaeus monodon | PHL-03-H8 | EU785015 | 2003 | |||||
| YHV (genotype 6) | Mozambique | Penaeus monodon | MOZ-04-H6 | pp1ab gene | EU785007 | 2004 | |||
| MOZ-04-H8 | EU785014 | 2004 | |||||||
| MOZ-04-H9 | EU785018 | 2004 | |||||||
| MOZ-04-H11 | EU785036 | 2004 | |||||||
| MOZ-04-H12 | EU785037 | 2004 | |||||||
| YHV (genotype 7) | Australia | Penaeus monodon | YHV7 (13-00169-01) PCR1 | ORF1b polyprotein gene | KP738160 | 2012 | [98,105] | ||
| YHV7 (13-00169-01) PCR2 | KP738161 | ||||||||
| YHV7 (13-00169-02) PCR2 | KP738162 | ||||||||
| YHV7 (13-00169-03) PCR2 | KP738163 | ||||||||
| YHV7 (13-00169-02) PCR3 | KP738164 | ||||||||
| YHV (genotype 8) | China | Fenneropenaeus chinensis | 20120706 | Complete genome | KX947267 | 2012 | [101] | ||
| TSV | Ecuador | Penaeid shrimp | EC1993a | Capsid protein 2 gene | FJ876460 | 1993 | [127] | ||
| EC1993b | FJ876461 | ||||||||
| EC1994 | FJ876466 | 1994 | |||||||
| EC2006a | FJ876512 | 2006 | |||||||
| EC2006b | FJ876513 | ||||||||
| Columbia | Penaeid shrimp | CO1994a | FJ876462 | 1994 | |||||
| CO1994b | FJ876463 | ||||||||
| CO1994c | FJ876464 | ||||||||
| CO1994d | FJ876465 | ||||||||
| CO1998 | FJ876477 | 1998 | |||||||
| Penaeus vannamei | CO-06A | JN194141 | 2006 | [138] | |||||
| CO-06B | JN194142 | ||||||||
| CO-06C | JN194143 | ||||||||
| CO-07A | JN194144 | 2007 | |||||||
| CO-07B | JN194145 | ||||||||
| CO-10 | JN194146 | 2010 | |||||||
| CO10 | Complete genome | JF966384 | 2010 | ||||||
| USA | Penaeus vannamei | 94USHI | Complete genome | AF277675 | 1994 | [62,132,194,195] | |||
| HI94TSV | Viral coat protein 2 gene | AY826054 | 1994 | [117] | |||||
| Viral coat protein 3 gene | AY826055 | ||||||||
| US-TX04 | Complete genome | GQ502201 | 2004 | [132] | |||||
| 2005-334 | MT877007 | 2019 | [119] | ||||||
| Penaeid shrimp | US1994 | Capsid protein 2 gene | FJ876468 | 1994 | [127] | ||||
| US1995 | FJ876469 | 1995 | |||||||
| US1996 | FJ876474 | 1996 | |||||||
| US1998 | FJ876476 | 1998 | |||||||
| US2004 | FJ876492 | 2004 | |||||||
| US2007 | FJ876517 | 2007 | |||||||
| Honduras | Penaeid shrimp | HO1994 | Capsid protein 2 gene | FJ876467 | 1994 | ||||
| HO1998 | FJ876475 | 1998 | |||||||
| HO2003 | FJ876483 | 2003 | |||||||
| Mexico | Penaeid shrimp | MX1995a | Capsid protein 2 gene | FJ876470 | 1995 | [127] | |||
| MX1995b | FJ876471 | ||||||||
| MX1995c | FJ876472 | ||||||||
| MX1996 | FJ876473 | 1996 | |||||||
| MX1998 | FJ876478 | 1998 | |||||||
| MX1999a | FJ876479 | 1999 | |||||||
| MX2000 | FJ876480 | 2000 | |||||||
| MX2004 | FJ876493 | 2004 | |||||||
| MX2005a | FJ876504 | 2005 | |||||||
| MX2005b | FJ876505 | ||||||||
| MX2005c | FJ876506 | ||||||||
| MX2006 | FJ876514 | 2006 | |||||||
| MX2007 | FJ876521 | 2007 | |||||||
| Penaeus vannamei | SIN98TSV | Viral coat protein 1 gene | AF510515 | 1998 | [125,195] | ||||
| MX99 | Coat protein gene | AF277378 | 1999 | [126,127] | |||||
| Mexico 10 | Capsid protein 2 gene | JN194147 | 2010 | [138] | |||||
| Penaeus stylirostris | MX99TSV | Viral coat protein 1 gene | AF510516 | 1999 | [125,195] | ||||
| SON2KTSV | AF510517 | 2000 | [131,195] | ||||||
| Penaeus stylirostris | HI94TSV | Viral coat protein 1 gene | AF510518 | 2000 | [117,125] | ||||
| Taiwan | Penaeus vannamei | TW99 | Coat protein gene | AF406789 | 1999 | [62,126,195] | |||
| Penaeus monodon | Tw2KPmTSV | Capsid protein precursor | AY355309 | 2000 | [126] | ||||
| Metapenaeus ensis | Tw2KMeTSV | Capsid protein precursor | AY355310 | 2000 | [196] | ||||
| Penaeus vannamei | Tw02PvTSV | Capsid protein precursor | AY355311 | 2002 | [127] | ||||
| Penaeid shrimp | TW2007 | Capsid protein 2 gene | FJ876520 | 2007 | |||||
| Thailand | Penaeus vannamei | Th03-1TSV | Capsid protein 2 gene | DQ000304 | 2003 | [196] | |||
| Th03-2TSV | DQ000305 | ||||||||
| ThOct03LvTSV | VP1 gene | AY912503 | 2003 | [126] | |||||
| ThMar04LvTSV | AY912504 | 2004 | |||||||
| ThJul04LvTSV | AY912508 | ||||||||
| Penaeus monodon | ThMar04Pm1TSV | VP1 gene | AY912505 | 2004 | |||||
| ThMar04Pm2TSV | AY912506 | ||||||||
| Penaeus monodon (post-larvae) | ThMay04PmPLTSV | VP1 gene | AY912507 | 2004 | |||||
| Penaeus vannamei | TH03-1 | Capsid protein 1 gene | AY755587 | 2003 | [125,196] | ||||
| TH03-2 | AY755588 | ||||||||
| TH03-3 | AY755589 | ||||||||
| TH03-4 | AY755590 | ||||||||
| TH03-5 | AY755591 | ||||||||
| TH03-7 | AY755593 | ||||||||
| TH03-9 | AY755595 | ||||||||
| TH04Lv | Complete genome | AY997025 | 2005 | [132,197] | |||||
| Macrobrachium rosenbergii | TH03-6 | Capsid protein 1 gene | AY755592 | 2003 | [125] | ||||
| Penaeus monodon | TH04Pm | Capsid protein 2 gene | DQ000306 | 2004 | [196] | ||||
| TH03-8 | Capsid protein 1 gene | AY755594 | 2003 | [125] | |||||
| Penaeid shrimp | TH2003a | Capsid protein 2 gene | FJ876484 | 2003 | [127] | ||||
| TH2003b | FJ876485 | ||||||||
| TH2004a | FJ876496 | 2004 | |||||||
| TH2004b | FJ876497 | ||||||||
| TH2006 | FJ876515 | 2006 | |||||||
| Myanmar | Penaeus monodon | Mm03Pm | Capsid protein 1 gene | AY755596 | 2003 | [125,196] | |||
| Vietnam | Penaeus vannamei | VN-TSV | Capsid protein gene | AY694136 | - | [198] | |||
| Belize | Penaeus vannamei | BZ01 | Non-structural polyprotein gene; Capsid protein precursor gene | AY590471 | 2001 | [62,124,132] | |||
| Penaeus vannamei | 2005-175 | Complete gene | MT877008 | 2019 | [119] | ||||
| BLZ02TSV | Viral coat protein 1 gene | AY826051 | 2002 | [117] | |||||
| Viral coat protein 2 gene | AY826052 | ||||||||
| Viral coat protein 3 gene | AY826053 | ||||||||
| Penaeid shrimp | BH2001 | Capsid protein 2 gene | FJ876481 | 2001 | [127] | ||||
| BH2002 | FJ876482 | 2002 | |||||||
| BH2004a | FJ876490 | 2004 | |||||||
| BH2004b | FJ876491 | ||||||||
| BH2005a | FJ876498 | 2005 | |||||||
| BH2005b | FJ876499 | ||||||||
| BH2005c | FJ876500 | ||||||||
| BH2008 | FJ876522 | 2008 | |||||||
| Indonesia | Penaeus vannamei | Id03TSV | Capsid protein 2 gene | DQ000303 | 2003 | [196] | |||
| Penaeus vannamei | Indonesia 10 | JN194148 | 2010 | [138] | |||||
| Penaeid shrimp | ID2003a | FJ876486 | 2003 | [127] | |||||
| ID2003b | FJ876487 | ||||||||
| ID2003c | FJ876488 | ||||||||
| ID2005 | FJ876501 | 2005 | |||||||
| ID2006 | FJ876510 | 2006 | |||||||
| China | Penaeus vannamei | ZHZC3TSV | Complete genome | DQ104696 | 2005 | [132,199] | |||
| Cn03TSV | Capsid protein 2 gene | DQ000301 | 2003 | [196] | |||||
| Ch-1 | Capsid protein 1 gene | AY755597 | [125,200] | ||||||
| Ch-2 | AY755598 | ||||||||
| Ch-3 | AY755599 | ||||||||
| Ch-4 | AY755600 | ||||||||
| Ch-6 | AY755602 | ||||||||
| Penaeus japonicus | Ch-5 | Capsid protein 1 gene | AY755601 | 2003 | [125] | ||||
| Penaeid shrimp | CH2003a | Capsid protein 2 gene | FJ876489 | 2003 | [127] | ||||
| CH2004 | FJ876494 | 2004 | |||||||
| CH2005a | FJ876509 | 2005 | |||||||
| CH2007 | FJ876518 | 2007 | |||||||
| Korea | Penaeus vannamei | KOR-CsPv04TSV | Capsid protein 1 mRNA | DQ099912 | 2004 | [131] | |||
| KOR-ImPv05TSV | DQ099913 | ||||||||
| Eritrea | Penaeus monodon | Er04PmTSV | Capsid protein 2 gene | DQ000302 | 2004 | [196] | |||
| Penaeid shrimp | ER2004 | FJ876495 | 2004 | [127] | |||||
| Venezuela | Penaeus vannamei | VE05 | Complete genome | DQ212790 | 2005 | [124] | |||
| 2005-194 | MT877006 | 2019 | [119] | ||||||
| Penaeid shrimp | VE2005a | Capsid protein 2 gene | FJ876502 | 2005 | [127] | ||||
| VE2005b | FJ876503 | ||||||||
| Saudi Arabia | Penaeid shrimp | SA2007 | Capsid protein 2 gene | FJ876519 | 2007 | ||||
| Penaeus indicus | SAPi | Complete genome | JX094350 | 2010 | [118] | ||||
| SA2010a | Capsid protein 2 gene | JQ356858 | |||||||
| SA2010b | JQ356859 | ||||||||
| SA2010c | JQ356860 | ||||||||
| SA2011a | JQ356861 | 2011 | |||||||
| SA2011b | JQ356862 | ||||||||
| SA2011c | JQ356863 | ||||||||
| SA2011d | JQ356864 | ||||||||
| SA2011e | JQ356865 | ||||||||
| Aruba | Penaeid shrimp | AW2005 | Capsid protein 2 gene | FJ876508 | 2005 | [127] | |||
| AW2006 | FJ876511 | 2006 | |||||||
| Nicaragua | Penaeid shrimp | NI2005 | Capsid protein 2 gene | FJ876507 | 2005 | ||||
| NI2006 | FJ876516 | 2006 | |||||||
| WTD (MrNV) | French West Indies | Macrobrachium rosenbergii | MrNV | Segment RNA-1 | AY222839 | 2003 | [143,154] | ||
| Segment RNA-2 | AY222840 | ||||||||
| RNA-1 | NC_005094 | 2009 | [201] | ||||||
| RNA-2 | NC_005095 | - | |||||||
| MrNV-Ant | Putative RNA-dependent RNA-polymerase gene | AY313773 | 2005 | [141] | |||||
| China | MrNV | RNA-directed RNA polymerase gene | AAQ54758 | - | [202] | ||||
| Chinese 1 | AY231436 | 2006 | [143,202] | ||||||
| Chinese 2 | Segment RNA-2 | FJ751225 | - | [143] | |||||
| MrNV | Segment RNA-1 RNA-dependent RNA polymerase gene; B2 protein gene | FJ751226 | 2006 | [201] | |||||
| Capsid protein gene | AY231437 | - | [143] | ||||||
| India | Nellore | Capsid protein gene | GU300102 | - | [203] | ||||
| B2 protein gene | GU300103 | 2011 | |||||||
| MrNV | Capsid protein-like gene | HM565741 | 2010 | [143] | |||||
| RNA-1 RNA-dependent RNA polymerase gene; B2 protein gene | JQ418295 | - | [153] | ||||||
| RNA-2 capsid protein gene | JQ418298 | - | [149,200] | ||||||
| Capsid protein | AM114036 | - | |||||||
| RNA-dependent RNA polymerase gene | AAO60068 | - | [152] | ||||||
| RNA-directed RNA polymerase gene | DQ146969 | - | [201] | ||||||
| Kakinada 1MrNV | Isolate Kakinada 1MrNV capsid protein gene | HQ637179 | 2008 | [149] | |||||
| Taiwan | AC06-016 | RNA-directed RNA polymerase gene | DQ459203 | - | [143] | ||||
| AC06-017 | DQ459204 | ||||||||
| AC06-024 | DQ459205 | ||||||||
| AC06-86 | DQ459206 | ||||||||
| AC06-088 | DQ459207 | ||||||||
| AC06-89 | DQ459208 | ||||||||
| MrNV | Segment RNA-1 nonfunctional polymerase gene | DQ521574 | - | [201] | |||||
| Segment RNA-2 capsid protein gene | DQ521575 | - | |||||||
| Malaysia | MrNV | Dependent RNA polymerase gene | JN187416 | 2009 | [143] | ||||
| Australia | 07-265.1 | Capsid protein gene | FJ379530 | 2007 | [204] | ||||
| 07-265.2 | A protein gene | FJ379531 | |||||||
| Australian | Segment RNA 1 | JN619369 | 2004 | [143] | |||||
| Segment RNA 2 | JN619370 | ||||||||
| Thailand | M298 | Capsid protein gene | EU150126 | - | [143] | ||||
| M299 | EU150127 | ||||||||
| M308 | EU150128 | ||||||||
| M12 | EU150129 | ||||||||
| MrNV | Capsid protein mRNA | DQ189990 | - | [201] | |||||
| WTD (XSV) | Taiwan | Macrobrachium rosenbergii | XSV | Nucleocapsid protein CP17 gene | DQ521573 | - | [205] | ||
| Thailand | M23 | Capsid protein gene | EU150133 | - | [204] | ||||
| M309 | EU150132 | - | |||||||
| 07-265.3 | FJ379532 | 2007 | |||||||
| India | Kakinada 1XSV | Isolate Kakinada 1XSV capsid protein gene | HQ637180 | 2008 | [149] | ||||
| XSV | Capsid protein gene | JQ418299 | - | ||||||
| Capsid protein, genomic RNA | AM114037 | - | |||||||
| Capsid protein gene | NC_043494 | - | |||||||
| Capsid protein gene | AY247793 | - | [198] | ||||||
| China | XSV | Nucleocapsid protein CP17 and CP16 genes | DQ174318 | - | [206] | ||||
| Type | Pathogen | Host Species | Characteristics | Reference | |
|---|---|---|---|---|---|
| DNA virus | ds DNA | WSSV | Penaeus monodon |
| [9,13,18,20,44,49,51,53,207,208,209,210] |
| Penaeus indicus | |||||
| Penaeus japonicas | |||||
| Penaeus chinensis | |||||
| Penaeus penicillatus | |||||
| Penaeus semisulcatus | |||||
| Penaeus aztecus | |||||
| Penaeus vannamei | |||||
| Penaeus merguiensis | |||||
| Penaeus duorarum | |||||
| Penaeus stylirostris | |||||
| Trachypenaeus curvirostris | |||||
| Metapenaeus ensis | |||||
| Exopalaemon orientalis | |||||
| Macrobrachium rosenbergii | |||||
| Marsupenaeus japonicus | |||||
| Metapenaeus dobsoni | |||||
| Parapenaeopsis stylifera | |||||
| Solenocera indica | |||||
| Squilla mantis | |||||
| Procambarus clarkii |
| [48,50,207,211] | |||
| Pacifastacus leniusculus | |||||
| Orconectes punctimanus | |||||
| Austropotamobius pallipes | |||||
| Panulirus versicolor |
| [212,213] | |||
| Panulirus penicillatus | |||||
| Panulirus homarus | |||||
| Panulirus ornatus | |||||
| Charybdis feriatus |
| [48,50,207,210,212,214] | |||
| Charybdis cruciata | |||||
| Portunus pelagicus | |||||
| Portunus sanguinolentus | |||||
| Charybdis granulata | |||||
| Scylla serrata | |||||
| Helice tridens | |||||
| Carcinus maenas | |||||
| Calappa lophos | |||||
| Paratelphusa hydrodomous | |||||
| Paratelphusa pulvinata | |||||
| Matuta planipes | |||||
| ss DNA | IHHNV | Penaeus vannamei |
| [57,58,59,66,85] | |
| Penaeus stylirostris | |||||
| Penaeus occidentalis | |||||
| Penaeus monodon | |||||
| Penaeus semisulcatus | |||||
| Penaeus californiensis | |||||
| Penaeus schmitti | |||||
| Penaeus japonicus | |||||
| Penaeus latisulcatus | |||||
| Penaeus chinensis | |||||
| Penaeus setiferus | |||||
| Penaeus aztecus | |||||
| Penaeus duorarum | |||||
| Penaeus subtilis | |||||
| Artemesia longinaris | |||||
| Macrobrachium rosenbergii | |||||
| Palaemon macrodactylus | |||||
| Procambarus clarkii | |||||
| Hemigrapsus penicillatus | |||||
| Neohelice granulate | |||||
| Corydoras arcuatus | |||||
| Mytilus edulis | |||||
| Mactra chinensis | |||||
| Tegillarca granosa | |||||
| Ruditapes philippinarum | |||||
| Sinonovacula constricta | |||||
| Meretrix meretrix | |||||
| Mactra veneriformis | |||||
| RNA virus | ds RNA | IMNV | Penaeus vannamei |
| [62,73,76,83,84,85,86,195] |
| Penaeus stylirostris | |||||
| Penaeus monodon | |||||
| Farfantepenaeus subtiltis | |||||
| ss RNA | YHD | Penaeus stylirostris |
| [68,91,100,101,106,113,215] | |
| Penaeus aztecus | |||||
| Penaeus duorarum | |||||
| Penaeus setiferus | |||||
| Penaeus vannamei | |||||
| Penaeus esculentus | |||||
| Penaeus stylirostris | |||||
| Penaeus monodon | |||||
| Fenneropenaeus merguiensis | |||||
| Farfantepenaeus aztecus | |||||
| Farfantepenaeus duorarum | |||||
| Metapenaeus ensis | |||||
| Metapenaeus affinis | |||||
| Marsupenaeus japonicus | |||||
| TSV | Penaeus stylirostris |
| [52,95,129,130,136,216] | ||
| Penaeus schmitti | |||||
| Penaeus setiferus | |||||
| Penaeus duorarum | |||||
| Penaeus aztecus | |||||
| Penaeus monodon | |||||
| Penaeus japonicus | |||||
| Penaeus chinensis | |||||
| WTD | Macrobrachium rosenbergii |
| [56,140,144,160,163] | ||
| Penaeus indicus | |||||
| Penaeus japonicus | |||||
| Penaeus monodon | |||||
| Penaeus vannamei | |||||
| Cherax quadricarinatus | |||||
| Type | Pathogen | PCR | Host | Tissue | Primer | Sequence 5′-3′ | Annealing Temperature (°C) | Amplicons (bp) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA virus | ds DNA | WSSV | Conventional PCR | Cherax quadricarinatus; Procambarus clarkii | Hepatopancreas, gills, cuticle, muscle | WSI3 | GTA ACT CCT TCC ATC TCC A | 62 | 941 | [217] | |
| WSI4 | TAC GGC AGC TGC TGC ACC TTG T | ||||||||||
| Penaeus monodon | Muscle | WSSV-VP28 F | TGT GAC CAA GAC CAT CGA AAC | 52 | 516 | [27] | |||||
| WSSV-VP28 R | TCG GTC TCA GTG CCA GAG TA | ||||||||||
| Real-time qPCR (EVA green) | Penaeus vannamei | Gills | VP24 F1 | AGG ACC CGA TCG CTT ACT TTG | - | 240 | [218] | ||||
| VP24 R1 | CTC CCT CCC TTG CGA ACT T | ||||||||||
| β-Actin F1 | GAA GTA GCC GCC CTG GTT G | 416 | |||||||||
| β-Actin R1 | CGG TTA GCC TTG GGG TTG AG | ||||||||||
| Real-time PCR (BRYT Green) | Penaeus monodon | Muscle | WSSV-qVP28 F | TGT GAC CAA GAC CAT CGA AA | 53 | 148 | [27] | ||||
| WSSV-qVP28 R | CTT GAT TTT GCC CAA GGT GT | ||||||||||
| Real-time PCR (TaqMan) | Cherax quadricarinatus; Procambarus clarkii | Hepatopancreas, gills, cuticle, muscle | WSS1011F | TGG TCC CGT CCT CAT CTC AG | 60 | 69 | [217] | ||||
| WSS1079R | GCT GCC TTG CCG GAA ATT A | ||||||||||
| Nested PCR | Fenneropenaeus indicus | Pleopod | 146F1 | First | ACT ACT AAC TTC AGC CTA TCT AG | 55 | 1447 | [150] | |||
| Second | GTA ACT GCC CCT TCC ATC TCC A | 941 | |||||||||
| ss DNA | IHHNV | Conventional PCR | Penaeus monodon | Tissues of infected samples | 77012F | ATC GGT GCA CTA CTC GGA | 53 | 356 | [58] | ||
| 77353R | TCG TAC TGG CTG TTC ATC | ||||||||||
| Penaeus vannamei | IHHNV389F | CGG AAC ACA ACC CGA CTT TA | 55 | 389 | |||||||
| IHHNV389R | GGC CAA GAC CAA AAT ACG AA | ||||||||||
| IHHNV392F | GGG CGA ACC AGA ATC ACT TA | 392 | |||||||||
| IHHNV392R | ATC CGG AGG AAT CTG ATG TG | ||||||||||
| Penaeus stylirostris; Penaeus vannamei | IHHNV721F | TCT ACT GCC TCT GCA ACG AG | 2000 | ||||||||
| IHHNV2860R | GTG GGT CTG GTC CAC TTG AT | ||||||||||
| Penaeus monodon | IHHNV3065F | GAC GAC GAA GAA TGG ACA GA | 3000 | ||||||||
| IHHNV3065R | TGC CTG GGT AGC TGG TAT GTA TA | ||||||||||
| IHHNV309F | TCC AAC ACT TAG TCA AAA CCA A | 309 | |||||||||
| IHHNV309R | TGT CTG CTA CGA TGA TTA TCC A | ||||||||||
| Penaeus vannamei | Hepatopancreas | IHHNV REPF | CGA TGT GCA ATA TAT ACC CGA TT | 52 | 442 | [57] | |||||
| IHHNV REPR | CTT CGC AGA AAC CGT TAA CTT | ||||||||||
| IHHNV472F | ACG AAC GAC CAC CCA TGG CA | 57 | 472 | ||||||||
| IHHNV472R | TCT GGT TCG CCC TGA CGT GT | ||||||||||
| IHHNV447F | CGA AGC GCG AGT ATC CAT CA | 55 | 447 | ||||||||
| IHHNV447R | TGA GTG ATG GAC GAA AGC GG | ||||||||||
| IHHNV-F | TCA TGA AGC GCG AGT ATC CAT CAT | 54 | 228 | ||||||||
| IHHNV-R1 | TGG GTG GTC GTT CGT ATC TT | ||||||||||
| Real-time PCR (TaqMan) | Penaeus monodon | Gills | IHHNV-q309F1 | CCT AAA GAA AAC AGT GCA GAA TAT GAC | 60.7 | 98 | [219] | ||||
| IHHNV-q309R1 | TCA TCG TCA AGT TTA TTG ACA AGT TC | 60.8 | |||||||||
| IHHNV-qEVEF1 | CCC ACA AAA AGC AAA TAT ATC TCA CTA T | 61.1 | 106 | ||||||||
| IHHNV-qEVER1 | GTC ATT ATG AGA TTA TTG TCC CAC CTT | 61.7 | |||||||||
| Pmon-EF1qF1 | GGC CGT GTG GAG ACT GGT AT | 62.3 | 110 | ||||||||
| Pmon-EF1qR1 | CGT GGT GCA TCT CCA CAG A | 62.0 | |||||||||
| Real-time PCR (SYBR Green) | Penaeus vannamei | Gillsm muscle, hepatopancreas, hemolymph | IHHNV 195F | GGG AGT TAC CTT TGC TGC | 56 | 195 | [220] | ||||
| IHHNV 195R | GGT CCG TCT ACT GCG TCT | ||||||||||
| RNA virus | ds RNA | IMNV | Reverse transcriptase PCR | Penaeus vannamei | Muscle | 389F | CGG AAC ACA ACC CGA CTT TA | 55 | 284 | [62] | |
| 389R | GGC CAA GAC CAA AAT ACG AA | ||||||||||
| Penaeus vannamei | Muscle | IMNV 105-297-F | CAT ATG GGG CAA TTA CGG TTA CAG GG | 60 | 600 | [74] | |||||
| IMNV 105-297-R | CGG GAT CCG TAT ACA TAC CAA ATG GCC | ||||||||||
| IMNV 300-527-F | CTC GAG ACT AAA CAA ACA ACA GAC AAT GC | 55 | 700 | [87] | |||||||
| IMNV 300-527-R | GGA TCC GGA GTC CCA TCA TAT AAC TGG | ||||||||||
| IMNVF22 | C CAT ATG ATT GTT TCA ATG GAA AAT C | 57 | 811 | [84] | |||||||
| IMNVR819 | G GAA TTC TTG TAG TGC AGT TGC TGG | ||||||||||
| IMNVF820 | CGG GA TCC GCT GCA AAA GAG GGT GCT CG | 924 | |||||||||
| IMNVR1728 | G GAA TTC TTG CAT TGA ACTCCACGAAAA C | ||||||||||
| IMNVF1729 | CG GGA TCC GGT AGT ATT GCA CCA GCA ATG | 1041 | |||||||||
| IMNVR | GGA ATT CTT ATA CTG TTG CTG T CG CTT G | ||||||||||
| IMNV 99372G09- F | CGA CGC TGC TAA CCA TAC A A | 62 | 372 | [221] | |||||||
| IMNV 99372 G10-R | ACT CGC CTG TTC GAT CAA GT | ||||||||||
| IMNV-NF | GGC ACA TGC TCA GAG ACA | 60 | 139 | [89] | |||||||
| IMNV-NR | AGC GCT GAG TCC AGT CTT G | ||||||||||
| ss RNA | YHD | RT-PCR | Penaeus monodon | Gills, hemolymph | YHV5f | CGT ATT GCA TCG AAC GTC ACT G | 60 | 885 | [222] | ||
| YHV5r | CAA GAT CAC TAA TAA CGC CTG ATG C | ||||||||||
| Nested PCR | YHV2s | CGG GGT TAC CCG CTT ATA TT | 400 | ||||||||
| YHV2as | GCC TGA GGT GAA GTC CAT GT | ||||||||||
| RT-PCR | Penaeus monodon | Gills, epidermis | YCF1a | ATC GTC GTC AGC TAC CGC AAT ACT GC | 60 | 359 | [98] | ||||
| YCF1b | ATC GTC GTC AGY TAY CGT AAC ACC GC | ||||||||||
| YCR1a | TCT TCR CGT GTG AAC ACY TTC TTR GC | ||||||||||
| YCR1b | TCT GCG TGG GTG AAC ACC TTC TTG GC | ||||||||||
| Nested PCR | YCF2a | CGC TTC CAA TGT ATC TGY ATG CAC CA | 66 | 147 | |||||||
| YCF2b | CGC TTY CAR TGT ATC TGC ATG CAC CA | ||||||||||
| YCR2a | RTC DGT GTA CAT GTT TGA GAG TTT GTT | ||||||||||
| YCR2b | GTC AGT GTA CAT ATT GGA GAG TTT RTT | ||||||||||
| Real time RT-qPCR (TaqMan) | Penaeus monodon | Pleopod | GAVQPF1 | GGG ATC CTA ACA TCG TCA ACG T | 60 | - | [223] | ||||
| GAVQPR1 | AGT AGT ATG GAT TAC CCT GGT GCA T | ||||||||||
| 6FAM-TAMRA probe | 6FAM-TCA GCC GCT TCC GCT TCC AAT G | ||||||||||
| RT-LAMP PCR | Penaeus vannamei | Pleopods | YHV-F3 | ACC CTG TAA TTG GCG ATG TT | 65 | 186 | [113] | ||||
| YHV-B3 | TGC AGT TAA GAT GGT CAC AG | ||||||||||
| YHV-FIP | AGA GCA CTG TAG ACT GGT GGG TTT TTG TGG AAC CTG AAG AAT GC | ||||||||||
| YHV-BIP-Biotin | Biotin-TCA GCA CCT GGG CTC GTC TCT TTT CGA CAG TGA TTG AAG ACT CG | ||||||||||
| YHV-LF | AAC TGT TGC AGA TCG GAT T | ||||||||||
| YHV-LB | ATG TGT CAT GAT ATT CTC | ||||||||||
| YHV FITC probe | CTC CAT CCA GAA A | ||||||||||
| YHV7-qPCR (TaqMan) | Penaeus monodon | Pleopods, gills | qYHV-F1 | CAT CCA ACC TAT CGC CTA CA | - | 79 | [91] | ||||
| qYHV-F2 | ACC TAT CGC CTA CAC AGC TA | 73 | |||||||||
| qYHV-R1 | TGT GAA GTC CAT GTG AAC GA | - | |||||||||
| qYHV7-Pr1 | 6FAM- CAA CGA CAG ACA CCT CAT CCG TGA-BHQ1 | - | |||||||||
| YH7-PCR | YHV7-F1a | CCT ACA CGC ATG CTC TCT CTA TG | - | 788 | |||||||
| YHV7-R1b | GGT GTC TGT CGT TGT GTA TAG CT | ||||||||||
| YHV7-nPCR | YHV7-F2a | CAA ACA CCA ACC GAC ATT CAG T | 58 | 412 | |||||||
| YHV7-R2a | GCG ACA GTG CTT GAA GAC TTT AG | ||||||||||
| TSV | ConventionalPCR | Penaeus monodon | Gills, tail, body cuticles, swimming feet | 9992F | AAG TAG ACA GCC GCG CTT | 60 | 231 | [129] | |||
| Real-time RT-PCR (TaqMan) | Davidson’s-fixed paraffin-embedded (DFPE) shrimp tissue | TSV1004F | TTG GGC ACC AAA CGA CAT T | 60 | 417 | [119] | |||||
| TSV1075R | GGG AGC TTA AAC TGG ACA CAC TGT | ||||||||||
| TSV-P1 | FAM-CAG CAC TGA CGC ACA ATA TTC GAG CAT C-TAMARA | ||||||||||
| TSV1004F | TTG GGC ACC AAA CGA CAT T | 122 | [120] | ||||||||
| TSV1075R | GGG AGC TTA AAC TGG ACA CAC TGT | ||||||||||
| TSV-probe | FAM-CAG CAC TGA CGC ACA ATA TTC GAG CAT C-TAMARA | ||||||||||
| Penaeus vannamei | Pleopods | TSV-55P1 | GGC GTA GTG AGT AAT GTA GC | 60 | 955 | [116] | |||||
| TSV-55P2 | CTT CAG TGA CCA CGG TAT AG | ||||||||||
| Real-time RT-PCR (SYBR green) | Penaeus vannamei | Cephalothorax | TSV-306F | CGT AAA TAG ACG GCC CAC AAA | 60 | 79 | [138] | ||||
| TSV384R | TGC ATC TAT ATA TCC AGG GAC TTA TCC | ||||||||||
| TSV-285F | TTC TAT AGG TCT GGT TTA AAA CGT AAA | 232 | |||||||||
| TSV-516R | CGG TTT TCT CCA TCA TCG TT | ||||||||||
| WTD | Reverse transcriptase PCR | Macrobrachium rosenbergii | Infected sample | Mr-RdRp-F | GCA TTT GTG AAG AAT GAA CCG | 50 | 729 | [56] | |||
| Mr-RdRp-R | CAT GTT CAACTTTCTCCACGT | ||||||||||
| qMrNV-F | AGG ATC CAC TAA GAA CGT GG | 211 | |||||||||
| qMrNV-R | CACGGTCACAATCCTTGCG | ||||||||||
| MrNv2F | GAT ACA GAT CCA CTA GAT GAC C | 55 | 681 | ||||||||
| MrNv2R | GAC GAT AGC TCT GAT AAT CC | ||||||||||
| Muscle | 1A775 | CCA CGT TCT TAG TGG ATC CT | 55 | 850 | [147] | ||||||
| 1B690 | CGT CCG CCT GGT AGT TCC | ||||||||||
| MrNV DBHF | ATG GCT AGA GGT AAA CAA AAT TC | 50 | 564 | [149] | |||||||
| MrNV DBHR | TCA TTG ATC ATC ACG CCT GAC A | ||||||||||
| MrNV PEF | GGG CCG GAT CCA TGG CTA GAG GTA AAC AAA ATT C | ||||||||||
| MrNV PER | GGC CAA GCT TTC ATT GAT CAT CAC GCC TGA CA | ||||||||||
| Infected sample | FL-XSV-F | CCA CGT CTA GCT GCT GAC GTT | 50 | 796 | [56] | ||||||
| FL-XSV-R | AAG GTC TTT ATT TAT CGA CGC | ||||||||||
| XSV-F | GGA GAA CCA TGA GAT CAC G | 55 | 507 | ||||||||
| XSV-R | CTG CTC ATT ACT GTT CGG AGT C | ||||||||||
| qXSV-F | AGC CAC ACT CTC GCA TCT GA | 50 | 68 | ||||||||
| qXSV-R | CTC CAG CAA AGT GCG ATA CG | ||||||||||
| Muscle | XSV DBHF | ATG AAT AAG CGC ATT AAT AAT | 50 | 525 | [149] | ||||||
| XSV DBHR | TTA CTG TTC GGA GTC CCA ATA | ||||||||||
| XSV PEF | GGG CCG GAT CCA TGA ATA AGC GCA TTA ATA AT | ||||||||||
| XSV PER | GGC CAA GCT TTT ACT GTT CGG AGT CCC AAT A | ||||||||||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, P.J.; Mohan, C.V. Viral disease emergence in shrimp aquaculture: Origins, impact and the effectiveness of health management strategies. Rev. Aquac. 2009, 1, 125–154. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.V.; Redman, R.M.; Pantoja, C.R.; Tang, K.F.J.; Noble, B.L.; Schofield, P.; Navarro, S.A. Historic emergence, impact and current status of shrimp pathogens in the Americas. J. Invertebr. Pathol. 2012, 110, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bossier, P.; Norouzitallab, P.; Vanrompay, D. Trained immunity and perspectives for shrimp aquaculture. Rev. Aquac. 2020, 12, 2351–2370. [Google Scholar] [CrossRef]
- Manan, H.; Ikhwanuddin, M. Triploid induction in penaeid shrimps aquaculture: A review. Rev. Aquac. 2021, 13, 619–631. [Google Scholar] [CrossRef]
- Morshed, M.; Islam, M.S.; Lohano, H.D.; Shyamsundar, P. Production externalities of shrimp aquaculture on paddy farming in coastal Bangladesh. Agric. Water. Manag. 2020, 238, 106213. [Google Scholar] [CrossRef]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef]
- Tacon, A.G. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- Flegel, T.W. Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 2012, 110, 166–173. [Google Scholar] [CrossRef]
- Sánchez-Paz, A. White spot syndrome virus: An overview on an emergent concern. Vet. Res. 2010, 41, 43. [Google Scholar] [CrossRef]
- Xiong, J. Progress in the gut microbiota in exploring shrimp disease pathogenesis and incidence. Appl. Microbiol. Biotechnol. 2018, 102, 7343–7350. [Google Scholar] [CrossRef]
- Flegel, T.W. Current status of viral diseases in Asian shrimp aquaculture. Isr. J. Aquac. Bamidgeh 2009, 60, 229–239. [Google Scholar] [CrossRef]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Itsathiphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Boonyakida, J.; Xu, J.; Satoh, J.; Nakanishi, T.; Mekata, T.; Kato, T.; Park, E.Y. Antigenic properties of VP15 from white spot syndrome virus in kuruma shrimp Marsupenaeus japonicus. Fish Shellfish. Immunol. 2020, 101, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, B.; Rai, P.; Mohan, S.A.; Shekhar, M.S.; Karunasagar, I. Biology, host range, pathogenesis and diagnosis of white spot syndrome virus. Indian. J. Virol. 2012, 23, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, S.; Hou, C.; Liang, X.; Dehwah, M.A.S.; Tan, B.; Shi, L. The T cell factor, pangolin, from Litopenaeus vannamei play a positive role in the immune responses against white spot syndrome virus infection. Dev. Comp. Immunol. 2021, 119, 104041. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Geng, R.; Zuo, H.; Weng, S.; He, J.; Xu, X. Toll receptor 2 (Toll2) positively regulates antibacterial immunity but promotes white spot syndrome virus (WSSV) infection in shrimp. Dev. Comp. Immunol. 2021, 115, 103878. [Google Scholar] [CrossRef]
- Panchal, V.; Kumar, S.; Hossain, S.N.; Vasudevan, D. Structure analysis of thymidylate synthase from white spot syndrome virus reveals WSSV-specific structural elements. Int. J. Biol. Macromol. 2021, 167, 1168–1175. [Google Scholar] [CrossRef]
- Verbruggen, B.; Bickley, L.K.; Van Aerle, R.; Bateman, K.S.; Stentiford, G.D.; Santos, E.M.; Tyler, C.R. Molecular mechanisms of white spot syndrome virus infection and perspectives on treatments. Viruses 2016, 8, 23. [Google Scholar] [CrossRef]
- Oakey, J.; Smith, C.; Underwood, D.; Afsharnasab, M.; Alday-Sanz, V.; Dhar, A.; Crook, A. Global distribution of white spot syndrome virus genotypes determined using a novel genotyping assay. Arch. Virol. 2019, 164, 2061–2082. [Google Scholar] [CrossRef]
- Dey, B.K.; Dugassa, G.H.; Hinzano, S.M.; Bossier, P. Causative agent, diagnosis and management of white spot disease in shrimp: A review. Rev. Aquac. 2020, 12, 822–865. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Zhang, J.; Bao, J. Intestinal microbiota in white spot syndrome virus infected red swamp crayfish (Procambarus clarkii) at different health statuses. Aquaculture 2021, 542, 736826. [Google Scholar] [CrossRef]
- Lai, Y.; Zhu, F.; Xu, Y. WSSV proteins and DNA genome released by ultrasonic rupture can infect cray fish as effectively as intact virions. J. Virol. Methods. 2020, 283, 113917. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.P.; de Souza, E.N.V.; Candido, J.R.; Dantas, M.D.; Nunes, A.R.; Ribeiro, K.; Lanza, D.C. Alternative PCR primers for genotyping of Brazilian WSSV isolates. J. Invertebr. Pathol. 2019, 162, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sathyabhama, A.B.; Puthumana, J.; Kombiyil, S.; Philip, R.; Singh, I.S.B. ‘PmLyO-Sf9-WSSV complex’could be a platform for elucidating the mechanism of viral entry, cellular apoptosis and replication impediments. Virology 2021, 553, 102–110. [Google Scholar] [CrossRef]
- Weerachatyanukul, W.; Chotwiwatthanakun, C.; Jariyapong, P. Dual VP28 and VP37 dsRNA encapsulation in IHHNV virus-like particles enhances shrimp protection against white spot syndrome virus. Fish Shellfish. Immunol. 2021, 113, 89–95. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Li, F.; Yang, F. Low-abundance envelope protein VP12 of white spot syndrome virus interacts with envelope protein VP150 and capsid protein VP51. Virus Res. 2013, 178, 206–210. [Google Scholar] [CrossRef]
- Talukder, A.S.; Punom, N.J.; Eshik, M.M.E.; Begum, M.K.; Islam, H.R.; Hossain, Z.; Rahman, M.S. Molecular identification of white spot syndrome virus (WSSV) and associated risk factors for white spot disease (WSD) prevalence in shrimp (Penaeus monodon) aquaculture in Bangladesh. J. Invertebr. Pathol. 2021, 179, 107535. [Google Scholar] [CrossRef]
- Ramos-Paredes, J.; Grijalva-Chon, J.M.; Ibarra-Gámez, J.C. Virulence and genotypes of white spot syndrome virus infecting Pacific white shrimp Litopenaeus vannamei in north-western Mexico. J. Fish Dis. 2017, 40, 425–435. [Google Scholar] [CrossRef]
- van Hulten, M.C.; Witteveldt, J.; Peters, S.; Kloosterboer, N.; Tarchini, R.; Fiers, M.; Vlak, J.M. The white spot syndrome virus DNA genome sequence. Virology 2001, 286, 7–22. [Google Scholar] [CrossRef]
- Yang, F.; He, J.; Lin, X.; Li, Q.; Pan, D.; Zhang, X.; Xu, X. Complete genome sequence of the shrimp white spot bacilliform virus. J. Virol. 2001, 75, 11811–11820. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, H.C.; Huang, C.J.; Peng, S.E.; Chen, Y.G.; Lin, S.J.; Chen, W.Y.; Dai, C.F.; Yu, H.T.; Wang, C.H.; et al. Transcriptional analysis of the DNA polymerase gene of shrimp white spot syndrome virus. Virology 2002, 301, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.Y.; Yoon, J.; Lee, Y.S.; Kim, Y.B.; Choi, T.J. Analysis of the complete nucleotide sequence of a white spot syndrome virus isolated from pacific white shrimp. J. Microbiol. 2013, 51, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Anaya, L.Z.; Gonzalez-Galaviz, J.R.; Casillas-Hernandez, R. Draft genome sequence of white spot syndrome virus isolated from cultured Litopenaeus vannamei in Mexico. Genome Announc. 2016, 4, e01674-15. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, M.; Xu, L.; Yang, F. Comparative genomic analysis of three white spot syndrome virus isolates of different virulence. Virus Genes. 2017, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, F.; Xu, L.; Yang, F.A. VP24-truncated isolate of white spot syndrome virus is inefficient in per os infection. Vet. Res. 2017, 48, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, J.; Liu, L.; Pan, Y.; Yan, S.; Wang, Y. Characterization and prevalence of a novel white spot syndrome viral genotype in naturally infected wild crayfish, Procambarus clarkii, in Shanghai, China. Virusdisease 2017, 28, 250–261. [Google Scholar] [CrossRef]
- Oakey, H.J.; Smith, C.S. Complete genome sequence of a white spot syndrome virus associated with a disease incursion in Australia. Aquaculture 2018, 484, 152–159. [Google Scholar] [CrossRef]
- Vinaya Kumar, K.; Shekhar, M.S.; Otta, S.K.; Karthic, K.; Ashok Kumar, J.; Gopikrishna, G.; Vijayan, K.K. First Report of a Complete Genome Sequence of White spot syndrome virus from India. Genome Announc. 2018, 6, e00055-18. [Google Scholar] [CrossRef]
- Restrepo, L.; Reyes, A.; Bajaña, L.; Betancourt, I.; Bayot, B. Draft genome sequence of a white spot syndrome virus isolate obtained in Ecuador. Genome Announc. 2018, 6, e00605-18. [Google Scholar] [CrossRef]
- Dantas, M.D.A.; Teixeira, D.G.; Silva-Portela, R.C.B.; Soares, P.E.T.; Lima, J.P.M.S.; Agnez-Lima, L.F.; Lanza, D.C.F. Direct sequencing of the white spot syndrome virus from Brazil: Genome assembly and new insights on phylogeny. Virus Res. 2018, 245, 52–61. [Google Scholar] [CrossRef]
- Cruz-Flores, R.; Mai, H.N.; Kanrar, S.; Caro, L.F.A.; Dhar, A.K. Genome reconstruction of white spot syndrome virus (WSSV) from archival Davidson’s-fixed paraffin embedded shrimp (Penaeus vannamei) tissue. Sci. Rep. 2020, 10, 13425. [Google Scholar] [CrossRef] [PubMed]
- Dashtiannasab, A. White Spot Syndrome Virus. In Emerging and Reemerging Viral Pathogens; Volume 1: Fundamental and Basic Viology Aspects of Human, Animal and Plant Pathogens; Ennaji, M.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 717–728. [Google Scholar] [CrossRef]
- Zwart, M.P.; Dieu, B.T.M.; Hemerik, L.; Vlak, J.M. Evolutionary trajectory of white spot syndrome virus (WSSV) genome shrinkage during spread in Asia. PLoS ONE 2010, 5, e13400. [Google Scholar] [CrossRef] [PubMed]
- Yoganandhan, K.; Thirupathi, S.; Hameed, A.S. Biochemical, physiological and hematological changes in white spot syndrome virus-infected shrimp, Penaeus indicus. Aquaculture 2003, 221, 1–11. [Google Scholar] [CrossRef]
- Tuyen, N.X.; Verreth, J.; Vlak, J.M.; de Jong, M.C.M. Horizontal transmission dynamics of White spot syndrome virus by cohabitation trials in juvenile Penaeus monodon and P. vannamei. Prev. Vet. Med. 2014, 117, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Arulmoorthy, M.P.; Anandajothi, E.; Vasudevan, S.; Suresh, E. Major viral diseases in culturable penaeid shrimps: A review. Aquac. Int. 2020, 28, 1939–1967. [Google Scholar] [CrossRef]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vijayan, K.K. Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 2021, 533, 736231. [Google Scholar] [CrossRef]
- Hameed, A.S.; Yoganandhan, K.; Sathish, S.; Rasheed, M.; Murugan, V.; Jayaraman, K. White spot syndrome virus (WSSV) in two species of freshwater crabs (Paratelphusa hydrodomous and P. pulvinate). Aquaculture 2001, 201, 179–186. [Google Scholar] [CrossRef]
- Escobedo-Bonilla, C.M.; Alday-Sanz, V.; Wille, M.; Sorgeloos, P.; Pensaert, M.B.; Nauwynck, H.J. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus. J. Fish Dis. 2008, 31, 1–18. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, F. Use of glycerol monolaurate as a treatment against white spot syndrome virus in crayfish (Procambarus clarkii). Aquaculture 2021, 541, 736853. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, Y.; Zhu, F. Effect of dietary sodium butyrate on the innate immune response of Procambarus clarkii and disease resistance against white spot syndrome virus. Aquaculture 2021, 541, 736784. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Bonami, J.R.; Alday-Sanz, V. A critical review of susceptibility of crustaceans to Taura syndrome, Yellowhead disease and White Spot Disease and implications of inclusion of these diseases in European legislation. Aquaculture 2009, 291, 1–17. [Google Scholar] [CrossRef]
- Hossain, A.; Nandi, S.P.; Siddique, M.A.; Sanyal, S.K.; Sultana, M.; Hossain, M.A. Prevalence and distribution of White Spot Syndrome Virus in cultured shrimp. Lett. Appl. Microbiol. 2015, 60, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Mohammadi, A.; Akbari, S.; Banaee, M. Molecular, histopathologic and electron microscopic analysis of white spot syndrome virus in wild shrimp (Fenneropenaeus indicus) in the coastal waters of Iran. Arch. Virol. 2020, 165, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Sritunyalucksana, K.; Srisala, J.; McColl, K.; Nielsen, L.; Flegel, T.W. Comparison of PCR testing methods for white spot syndrome virus (WSSV) infections in penaeid shrimp. Aquaculture 2006, 255, 95–104. [Google Scholar] [CrossRef]
- Gangnonngiw, W.; Bunnontae, M.; Phiwsaiya, K.; Senapin, S.; Dhar, A.K. In experimental challenge with infectious clones of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), MrNV alone can cause mortality in freshwater prawn (Macrobrachium rosenbergii). Virology 2020, 540, 30–37. [Google Scholar] [CrossRef]
- Chai, C.; Liu, Y.; Xia, X.; Wang, H.; Pan, Y.; Yan, S.; Wang, Y. Prevalence and genomic analysis of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei shrimp farmed in Shanghai, China. Arch. Virol. 2016, 161, 3189–3201. [Google Scholar] [CrossRef]
- Rai, P.; Safeena, M.P.; Krabsetsve, K.; La Fauce, K.; Owens, L.; Karunasagar, I. Genomics, molecular epidemiology and diagnostics of infectious hypodermal and hematopoietic necrosis virus. Indian J. Virol. 2012, 23, 203–214. [Google Scholar] [CrossRef]
- Yu, J.Y.; Yang, N.; Hou, Z.H. Research progress on hosts and carriers, prevalence, virulence of infectious hypodermal and hematopoietic necrosis virus (IHHNV). J. Invertebr. Pathol. 2021, 183, 107556. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, W.; Shao, S. Phylogenetic and recombination analysis of genomic sequences of IHHNV. J. Basic Microbiol. 2015, 55, 1048–1052. [Google Scholar] [CrossRef]
- Nita, M.K.H.; Kua, B.C.; Bhassu, S.; Othman, R.Y. Detection and genetic profiling of infectious hypodermal and haematopoietic necrosis virus (IHHNV) infections in wild berried freshwater prawn, Macrobrachium rosenbergii collected for hatchery production. Mol. Biol. Rep. 2012, 39, 3785–3790. [Google Scholar] [CrossRef]
- Lightner, D.V. Virus diseases of farmed shrimp in the Western Hemisphere (the Americas): A review. J. Invertebr. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Madrigal, K.Y.; Luna-González, A.; Escobedo-Bonilla, C.M.; Fierro-Coronado, J.A.; Maldonado-Mendoza, I.E. Screening for potential probiotic bacteria to reduce prevalence of WSSV and IHHNV in whiteleg shrimp (Litopenaeus vannamei) under experimental conditions. Aquaculture 2011, 322, 16–22. [Google Scholar] [CrossRef]
- Rai, P.; Pradeep, B.; Karunasagar, I.; Karunasagar, I. Detection of viruses in Penaeus monodon from India showing signs of slow growth syndrome. Aquaculture 2009, 289, 231–235. [Google Scholar] [CrossRef]
- Montgomery-Brock, D.; Tacon, A.G.J.; Poulos, B.; Lightner, D. Reduced replication of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei held in warm water. Aquaculture 2007, 265, 41–48. [Google Scholar] [CrossRef]
- Tang, K.F.; Lightner, D.V. Infectious hypodermal and hematopoietic necrosis virus (IHHNV)-related sequences in the genome of the black tiger prawn Penaeus monodon from Africa and Australia. Virus Res. 2006, 118, 185–191. [Google Scholar] [CrossRef]
- Motte, E.; Yugcha, E.; Luzardo, J.; Castro, F.; Leclercq, G.; Rodríguez, J.; Boulo, V. Prevention of IHHNV vertical transmission in the white shrimp Litopenaeus vannamei. Aquaculture 2003, 219, 57–70. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Wang, X.; Zhang, Q.; Ren, Y.; Song, J.; Wang, X.; Dong, X.; Huang, J. First detection of yellow head virus genotype 3 (YHV-3) in cultured Penaeus monodon, mainland China. J. Fish Dis. 2018, 41, 1449–1451. [Google Scholar] [CrossRef]
- Encinas-García, T.; Mendoza-Cano, F.; Enríquez-Espinoza, T.; Luken-Vega, L.; Vichido-Chávez, R.; Sánchez-Paz, A. An improved validated SYBR green-based real-time quantitative PCR assay for the detection of the Penaeus stylirostris densovirus in penaeid shrimp. J. Virol. Methods 2015, 212, 53–58. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Li, C.; Wan, X.Y.; Yang, Q.; Xie, G.S.; Huang, J. Delivery of plasmid DNA to shrimp hemocytes by Infectious hypodermal and hematopoietic necrosis virus (IHHNV) nanoparticles expressed from a baculovirus insect cell system. J. Invertebr. Pathol. 2019, 166, 107231. [Google Scholar] [CrossRef]
- Chen, B.K.; Dong, Z.; Liu, D.P.; Yan, Y.B.; Pang, N.Y.; Nian, Y.Y.; Yan, D.C. Infectious hypodermal and haematopoietic necrosis virus (IHHNV) infection in freshwater crayfish Procambarus clarkii. Aquaculture 2017, 477, 76–79. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests for Aquatic Animals. Chapter 2.2.4, Infection with Infectious Hypodermal and Haematopoietic Necrosis Virus. 2019. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 11 May 2018).
- Andrade, T.P.D.; Redman, R.M.; Lightner, D.V. Evaluation of the preservation of shrimp samples with Davidson’s AFA fixative for infectious myonecrosis virus (IMNV) in situ hybridization. Aquaculture 2008, 278, 179–183. [Google Scholar] [CrossRef]
- Borsa, M.; Seibert, C.H.; Rosa, R.D.; Stoco, P.H.; Cargnin-Ferreira, E.; Pereira, A.M.L.; Grisar, E.C.; Zanetti, C.R.; Pinto, A.R. Detection of infectious myonecrosis virus in penaeid shrimps using immunoassays: Usefulness of monoclonal antibodies directed to the viral major capsid protein. Arch. Virol. 2011, 156, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.G.L.; Silva, A.C.G.; Nova, C.M.V.V.; Neto, J.M.O.; Lima, A.C.N.; Feijó, R.G.; Apolinário, D.F.; Maggioni, R.; Gesteira, T.C.V. Susceptibility of the wild southern brown shrimp (Farfantepenaeus subtilis) to infectious hypodermal and hematopoietic necrosis (IHHN) and infectious myonecrosis (IMN). Aquaculture 2009, 294, 1–4. [Google Scholar] [CrossRef]
- Prasad, K.P.; Shyam, K.U.; Banu, H.; Jeena, K.; Krishnan, R. Infectious Myonecrosis Virus (IMNV)–An alarming viral pathogen to Penaeid shrimps. Aquaculture 2017, 477, 99–105. [Google Scholar] [CrossRef]
- Mai, H.N.; Hanggono, B.; Caro, L.F.A.; Komaruddin, U.; Nur’aini, Y.L.; Dhar, A.K. Novel infectious myonecrosis virus (IMNV) genotypes associated with disease outbreaks on Penaeus vannamei shrimp farms in Indonesia. Arch. Virol. 2019, 164, 3051–3057. [Google Scholar] [CrossRef]
- Jithendran, K.P.; Krishnan, A.N.; Jagadeesan, V.; Anandaraja, R.; Ezhil Praveena, P.; Anushya, S.; Bhuvaneswari, T. Co-infection of infectious myonecrosis virus and Enterocytozoon hepatopenaei in Penaeus vannamei farms in the east coast of India. Aquac. Res. 2021, 52, 4701–4710. [Google Scholar] [CrossRef]
- Santhosh Kumar, S.; Sivakumar, S.; Abdul Majeed, S.; Vimal, S.; Taju, G.; Sahul Hameed, A.S. In vitro propagation of infectious myonecrosis virus in C6/36 mosquito cell line. J. Fish Dis. 2021, 44, 987–992. [Google Scholar] [CrossRef]
- Kokkattunivarthil, S.; Krishnan, R.; Kezhedath, J.; Prasad, K.P.; Naik, T.V. New set of PCR primers for SYBR green-based qPCR detection of IMNV in India. Aquaculture 2018, 495, 726–730. [Google Scholar] [CrossRef]
- Senapin, S.; Phiwsaiya, K.; Gangnonngiw, W.; Flegel, T.W. False rumours of disease outbreaks caused by infectious myonecrosis virus (IMNV) in the whiteleg shrimp in Asia. J. Negat. Results. Biomed. 2011, 10, 10. [Google Scholar] [CrossRef]
- Jha, R.K.; Babikian, H.; Kristina; Srisombat, S. Managing infectious myonecrosis virus (IMNV) in Vannamei shrimp culture: Learning by doing. Int. J. Fish. Aquat. Stud. 2021, 9, 385–391. [Google Scholar] [CrossRef]
- Sahul Hameed, A.S.; Abdul Majeed, S.; Vimal, S.; Madan, N.; Rajkumar, T.; Santhoshkumar, S.; Sivakumar, S. Studies on the occurrence of infectious myonecrosis virus in pond-reared Litopenaeus vannamei (Boone, 1931) in India. J. Fish Dis. 2017, 40, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Kunanopparat, A.; Chaivisuthangkura, P.; Senapin, S.; Longyant, S.; Rukpratanporn, S.; Flegel, T.W.; Sithigorngul, P. Detection of infectious myonecrosis virus using monoclonal antibody specific to N and C fragments of the capsid protein expressed heterologously. J. Virol. Methods 2011, 171, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Lopes, M.A.; Vieira-Girão, P.R.N.; da Cruze Freire, J.E.; Rocha, I.R.C.B.; Costa, F.H.F.; Rádis-Baptista, G. Natural co-infection with infectious hypodermal and hematopoietic necrosis virus (IHHNV) and infectious myonecrosis virus (IMNV) in Litopenaeus vannamei in Brazil. Aquaculture 2011, 312, 212–216. [Google Scholar] [CrossRef]
- Poulos, B.T.; Tang, K.F.J.; Pantoja, C.R.; Bonami, J.R.; Lightner, D.V. Purification and characterization of infectious myonecrosis virus of penaeid shrimp. J. Gen. Virol. 2006, 87, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Seibert, C.H.; Borsa, M.; Rosa, R.D.; Cargnin-Ferreira, E.; Pereira, A.M.L.; Grisard, E.C.; Zanetti, C.R.; Pinto, A.R. Detection of major capsid protein of infectious myonecrosis virus in shrimps using monoclonal antibodies. J. Virol. Methods 2010, 169, 169–175. [Google Scholar] [CrossRef]
- Vanpatten, K.A.; Nunan, L.M.; Lightner, D.V. Seabirds as potential vectors of penaeid shrimp viruses and the development of a surrogate laboratory model utilizing domestic chickens. Aquaculture 2004, 241, 31–46. [Google Scholar] [CrossRef]
- Srisala, J.; Sanguanrut, P.; Laiphrom, S.; Siriwattano, J.; Khudet, J.; Thaiue, D.; Sritunyalucksana, K. Infectious myonecrosis virus (IMNV) and decapod iridescent virus 1 (DIV1) detected in Penaeus monodon from the Indian Ocean. Aquaculture 2021, 545, 1–26. [Google Scholar] [CrossRef]
- Feijó, R.G.; Kamimura, M.T.; Oliveira-Neto, J.M.; Vila-Nova, C.M.V.M.; Gomes, A.C.S.; Coelho, M.G.L.; Vasconcelos, R.F.; Gesteira, T.C.V.; Marins, L.F.; Maggioni, R. Infectious myonecrosis virus and white spot syndrome virus co-infection in Pacific white shrimp (Litopenaeus vannamei) farmed in Brazil. Aquaculture 2013, 380, 1–5. [Google Scholar] [CrossRef]
- Cowley, J.A.; Rao, M.; Mohr, P.; Moody, N.J.; Sellars, M.J.; Crane, M.S.J. TaqMan real-time and conventional nested PCR tests specific to yellow head virus genotype 7 (YHV7) identified in giant tiger shrimp in Australia. J. Virol. Methods 2019, 273, 113689. [Google Scholar] [CrossRef]
- Sittidilokratna, N.; Dangtip, S.; Cowley, J.A.; Walker, P.J. RNA transcription analysis and completion of the genome sequence of yellow head nidovirus. Virus Res. 2008, 136, 157–165. [Google Scholar] [CrossRef]
- Li, C.; Ren, Y.; Dong, X.; Wang, C.; Huang, J. Extraction of assembling complexes of viral capsomers from shrimp tissue infected with yellow head virus genotype 8 (YHV-8). J. Fish Dis. 2019, 42, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Soowannayan, C.; Nguyen, G.T.; Pham, L.N.; Phanthura, M.; Nakthong, N. Australian red claw crayfish (Cherax quadricarinatus) is susceptible to yellow head virus (YHV) infection and can transmit it to the black tiger shrimp (Penaeus monodon). Aquaculture 2015, 445, 63–69. [Google Scholar] [CrossRef]
- Dhar, A.K.; Cowley, J.A.; Hasson, K.W.; Walker, P.J. Genomic organization, biology, and diagnosis of Taura syndrome virus and yellowhead virus of penaeid shrimp. Adv. Virus Res. 2004, 63, 353. [Google Scholar] [CrossRef] [PubMed]
- Bateman, K.S.; Stentiford, G.D. A taxonomic review of viruses infecting crustaceans with an emphasis on wild hosts. J. Invertebr. Pathol. 2017, 147, 86–110. [Google Scholar] [CrossRef]
- Wijegoonawardane, P.K.; Cowley, J.A.; Phan, T.; Hodgson, R.A.J.; Nielsen, L.; Kiatpathomchai, W.; Walker, P.J. Genetic diversity in the yellow head nidovirus complex. Virology 2008, 380, 213–225. [Google Scholar] [CrossRef]
- Mohr, P.G.; Moody, N.J.G.; Hoad, J.; Williams, L.M.; Bowater, R.O.; Cummins, D.M.; Crane, M.S.J. New yellow head virus genotype (YHV7) in giant tiger shrimp Penaeus monodon indigenous to northern Australia. Dis. Aquat. Org. 2015, 115, 263–268. [Google Scholar] [CrossRef]
- Elliott, L.; Owens, L. CART analysis of environmental factors, biomarkers and gill-associated virus to predict production outcomes for farmed Penaeus monodon. Aquaculture 2015, 448, 298–305. [Google Scholar] [CrossRef]
- Walker, P.J.; Sittidilokratna, N. Yellow Head Virus. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 476–483. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Zhu, L.; Wan, X.; Liu, Q.; Qiu, L.; Zou, P.; Zhang, Q.; Huang, J. Complete genome sequence of an isolate of a novel genotype of yellow head virus from Fenneropenaeus chinensis indigenous in China. Arch. Virol. 2017, 162, 1149–1152. [Google Scholar] [CrossRef]
- Anantasomboon, G.; Poonkhum, R.; Sittidilokratna, N.; Flegel, T.W.; Withyachumnarnkul, B. Low viral loads and lymphoid organ spheroids are associated with yellow head virus (YHV) tolerance in whiteleg shrimp Penaeus vannamei. Dev. Comp. Immunol. 2008, 32, 613–626. [Google Scholar] [CrossRef]
- Havanapan, P.O.; Taengchaiyaphum, S.; Paemanee, A.; Phungthanom, N.; Roytrakul, S.; Sritunyalucksana, K.; Krittanai, C. Caspase-3, a shrimp phosphorylated hemocytic protein is necessary to control YHV infection. Fish Shellfish Immunol. 2021, 114, 36–48. [Google Scholar] [CrossRef]
- Walker, P.J.; Cowley, J.A.; Dong, X.; Huang, J.; Moody, N.; Ziebuhr, J. ICTV Virus Taxonomy Profile: Roniviridae. J. Gen. Virol. 2021, 102, 001514. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Qiu, L.; Liu, Q.; Wan, X.Y.; Liu, S.; Zhu, L.L.; Huang, J. A novel method of real-time reverse-transcription loop-mediated isothermal amplification developed for rapid and quantitative detection of a new genotype (YHV-8) of yellow head virus. Lett. Appl. Microbiol. 2016, 63, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Senapin, S.; Thaowbut, Y.; Gangnonngiw, W.; Chuchird, N.; Sriurairatana, S.; Flegel, T.W. Impact of yellow head virus outbreaks in the whiteleg shrimp, Penaeus vannamei (Boone), in Thailand. J. Fish Dis. 2010, 33, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.A.; Cadogan, L.C.; Spann, K.M.; Sittidilokratna, N.; Walker, P.J. The Gene Encoding the Nucleocapsid Protein of Gill-Associated Nidovirus of Penaeus monodon Prawns Is Located Upstream of the Glycoprotein Gene. J. Virol. 2004, 78, 8935–8941. [Google Scholar] [CrossRef][Green Version]
- Cedano-Thomas, Y.; de la Rosa-Vélez, J.; Bonami, J.R.; Vargas-Albores, F. Gene expression kinetics of the yellow head virus in experimentally infected Litopenaeus vannamei. Aquac. Res. 2010, 41, 1432–1443. [Google Scholar] [CrossRef]
- Samocha, T.M. Sustainable Biofloc Systems for Marine Shrimp; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Prapavorarat, A.; Pongsomboon, S.; Tassanakajon, A. Identification of genes expressed in response to yellow head virus infection in the black tiger shrimp, Penaeus monodon, by suppression subtractive hybridization. Dev. Comp. Immunol. 2010, 34, 611–617. [Google Scholar] [CrossRef]
- Cowley, J.A. Nidoviruses of Fish and Crustaceans. In Aquaculture Virology; Kibenge, F.S.B., Godoy, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 443–472. [Google Scholar] [CrossRef]
- Thedcharoen, P.; Pewkliang, Y.; Kiem, H.K.T.; Nuntakarn, L.; Taengchaiyaphum, S.; Sritunyalucksana, K.; Borwornpinyo, S. Effective suppression of yellow head virus replication in Penaeus monodon hemocytes using constitutive expression vector for long-hairpin RNA (lhRNA). J. Invertebr. Pathol. 2020, 175, 107442. [Google Scholar] [CrossRef]
- Khunthong, S.; Jaroenram, W.; Arunrut, N.; Suebsing, R. Rapid and sensitive detection of shrimp yellow head virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. J. Virol. Methods 2013, 188, 51–56. [Google Scholar] [CrossRef]
- Wijegoonawardane, P.K.M.; Cowley, J.A.; Walker, P.J. Consensus RT-nested PCR detection of yellow head complex genotypes in penaeid shrimp. J. Virol. Methods 2008, 153, 168–175. [Google Scholar] [CrossRef]
- Sanitt, P.; Attasart, P.; Panyim, S. Protection of yellow head virus infection in shrimp by feeding of bacteria expressing dsRNAs. J. Biotechnol. 2014, 179, 26–31. [Google Scholar] [CrossRef]
- Tang, K.F.; Aranguren, L.F.; Piamsomboon, P.; Han, J.E.; Maskaykina, I.Y.; Schmidt, M.M. Detection of the microsporidian Enterocytozoon hepatopenaei (EHP) and Taura syndrome virus in Penaeus vannamei cultured in Venezuela. Aquaculture 2017, 480, 17–21. [Google Scholar] [CrossRef]
- Cruz-Flores, R.; Mai, H.N.; Dhar, A.K. Complete genome reconstruction and genetic analysis of Taura syndrome virus of shrimp from archival Davidson’s-fixed paraffin embedded tissue. Virology 2021, 553, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, L.M.; Cruz-Flores, R.; Dhar, A.K. Detection and Phylogenetic Analyses of Taura Syndrome Virus from Archived Davidson’s-Fixed Paraffin-Embedded Shrimp Tissue. Viruses 2020, 12, 1030. [Google Scholar] [CrossRef]
- Kiatpathomchai, W.; Jareonram, W.; Jitrapakdee, S.; Flegel, T.W. Rapid and sensitive detection of Taura syndrome virus by reverse transcription loop-mediated isothermal amplification. J. Virol. Methods 2007, 146, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Boube, I.; Lotz, J.M.; Pozhitkov, A.E.; Li, S.; Griffitt, R.J. Identification of genes involved in Taura Syndrome Virus resistance in Litopenaeus vannamei. J. Aquat. Anim. Health 2014, 26, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.T.; Lin, W.H.; Wang, P.C.; Tsai, M.A.; Ho, P.Y.; Hsu, J.P.; Chern, R.S.; Chen, S.C. Epidemiology and phylogenetic analysis of Taura syndrome virus in cultured Pacific white shrimp Litopenaeus vannamei B. in Taiwan. Dis. Aquat. Org. 2011, 97, 17–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, K.F.; Navarro, S.A.; Pantoja, C.R.; Aranguren, F.L.; Lightner, D.V. New genotypes of white spot syndrome virus (WSSV) and Taura syndrome virus (TSV) from the Kingdom of Saudi Arabia. Dis. Aquat. Org. 2012, 99, 179–185. [Google Scholar] [CrossRef]
- Moss, D.R.; Moss, S.M.; Lotz, J.M. Estimation of genetic parameters for survival to multiple isolates of Taura syndrome virus in a selected population of Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture 2013, 416, 78–84. [Google Scholar] [CrossRef]
- Nielsen, L.; Sang-Oum, W.; Cheevadhanarak, S.; Flegel, T.W. Taura syndrome virus (TSV) in Thailand and its relationship to TSV in China and the Americas. Dis. Aquat. Org. 2005, 63, 101–106. [Google Scholar] [CrossRef]
- Phalitakul, S.; Wongtawatchai, J.; Sarikaputi, M.; Viseshakul, N. The molecular detection of Taura syndrome virus emerging with White spot syndrome virus in penaeid shrimps of Thailand. Aquaculture 2006, 260, 77–85. [Google Scholar] [CrossRef]
- Erickson, H.S.; Poulos, B.T.; Tang, K.F.J.; Bradley-dunlop, D.; Lightner, D.V. Taura syndrome virus from Belize represents a unique variant. Dis. Aquat. Org. 2005, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Tang, K.F.; Navarro, S.A.; Lightner, D.V. A quick fuse and the emergence of Taura syndrome virus. Virology 2009, 390, 324–329. [Google Scholar] [CrossRef] [PubMed]
- George, S.K.; Kaizer, K.N.; Betz, Y.M.; Dhar, A.K. Multiplication of Taura syndrome virus in primary hemocyte culture of shrimp (Penaeus vannamei). J. Virol. Methods 2011, 172, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, A.N.; Fasya, A.H. Examination of Taura Syndrome Virus (TSV) in white shrimp (Litopenaeus vannamei) and tiger prawn (Penaeus monodon) with Polymerase Chain Reaction (PCR) method. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 0212069. [Google Scholar] [CrossRef]
- Dhar, A.K.; Allnutt, F.T. Taura Syndrome Virus. In Encyclopedia of Virology; Academic Press: Cambridge, MA, USA, 2008; pp. 1–8. [Google Scholar] [CrossRef]
- Do, J.W.; Cha, S.J.; Lee, N.S.; Kim, Y.C.; Kim, J.D.; Park, J.W. Taura syndrome virus from Penaeus vannamei shrimp cultured in Korea. Dis. Aquat. Org. 2006, 70, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Lakshman, D.K.; Amundsen, K.; Robles-Sikisaka, R.; Kaizer, K.N.; Roy, S.; Hasson, K.W.; Allnutt, F.C.T. Characterization of a Taura syndrome virus isolate originating from the 2004 Texas epizootic in cultured shrimp. Arch. Virol. 2010, 155, 315–327. [Google Scholar] [CrossRef]
- Tang, K.F.; Wang, J.; Lightner, D.V. Quantitation of Taura syndrome virus by real-time RT-PCR with a TaqMan assay. J. Virol. Methods 2004, 115, 109–114. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, S.Y.; Breeland, V.; Moore, A.M.; Lotz, J.M. Taura syndrome virus loads in Litopenaeus vannamei hemolymph following infection are related to differential mortality. Dis. Aquat. Org. 2010, 91, 97–103. [Google Scholar] [CrossRef]
- Tumburu, L.; Shepard, E.F.; Strand, A.E.; Browdy, C.L. Effects of endosulfan exposure and Taura Syndrome Virus infection on the survival and molting of the marine penaeid shrimp, Litopenaeus vannamei. Chemosphere 2012, 86, 912–918. [Google Scholar] [CrossRef]
- Côté, I.; Navarro, S.; Tang, K.F.J.; Noble, B.; Lightner, D.V. Taura syndrome virus from Venezuela is a new genetic variant. Aquaculture 2008, 284, 62–67. [Google Scholar] [CrossRef]
- Vergel, J.C.V.; Cabawatan, L.D.P.; Madrona, V.A.C.; Rosario, A.F.T.; Tare, M.V.R.; Maningas, M.B.B. Detection of Taura Syndrome Virus (TSV) in Litopenaeus vannamei in the Philippines. Philipp. J. Fish. 2019, 26, 8–14. [Google Scholar] [CrossRef]
- Aranguren, L.F.; Salazar, M.; Tang, K.; Caraballo, X.; Lightner, D. Characterization of a new strain of Taura syndrome virus (TSV) from Colombian shrimp farms and the implication in the selection of TSV resistant lines. J. Invertebr. Pathol. 2013, 112, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Phromjai, J.; Mathuros, T.; Phokharatkul, D.; Prombun, P.; Suebsing, R.; Tuantranont, A.; Kiatpathomchai, W. RT-LAMP detection of shrimp Taura syndrome virus (TSV) by combination with a nanogold-oligo probe. Aquac. Res. 2015, 46, 1902–1913. [Google Scholar] [CrossRef]
- Hameed, A.S.; Bonami, J.R. White tail disease of freshwater prawn, macrobrachium rosenbergii. Indian. J. Virol. 2012, 23, 134–140. [Google Scholar] [CrossRef]
- Bonami, J.R.; Shi, Z.; Qian, D.; Widada, J.S. White tail disease of the giant freshwater prawn, Macrobrachium rosenbergii: Separation of the associated virions and characterization of MrNV as a new type of nodavirus. J. Fish Dis. 2005, 28, 23–31. [Google Scholar] [CrossRef]
- Chen, K.F.; Tan, W.S.; Ong, L.K.; Abidin, S.A.Z.; Othman, I.; Tey, B.T.; Lee, R.F.S. The Macrobrachium rosenbergii nodavirus: A detailed review of structure, infectivity, host immunity, diagnosis and prevention. Rev Aquac. 2021, 13, 2117–2141. [Google Scholar] [CrossRef]
- Hayakijkosol, O.; Burgess, G.; La Fauce, K.; Owens, L. The complete sequence of the Australia recognizate of Macrobrachium rosenbergii nodavirus which causes white tail disease. Aquaculture 2012, 366, 98–104. [Google Scholar] [CrossRef]
- Ravi, M.; Nazeer Basha, A.; Sarathi, M.; Idalia, H.R.; Widada, J.S.; Bonami, J.R.; Hameed, A.S. Studies on the occurrence of white tail disease (WTD) caused by MrNV and XSV in hatchery-reared post-larvae of Penaeus indicus and P. monodon. Aquaculture 2009, 292, 117–120. [Google Scholar] [CrossRef]
- Pillai, D.; Bonami, J.R.; Sri Widada, J. Rapid detection of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), the pathogenic agents of white tail disease of Macrobrachium rosenbergii (De Man), by loop-mediated isothermal amplification. J. Fish Dis. 2006, 29, 275–283. [Google Scholar] [CrossRef]
- Sudhakaran, R.; Haribabu, P.; Kumar, S.R.; Sarathi, M.; Ahmed, V.I.; Babu, V.S.; Hameed, A.S. Natural aquatic insect carriers of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV). Dis. Aquat. Org. 2008, 79, 141–145. [Google Scholar] [CrossRef][Green Version]
- Murwantoko, M.; Bimantara, A.; Roosmanto, R.; Kawaichi, M. Macrobrachium rosenbergii nodavirus infection in a giant freshwater prawn hatchery in Indonesia. Springerplus 2016, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, L.; Hao, G.J.; Sheng, P.C.; Cao, Z.; Zhou, Y.; Chen, K. The development and application of a duplex reverse transcription loop-mediated isothermal amplification assay combined with a lateral flow dipstick method for Macrobrachium rosenbergii nodavirus and extra small virus isolated in China. Mol. Cell. Probes. 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.N.; Rai, P.; Karunasagar, I.; Karunasagar, I. Genomic and antibody-based assays for the detection of Indian strains of Macrobrachium rosenbergii nodavirus and extra small virus associated with white tail disease of Macrobrachium rosenbergii. VirusDisease 2020, 31, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Bonami, J.R.; Widada, J.S. Viral diseases of the giant fresh water prawn Macrobrachium rosenbergii: A review. J. Invertebr. Pathol. 2011, 106, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Pillai, D.; Bonami, J.R. A review on the diseases of freshwater prawns with special focus on white tail disease of Macrobrachium rosenbergii. Aquac. Res. 2012, 43, 1029–1037. [Google Scholar] [CrossRef]
- NaveenKumar, S.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Genetic analysis of RNA1 and RNA2 of Macrobrachium rosenbergii nodavirus (MrNV) isolated from India. Virus Res. 2013, 173, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Pasookhush, P.; Hindmarch, C.; Sithigorngul, P.; Longyant, S.; Bendena, W.G.; Chaivisuthangkura, P. Transcriptomic analysis of Macrobrachium rosenbergii (giant fresh water prawn) post-larvae in response to M. rosenbergii nodavirus (MrNV) infection: De novo assembly and functional annotation. BMC Genom. 2019, 20, 762. [Google Scholar] [CrossRef]
- Widada, J.S.; Durand, S.; Cambournac, I.; Qian, D.; Shi, Z.; Dejonghe, E.; Bonami, J.R. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: Dot-blot, in situ hybridization and RT-PCR. J. Fish Dis. 2003, 26, 583–590. [Google Scholar] [CrossRef]
- Kumar, B.T.N.; Murthy, H.S.; Patil, P.; Doddamani, P.L.; Patil, R. Enhanced immune response and resistance to white tail disease in chitin-diet fed freshwater prawn, Macrobrachium rosenbergii. Aquac. Rep. 2015, 2, 34–38. [Google Scholar] [CrossRef]
- Saedi, T.A.; Moeini, H.; Tan, W.S.; Yusoff, K.; Daud, H.M.; Chu, K.B.; Bhassu, S. Detection and phylogenetic profiling of nodavirus associated with white tail disease in Malaysian Macrobrachium rosenbergii de Man. Mol. Biol. Rep. 2012, 39, 5785–5790. [Google Scholar] [CrossRef]
- Sudhakaran, R.; Ishaq Ahmed, V.P.; Haribabu, P.; Mukherjee, S.C.; Sri Widada, J.; Bonami, J.R.; Sahul Hameed, A.S. Experimental vertical transmission of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) from brooders to progeny in Macrobrachium rosenbergii and Artemia. J. Fish Dis. 2007, 30, 27–35. [Google Scholar] [CrossRef] [PubMed]
- OIE. Manual of Diagnostic Tests for Aquatic Animals. Chapter 2.2.6, White Tail Disease. 2018. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 11 May 2017).
- Jariyapong, P.; Pudgerd, A.; Weerachatyanukul, W.; Hirono, I.; Senapin, S.; Dhar, A.K.; Chotwiwatthanakun, C. Construction of an infectious Macrobrachium rosenbergii nodavirus from cDNA clones in Sf9 cells and improved recovery of viral RNA with AZT treatment. Aquaculture 2018, 483, 111–119. [Google Scholar] [CrossRef]
- Hayakijkosol, O.; La Fauce, K.; Owens, L. Experimental infection of redclaw crayfish (Cherax quadricarinatus) with Macrobrachium rosenbergii nodavirus, the aetiological agent of white tail disease. Aquaculture 2011, 319, 25–29. [Google Scholar] [CrossRef]
- Palmer, P.J.; Rao, M.; Cowley, J.A. Reduced transmission of IHHNV to Penaeus monodon from shrimp pond wastewater filtered through a polychaete-assisted sand filter (PASF) system. Aquaculture 2021, 535, 736359. [Google Scholar] [CrossRef]
- Sun, Z.F.; Hu, C.Q.; Ren, C.H.; Shen, Q. Sensitive and rapid detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in shrimps by loop-mediated isothermal amplification. J. Virol. Methods 2006, 131, 41–46. [Google Scholar] [CrossRef]
- Sudhakaran, R.; Syed Musthaq, S.; Haribabu, P.; Mukherjee, S.C.; Gopal, C.; Sahul Hameed, A.S. Experimental transmission of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) in three species of marine shrimp (Penaeus indicus, Penaeus japonicus and Penaeus monodon). Aquaculture 2006, 257, 136–141. [Google Scholar] [CrossRef]
- Chaivisuthangkura, P.; Phattanapaijitkul, P.; Thammapalerd, N.; Rukpratanporn, S.; Longyant, S.; Sithigorngul, W.; Sithigorngul, P. Development of a polyclonal antibody specific to VP19 envelope protein of white spot syndrome virus (WSSV) using a recombinant protein preparation. J. Virol. Methods 2006, 133, 180–184. [Google Scholar] [CrossRef]
- de Jesús Durán-Avelar, M.; Pérez-Enríquez, R.; Zambrano-Zaragoza, J.F.; Montoya-Rodríguez, L.; Vázquez-Juárez, R.; Vibanco-Pérez, N. Genotyping WSSV isolates from northwestern Mexican shrimp farms affected by white spot disease outbreaks in 2010-2012. Dis. Aquat. Org. 2015, 114, 11–20. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Li, F.; Han, Y.; Xu, L.; Yang, F. VP24 is a chitin-binding protein involved in white spot syndrome virus infection. J. Virol. 2016, 90, 842–850. [Google Scholar] [CrossRef]
- Liu, Q.H.; Ma, F.F.; Guan, G.K.; Wang, X.F.; Li, C.; Huang, J. White spot syndrome virus VP51 interact with ribosomal protein L7 of Litopenaeus vannamei. Fish Shellfish Immunol. 2015, 44, 382–388. [Google Scholar] [CrossRef]
- Mendoza-cano, F.; Sánchez-paz, A. Development and validation of a quantitative real-time polymerase chain assay for universal detection of the White Spot Syndrome Virus in marine crustaceans. Virol. J. 2013, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.C.; Andrade, T.P.D.; Tang-Nelson, K.F.J.; Marques, M.R.F.; Lightner, D.V. Genotyping of White spot syndrome virus (WSSV) geographical isolates from Brazil and comparison to other isolates from the Americas. Dis. Aquat. Org. 2010, 88, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sindhupriya, M.; Saravanan, P.; Otta, S.wK.; Amarnath, C.B.; Arulraj, R.; Bhuvaneswari, T.; Ponniah, A.G. White spot syndrome virus (WSSV) genome stability maintained over six passages through three different penaeid shrimp species. Dis. Aquat. Org. 2014, 111, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.F.; Groumellec, L.M.; Lightner, D.V. Novel, closely related, white spot syndrome virus (WSSV) genotypes from Madagascar, Mozambique and the Kingdom of Saudi Arabia. Dis. Aquat. Org. 2013, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xu, L.; Yang, F. VP26 of white spot syndrome virus functions as a linker protein between the envelope and nucleocapsid of virions by binding with VP51. J. Virol. 2008, 82, 12598–12601. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.K.; Nguyen, V.G.; Park, B.K.; Choresca, C.H.; Shin, S.P.; Park, S.C. Genomic sequence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) KLV-2010-01 originating from the first Korean outbreak in cultured Litopenaeus vannamei. Arch. Virol. 2012, 157, 369–373. [Google Scholar] [CrossRef]
- Rai, P.; Safeena, M.P.; Karunasagar, I.; Karunasagar, I. Complete nucleic acid sequence of Penaeus stylirostris densovirus (PstDNV) from India. Virus Res. 2011, 158, 37–45. [Google Scholar] [CrossRef]
- Sittidilokratna, N.; Chotwiwatthanakun, C.; Wijegoonawardane, P.K.; Unajak, S.; Boonnad, A.; Wangnai, W.; Walker, P.J. A virulent isolate of yellow head nidovirus contains a deformed envelope glycoprotein gp116. Virology 2009, 384, 192–200. [Google Scholar] [CrossRef]
- Sriphaijit, T.; Flegel, T.W.; Senapin, S. Characterization of a shrimp serine protease homolog, a binding protein of yellow head virus. Dev. Comp. Immunol. 2007, 31, 1145–1158. [Google Scholar] [CrossRef]
- Cowley, J.A.; Walker, P.J. The complete genome sequence of gill-associated virus of Penaeus monodon prawns indicates a gene organisation unique among nidoviruses. Arch. Virol. 2002, 147, 1977–1987. [Google Scholar] [CrossRef]
- Aranguren, L.F.; Tang, K.F.J.; Lightner, D.V. Protection from yellow head virus (YHV) infection in Penaeus vannamei pre-infected with Taura syndrome virus (TSV). Dis. Aquat. Org. 2012, 98, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Paredes, J.; Grijalva-Chon, J.M.; Rosa-Vélez, J.D.L.; Enríquez-Paredes, L.M. New genetic recombination in hypervariable regions of the white spot syndrome virus isolated from Litopenaeus vannamei (Boone) in northwest Mexico. Aquac. Res. 2012, 43, 339–348. [Google Scholar] [CrossRef]
- Parrilla-Taylor, D.P.; Vibanco-Porez, N.; Durán-Avelar, M.D.J.; Gomez-Gil, B.; Llera-Herrera, R.; Vázquez-Juárez, R. Molecular variability and genetic structure of white spot syndrome virus strains from northwest Mexico based on the analysis of genomes. FEMS Microbiol. Lett. 2018, 365, fny216. [Google Scholar] [CrossRef]
- Saravanan, K.; Kumar, P.P.; Praveenraj, J.; Baruah, A.; Sivaramakrishnan, T.; Kumar, T.S.; Roy, S.D. Investigation and confirmation of white spot syndrome virus (WSSV) infection in wild caught penaeid shrimps of Andaman and Nicobar Islands, India. VirusDisease 2017, 28, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, B.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Characterization of variable genomic regions of Indian white spot syndrome virus. Virology 2008, 376, 24–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, Y. Molecular epidemiology of white spot syndrome virus in the world. Aquaculture 2021, 537, 736509. [Google Scholar] [CrossRef]
- Marks, H.; van Duijse, J.J.A.; Zuidema, D.; van Hulten, M.C.W.; Vlak, J.M. Fitness and virulence of an ancestral White Spot Syndrome Virus isolate from shrimp. Virus Res. 2005, 110, 9–20. [Google Scholar] [CrossRef]
- Tang, K.F.; Poulos, B.T.; Wang, J.; Redman, R.M.; Shih, H.H.; Lightner, D.V. Geographic variations among infectious hypodermal and hematopoietic necrosis virus (IHHNV) isolates and characteristics of their infection. Dis. Aquat. Org. 2003, 53, 91–99. [Google Scholar] [CrossRef]
- Hsia, H.L.; Chen, L.L.; Peng, S.E.; Yu, H.T.; Lo, C.F.; Kou, G.H. Comparison of genomic sequence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) between taiwan and other geographical isolates. Fish Pathol. 2003, 38, 177–179. [Google Scholar] [CrossRef][Green Version]
- Park, S.C.; Choi, S.K.; Han, S.H.; Park, S.; Jeon, H.J.; Lee, S.C.; Han, J.E. Detection of infectious hypodermal and hematopoietic necrosis virus and white spot syndrome virus in whiteleg shrimp (Penaeus vannamei) imported from Vietnam to South Korea. J. Vet. Sci. 2020, 21, e31. [Google Scholar] [CrossRef]
- Saksmerprome, V.; Puiprom, O.; Noonin, C.; Flegel, T.W. Detection of infectious hypodermal and haematopoietic necrosis virus (IHHNV) in farmed Australian Penaeus monodon by PCR analysis and DNA sequencing. Aquaculture 2010, 298, 190–193. [Google Scholar] [CrossRef]
- Wei, Y.W.; Fan, D.D.; Chen, J. The mussel Mytilus edulis L. as an important reservoir of infectious hypodermal and hematopoietic necrosis virus (IHHNV). Aquac. Res. 2017, 48, 1346–1350. [Google Scholar] [CrossRef]
- Hou, L.; Wu, H.; Xu, L.; Yang, F. Expression and self-assembly of virus-like particles of infectious hypodermal and hematopoietic necrosis virus in Escherichia coli. Arch. Virol. 2009, 154, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choresca Jr, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Han, S.Y.; Park, S.C. Detection of infectious hypodermal and hematopoietic necrosis virus (IHHNV) in Litopenaeus vannamei shrimp cultured in South Korea. Aquaculture 2011, 313, 161–164. [Google Scholar] [CrossRef]
- Dantas, M.D.A.; Chavante, S.F.; Teixeira, D.I.A.; Lima, J.P.M.; Lanza, D.C. Analysis of new isolates reveals new genome organization and a hypervariable region in infectious myonecrosis virus (IMNV). Virus Res. 2015, 203, 66–71. [Google Scholar] [CrossRef]
- Gangnonngiw, W.; Anantasomboon, G.; Sang-oum, W.; Sriurairatana, S.; Sritunyalucksana, K.; Flegel, T.W. Non-virulence of a recombinant shrimp nidovirus is associated with its non structural gene sequence and not a large structural gene deletion. Virology 2009, 385, 161–168. [Google Scholar] [CrossRef]
- Mari, J.; Poulos, B.T.; Lightner, D.V.; Bonami, J. Shrimp Taura syndrome virus: Genomic characterization and similarity with members of the genus Cricket paralysis-like viruses. J. Gen. Virol. 2002, 83, 915–926. [Google Scholar] [CrossRef]
- Tang, K.F.; Lightner, D.V. Phylogenetic analysis of Taura syndrome virus isolates collected between 1993 and 2004 and virulence comparison between two isolates representing different genetic variants. Virus Res. 2005, 112, 69–76. [Google Scholar] [CrossRef]
- Srisuvan, T.; Tang, K.F.; Lightner, D.V. Experimental infection of Penaeus monodon with Taura syndrome virus (TSV). Dis. Aquat. Org. 2005, 67, 1–8. [Google Scholar] [CrossRef][Green Version]
- Ongvarrasopone, C.; Saejia, P.; Chanasakulniyom, M.; Panyim, S. Inhibition of Taura syndrome virus replication in Litopenaeus vannamei through silencing the LvRab7 gene using double-stranded RNA. Arch. Virol. 2011, 156, 1117–1123. [Google Scholar] [CrossRef]
- Widada, J.S.; Bonami, J.R. Characteristics of the monocistronic genome of extra small virus, a virus-like particle associated with Macrobrachium rosenbergii nodavirus: Possible candidate for a new species of satellite virus. J. Gen. Virol. 2004, 85, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Ueda, R.; Krabsetsve, K.; Owens, L. Polymerase chain reaction detection of Taura Syndrome Virus and infectious hypodermal and haematopoietic necrosis virus in frozen commodity tails of Penaeus vannamei Boone. Aquac. Res. 2008, 39, 1606–1611. [Google Scholar] [CrossRef]
- Tripathy, S.; Sahoo, P.K.; Kumari, J.; Mishra, B.K.; Sarangi, N.; Ayyappan, S. Multiplex RT-PCR detection and sequence comparison of viruses MrNV and XSV associated with white tail disease in Macrobrachium rosenbergii. Aquaculture 2006, 258, 134–139. [Google Scholar] [CrossRef]
- Senapin, S.; Jaengsanong, C.; Phiwsaiya, K.; Prasertsri, S.; Laisutisan, K.; Chuchird, N.; Flegel, T.W. Infections of MrNV (Macrobrachium rosenbergii nodavirus) in cultivated whiteleg shrimp Penaeus vannamei in Asia. Aquaculture 2012, 338, 41–46. [Google Scholar] [CrossRef]
- Shekhar, M.S.; Azad, I.S.; Jithendran, K.P. RT-PCR and sequence analysis of Macrobrachium rosenbergii nodavirus: Indian isolate. Aquaculture 2006, 252, 128–132. [Google Scholar] [CrossRef]
- Shekhar, M.S.; Sahoo, P.K.; Dillikumar, M.; Das, A. Cloning, expression and sequence analysis of Macrobrachium rosenbergii nodavirus genes: Indian isolate. Aquac. Res. 2011, 42, 1778–1788. [Google Scholar] [CrossRef]
- Owens, L.; Fauce, L.K.; Juntunen, K.; Hayakijkosol, O.; Zeng, C. Macrobrachium rosenbergii nodavirus disease (white tail disease) in Australia. Dis. Aquat. Org. 2009, 85, 175–180. [Google Scholar] [CrossRef]
- Wang, C.S.; Chang, J.S.; Shih, H.H.; Chen, S.N. RT-PCR amplification and sequence analysis of extra small virus associated with white tail disease of Macrobrachium rosenbergii (de Man) cultured in Taiwan. J. Fish Dis. 2007, 30, 127–132. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhang, H.J.; Shi, Z.L. Expression and assembly mechanism of the capsid proteins of a satellite virus (XSV) associated with Macrobrachium rosenbergii nodavirus. Virol. Sin. 2008, 23, 73–77. [Google Scholar] [CrossRef]
- Bateman, K.S.; Tew, I.; French, C.; Hicks, R.J.; Martin, P.; Munro, J.; Setentiford, G.D. Susceptibility to infection and pathogenicity of White Spot Disease (WSD) in non-model crustacean host taxa from temperate regions. J. Invertebr. Pathol. 2012, 110, 340–351. [Google Scholar] [CrossRef]
- Hameed, A.S.; Charles, M.X.; Anilkumar, M. Tolerance of Macrobrachium rosenbergii to white spot syndrome virus. Aquaculture 2000, 183, 207–213. [Google Scholar] [CrossRef]
- Jin, W.; Lai, Y.; Zhu, F. Effect of dietary fucoidan on innate immune response of Procambarus clarkii and disease resistance against white spot syndrome virus. Aquaculture 2021, 534, 736233. [Google Scholar] [CrossRef]
- Kono, T.; Savan, R.; Sakai, M.; Itami, T. Detection of white spot syndrome virus in shrimp by loop-mediated isothermal amplification. J. Virol. Methods 2004, 115, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zhu, L.; Jiang, X.; Zhang, J.; Pei, C.; Zhao, X.; Kong, X. Diagnosis of co-infection with white spot syndrome virus and Aeromonas veronii in red swamp crayfish Procambarus clarkii. Aquaculture 2021, 532, 736010. [Google Scholar] [CrossRef]
- Chang, P.S.; Chen, L.J.; Wang, Y.C. The effect of ultraviolet irradiation, heat, pH, ozone, salinity and chemical disinfectants on the infectivity of white spot syndrome baculovirus. Aquaculture 1998, 166, 1–17. [Google Scholar] [CrossRef]
- Musthaq, S.S.; Sudhakaran, R.; Ahmed, V.I.; Balasubramanian, G.; Hameed, A.S. Variability in the tandem repetitive DNA sequences of white spot syndrome virus (WSSV) genome and suitability of VP28 gene to detect different isolates of WSSV from India. Aquaculture 2006, 256, 34–41. [Google Scholar] [CrossRef]
- Mathew, S.; Kumar, K.A.; Anandan, R.; Nair, P.G.V.; Devadasan, K. Changes in tissue defence system in white spot syndrome virus (WSSV) infected Penaeus monodon. Comp. Biochem. Phys. C 2007, 145, 315–320. [Google Scholar] [CrossRef]
- Jatuyosporn, T.; Supungul, P.; Tassanakajon, A.; Krusong, K. The essential role of clathrin-mediated endocytosis in yellow head virus propagation in the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2014, 44, 100–110. [Google Scholar] [CrossRef]
- Senapin, S.; Phongdara, A. Binding of shrimp cellular proteins to Taura syndrome viral capsid proteins VP1, VP2 and VP3. Virus Res. 2006, 122, 69–77. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.H.; Choi, S.K.; Jeon, H.J.; Lee, S.H.; Kim, B.K.; Kim, Y.K.; Lee, K.J.; Han, J.E. Detection of infectious white spot syndrome virus in red claw crayfish (Cherax quadricarinatus) and red swamp crayfish (Procambarus clarkii) imported into Korea. Aquaculture 2021, 544, 737117. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.R.; Álvarez-Sánchez, A.R.; Romo-Quiñonez, C.R.; Barraza, A.; Magallón-Barajas, F.J.; Chávez-Sánchez, A.; Mejía-Ruiz, C.H. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.A.; Rao, M.; Coman, G.J. Real-time PCR tests to specifically detect Infectious hypodermal and haemopoietic necrosis virus (IHHNV) lineages and an IHHNV endogenous viral element (EVE) integrated in the genome of Black Tiger shrimp (Penaeus monodon). Dis. Aquat. Org. 2018, 129, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Dong, Z.; Pang, N.Y.; Nian, Y.Y.; Yan, D.C. A novel real-time PCR approach for detection of infectious hypodermal and haematopoietic necrosis virus (IHHNV) in the freshwater crayfish Procambarus clarkii. J. Invertebr. Pathol. 2018, 157, 100–103. [Google Scholar] [CrossRef]
- Coelho-Melo, M.V.; Florindo-Guedes, M.I.; Rodriguez-Málaga, S.; de Almeida, L.M.; de Freitas Moreira, M.; de Oliveira, T.R. Molecular characterization of Infectious Myonecrosis Virus (IMNV) isolated from the shrimp Litopenaeus vannamei farmed in Ceará state, Brazil. Lat. Am. J. Aquat. Res. 2014, 42, 649–652. [Google Scholar] [CrossRef]
- Hamano, K.; Miyoshi, T.; Aue-umneoy, D.; Srisapoome, P.; Maeno, Y.; Tsutsui, I. Waterborne and cannibalism-mediated transmission of the Yellow head virus in Penaeus monodon. Aquaculture 2015, 437, 161–166. [Google Scholar] [CrossRef]
- Noble, T.H.; Coman, G.J.; Cowley, J.A.; Wade, N.; Sellars, M.J.; Jerry, D.R. Comparison of methods for uniformly challenging Black Tiger shrimp (Penaeus monodon) with gill-associated virus. Aquaculture 2017, 473, 191–196. [Google Scholar] [CrossRef]
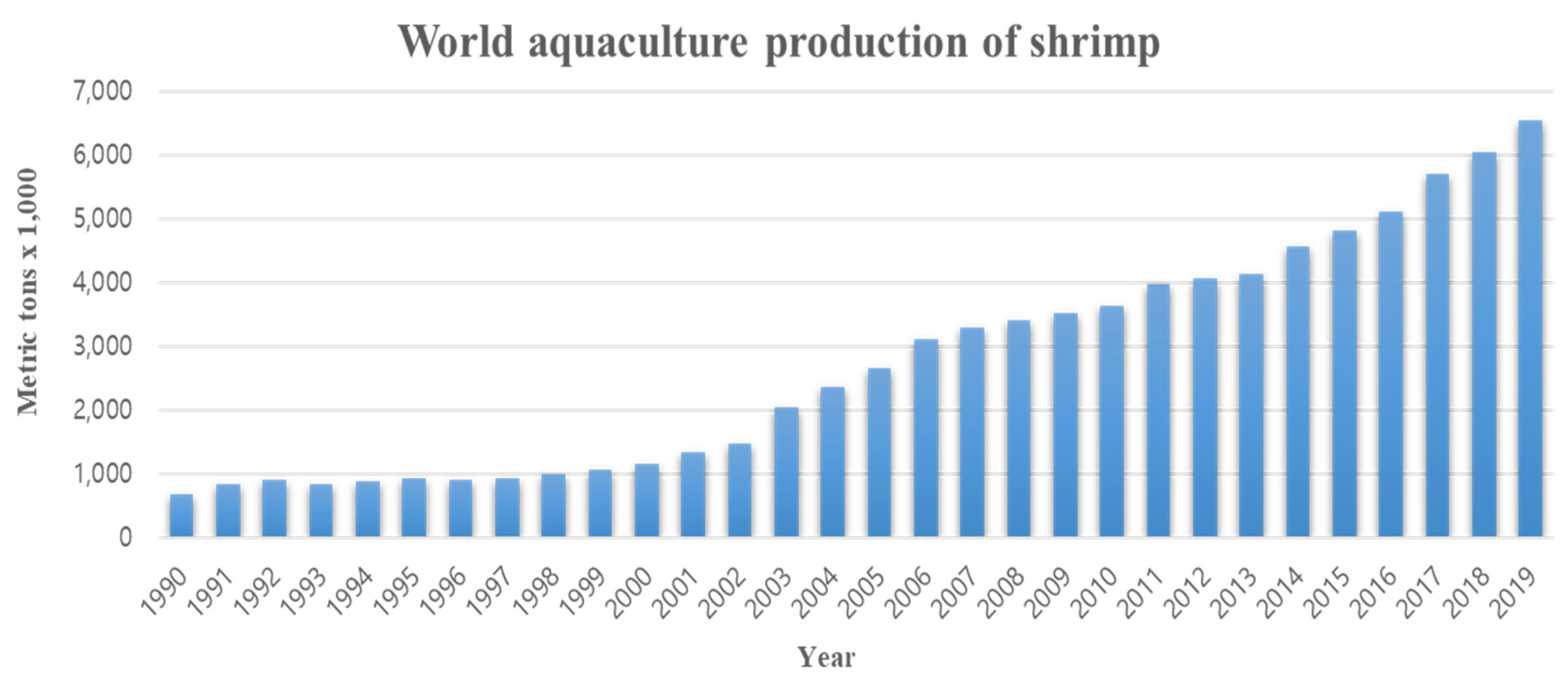
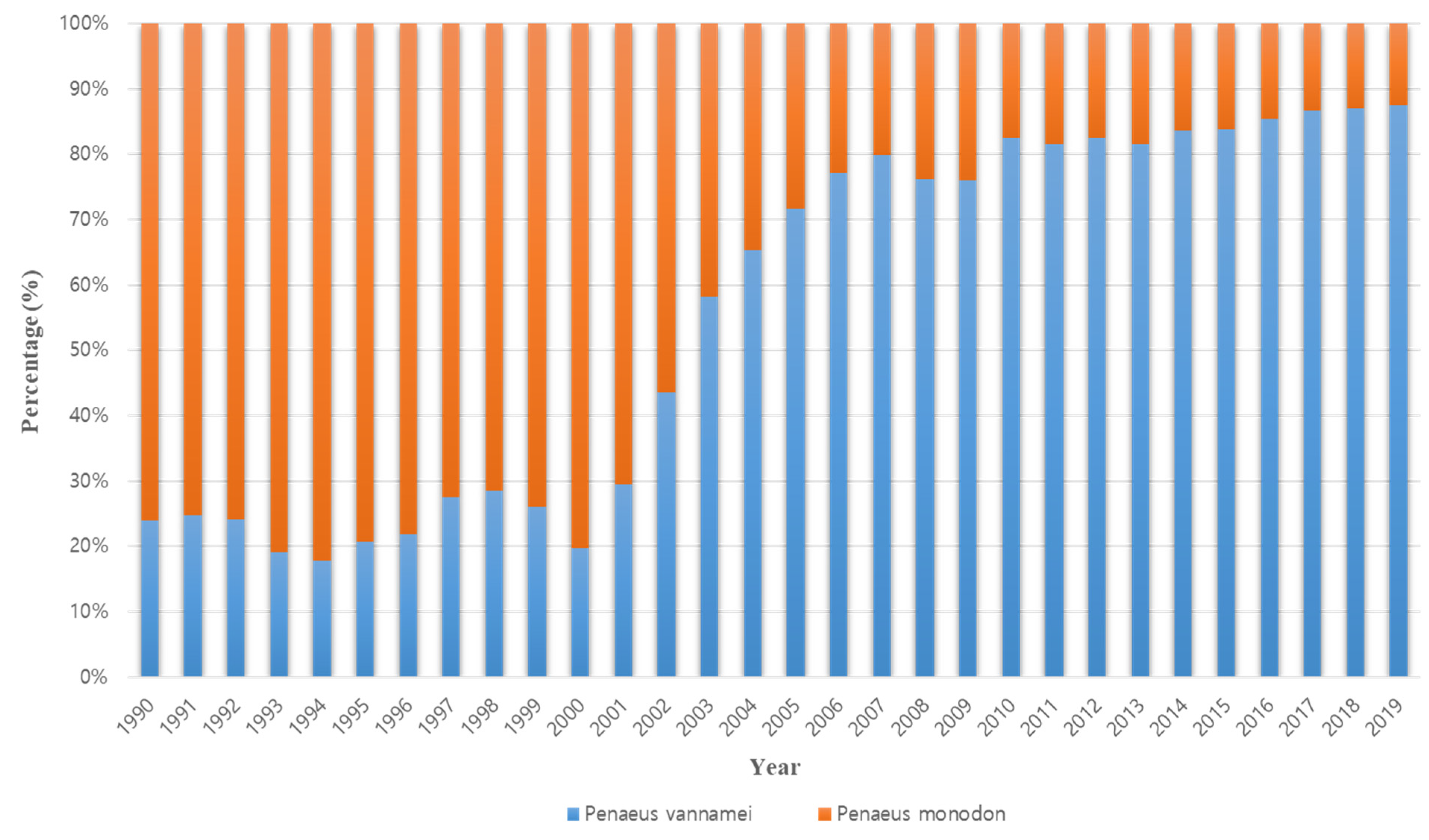
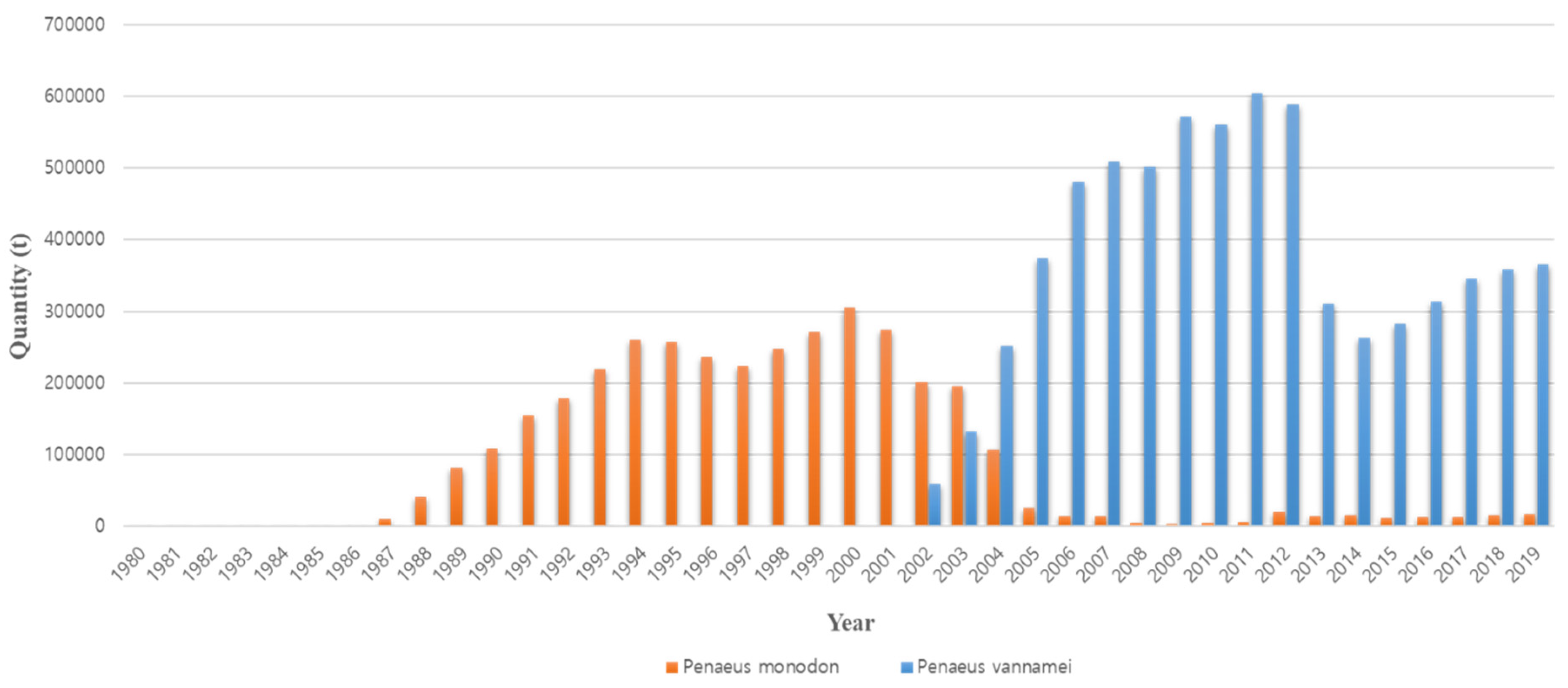
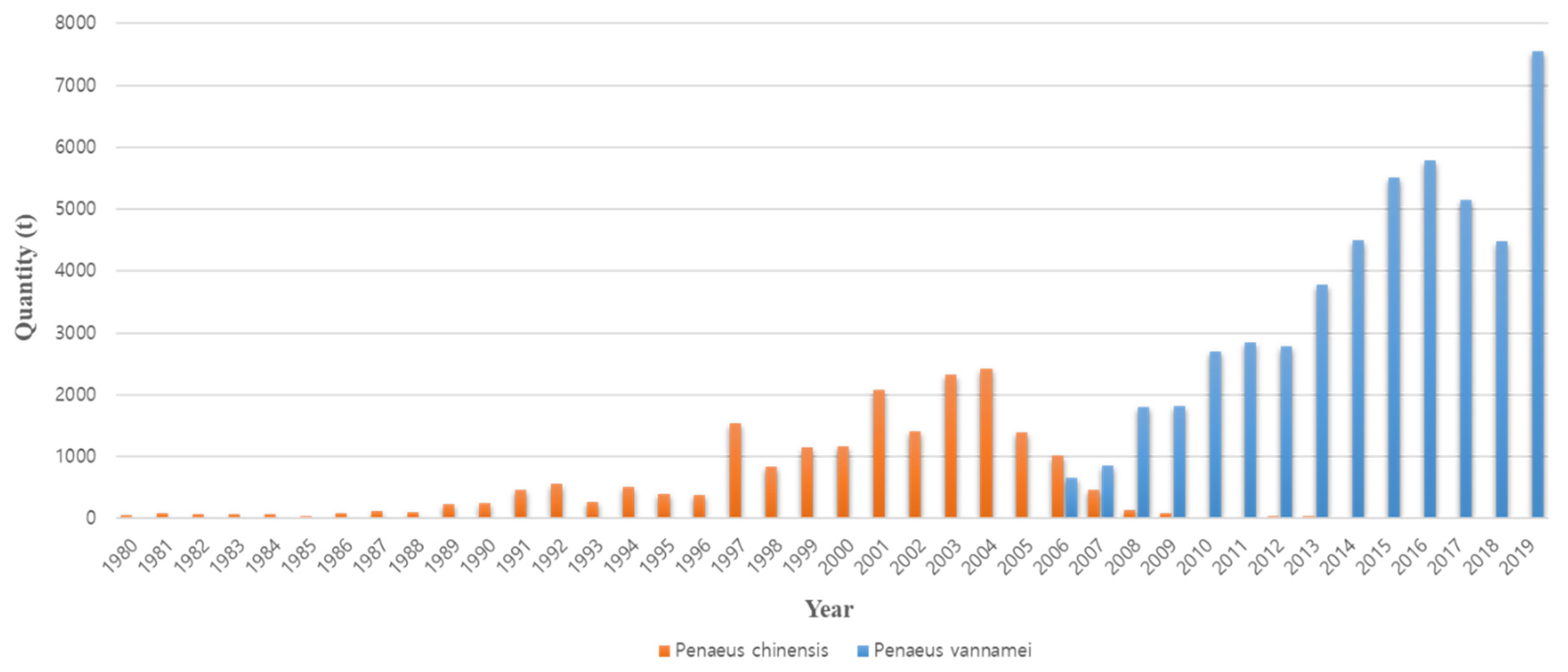
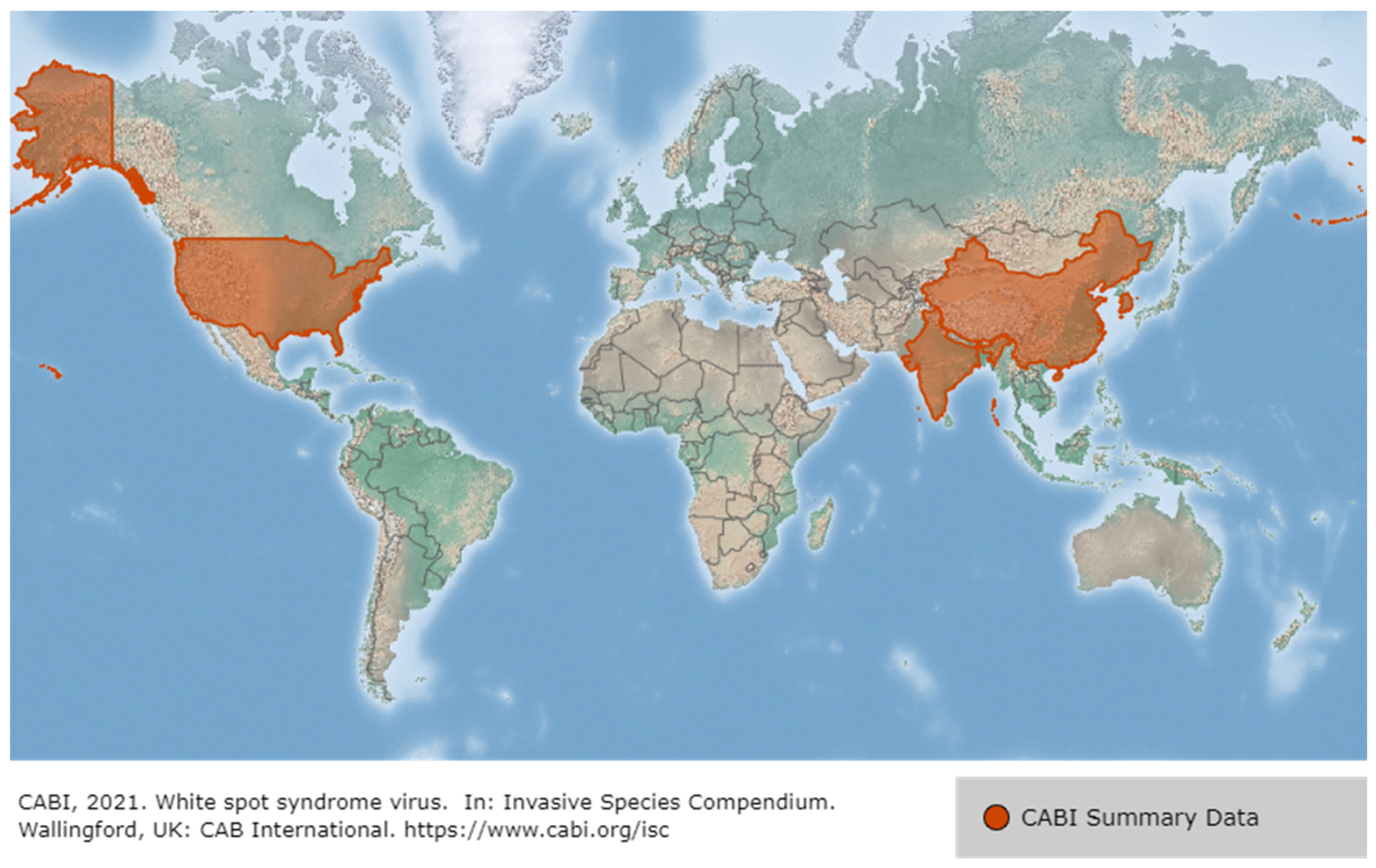
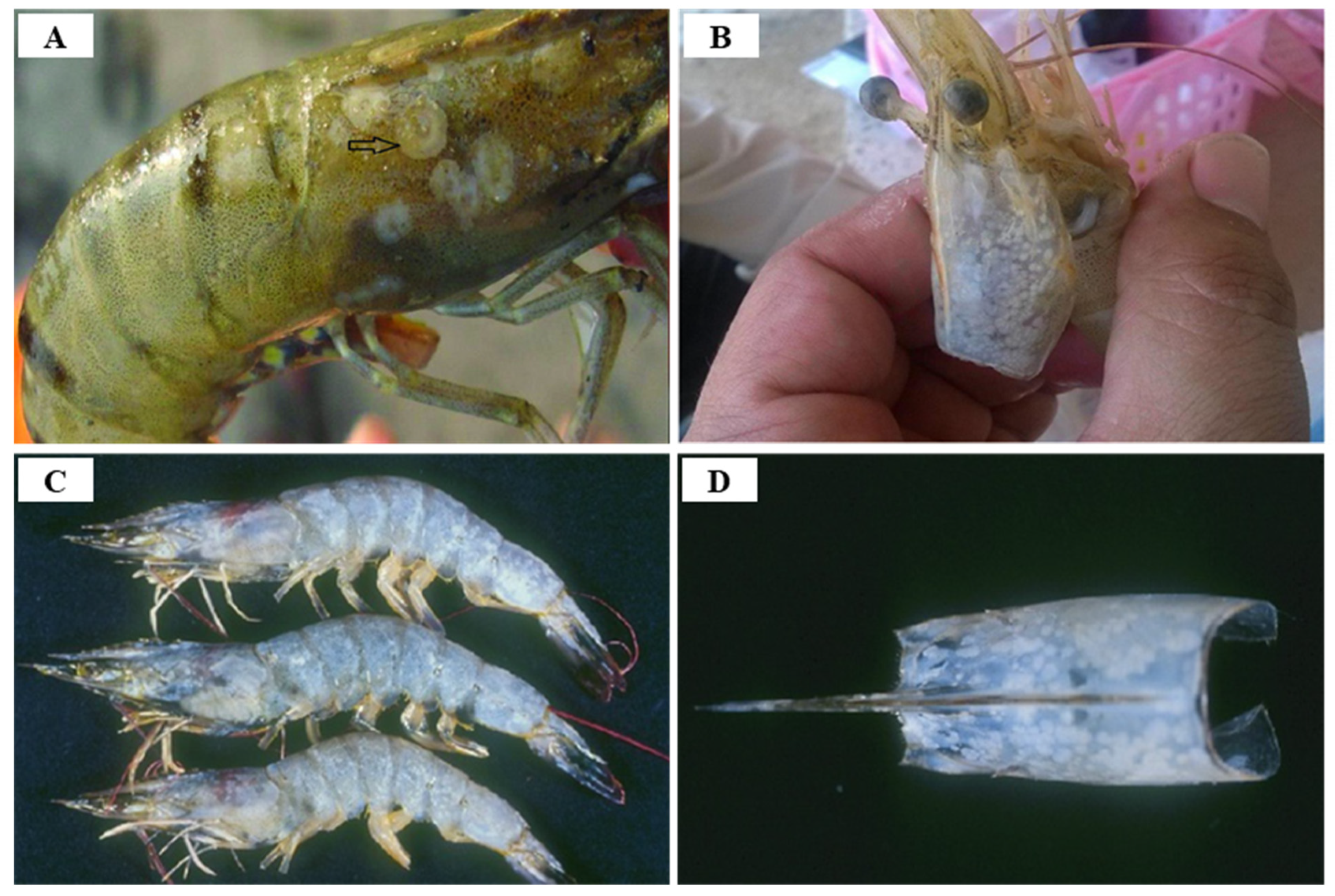
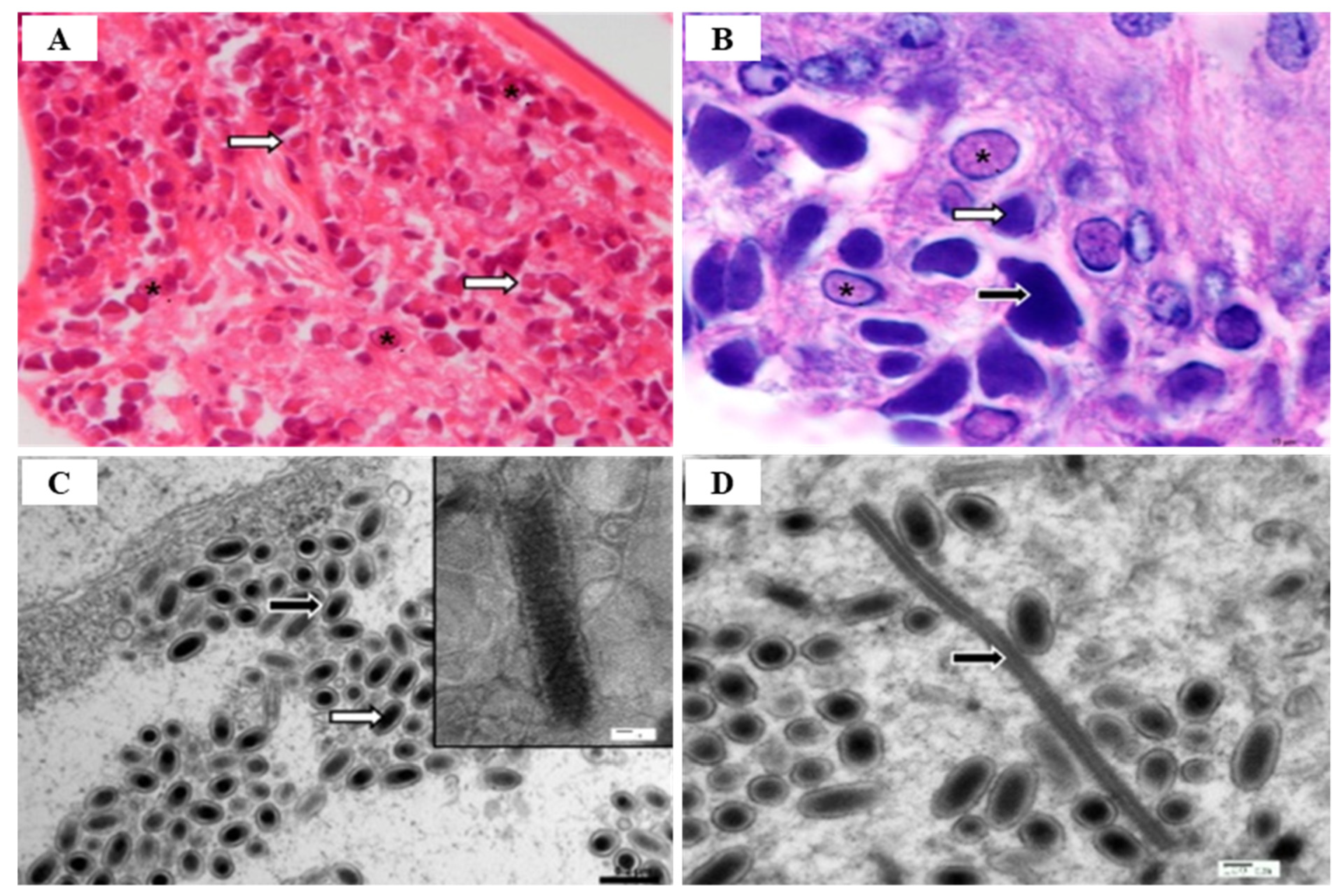
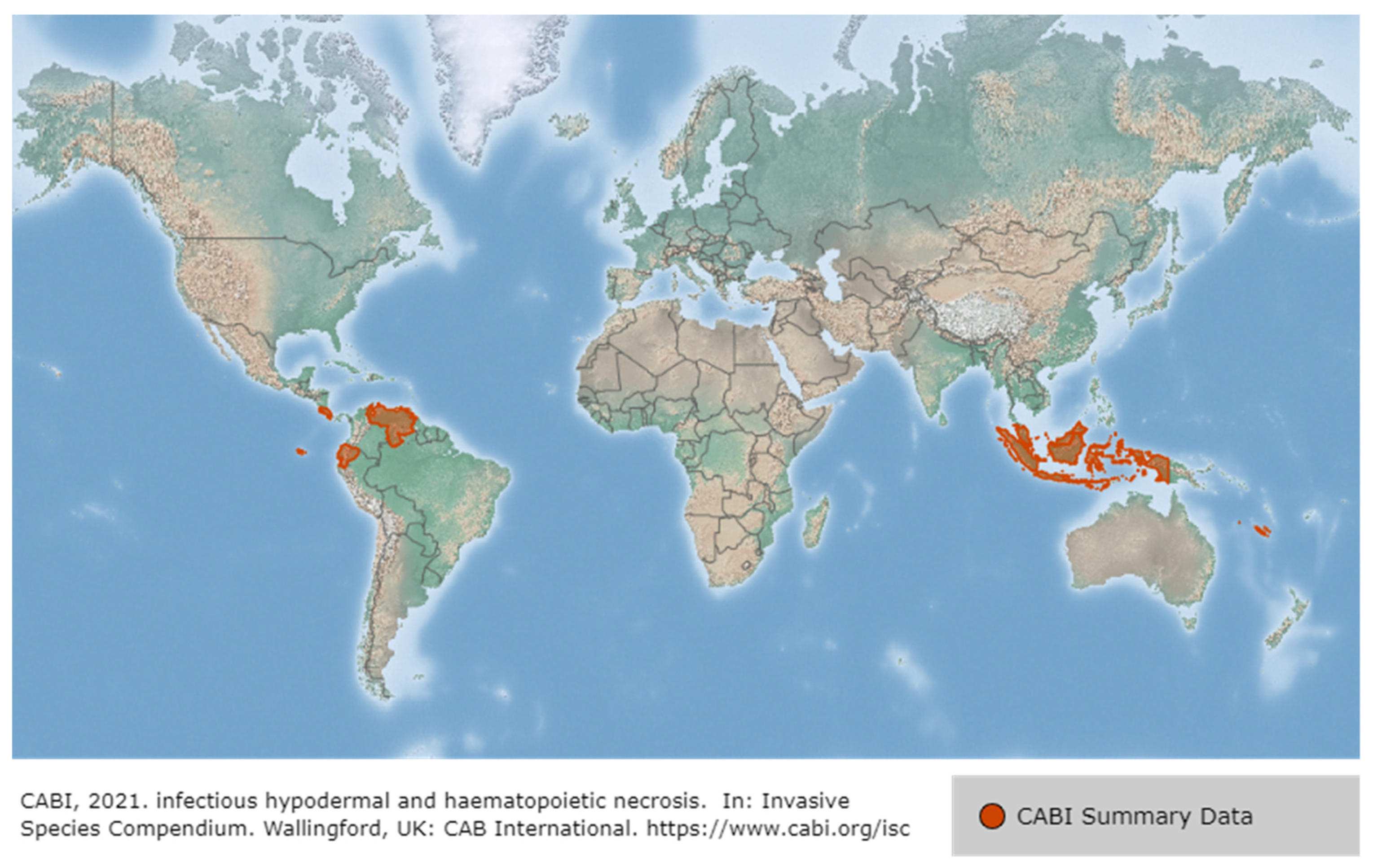

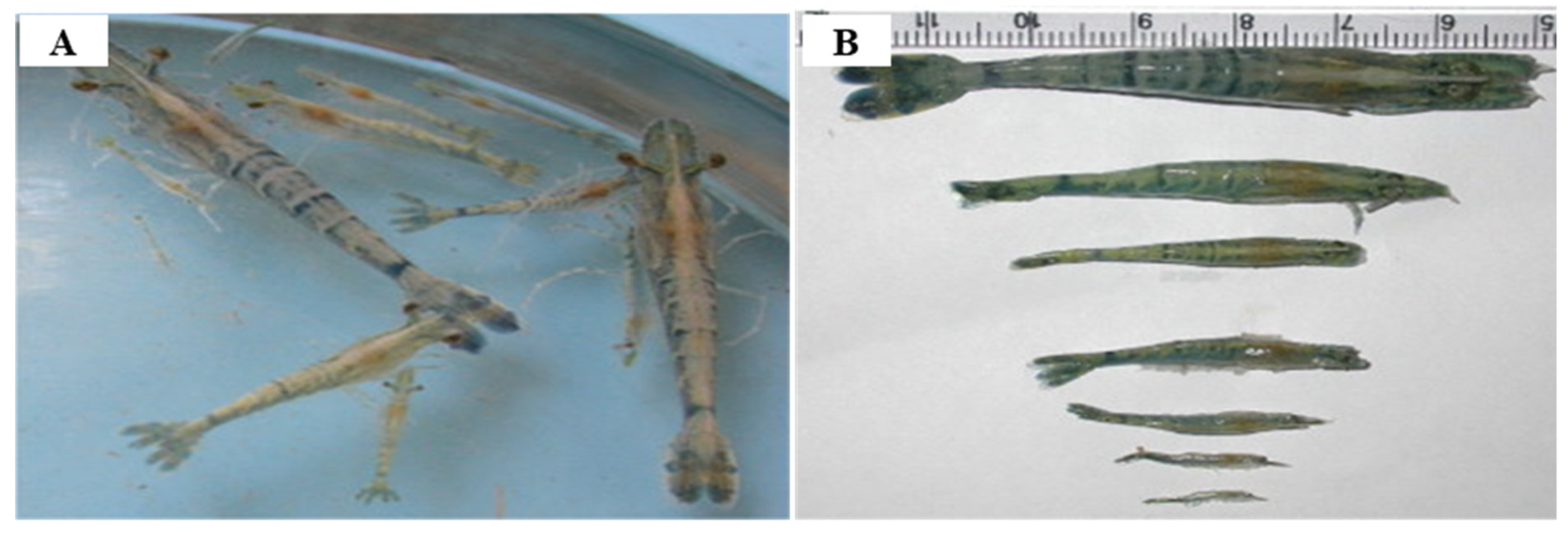
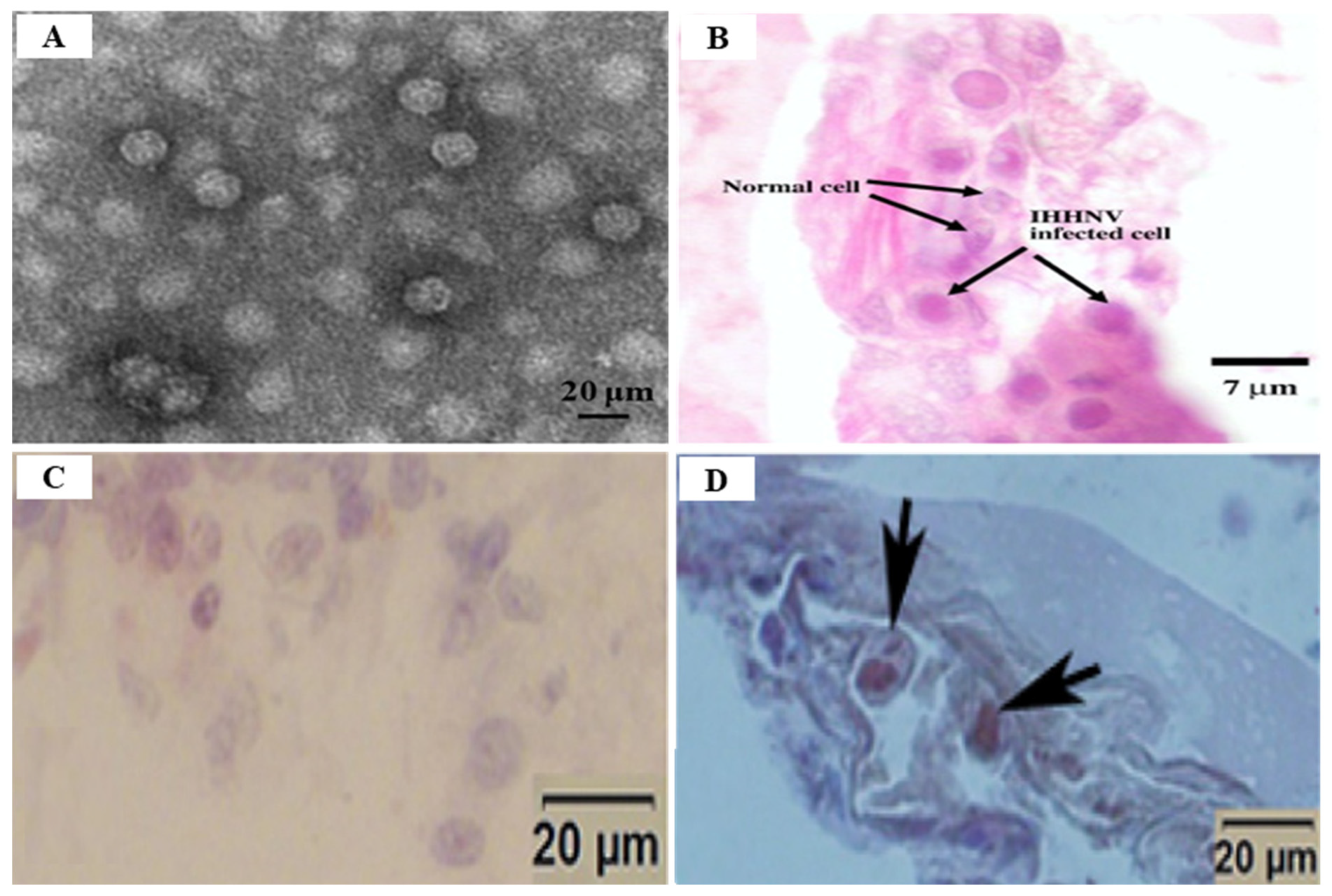
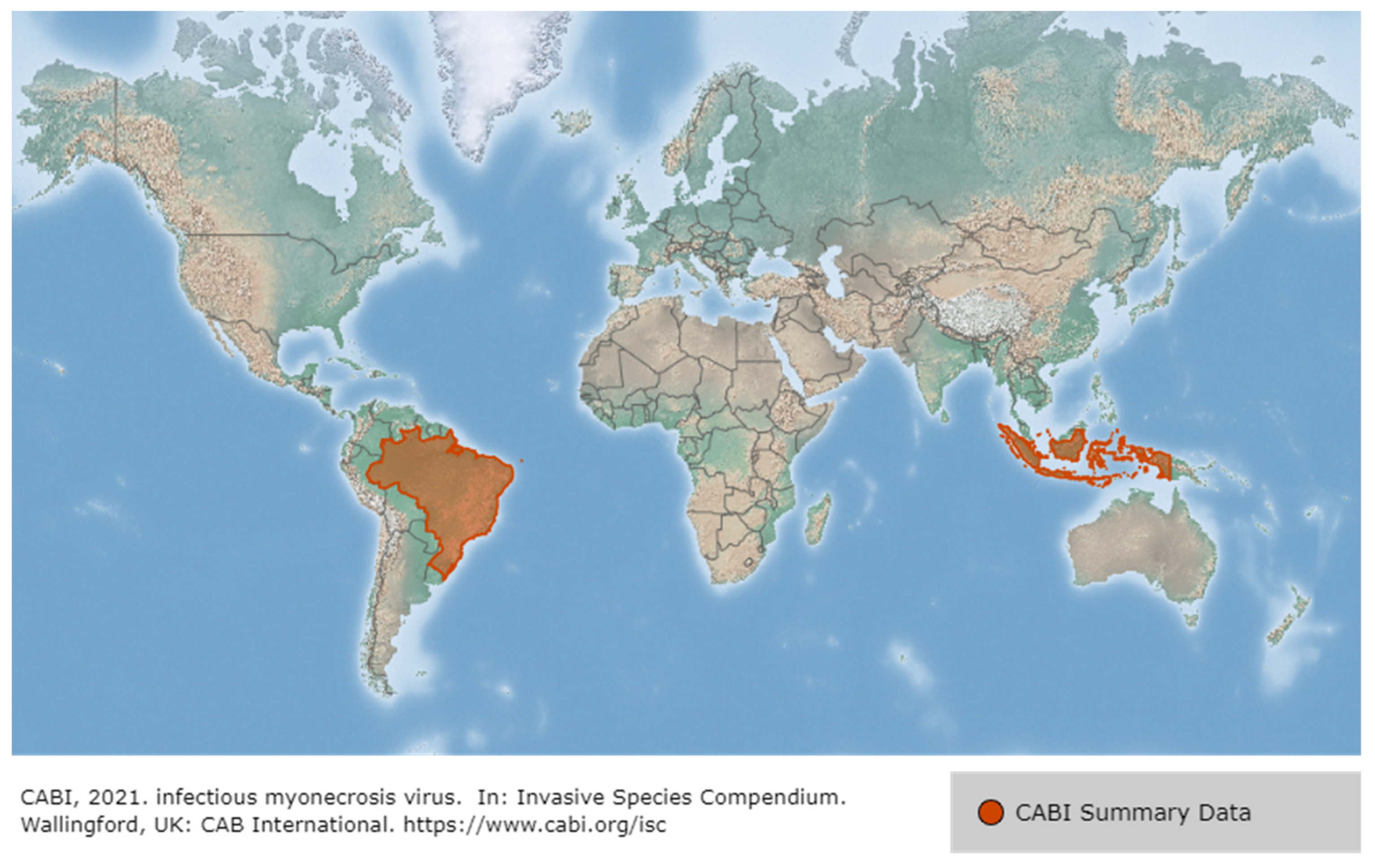
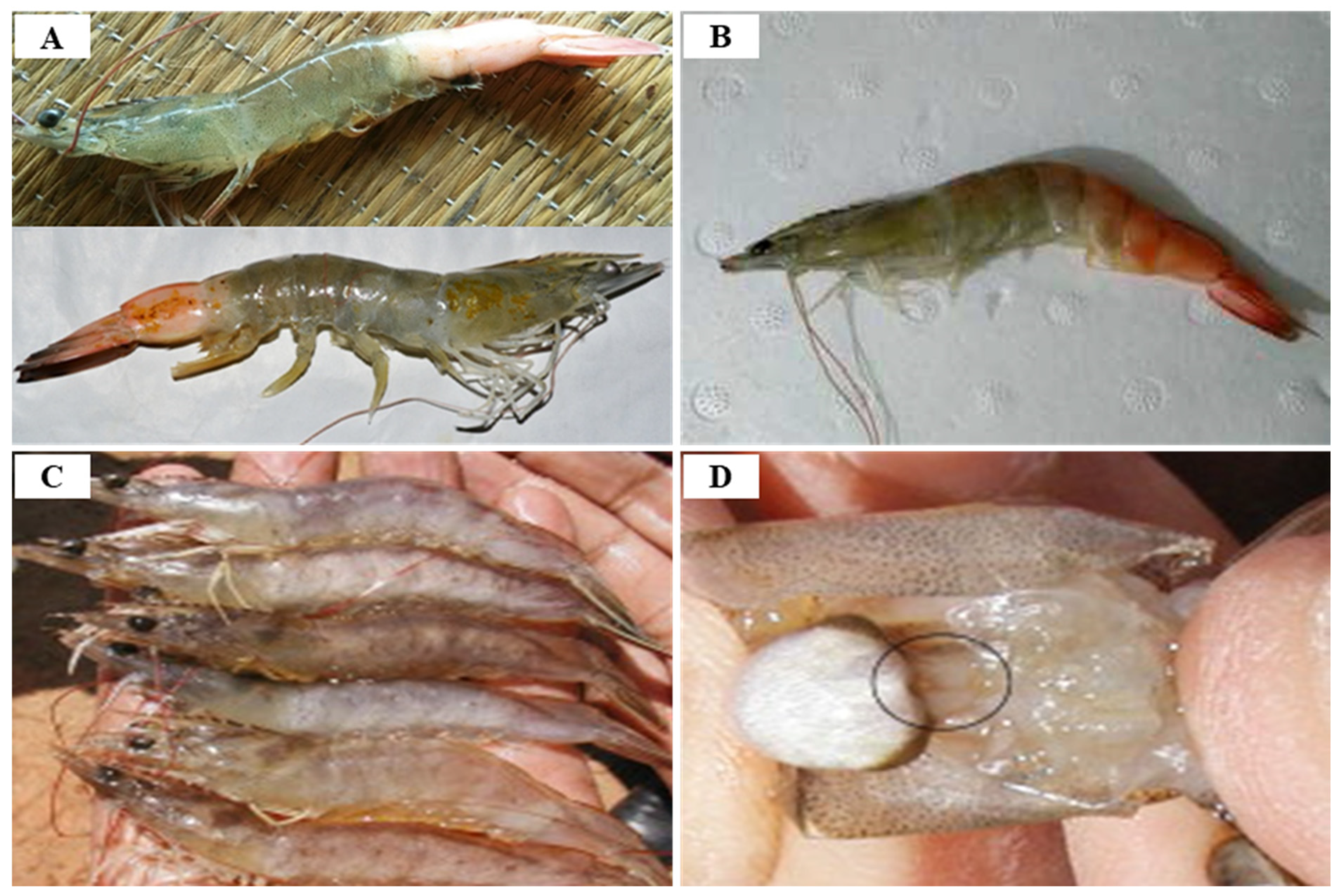

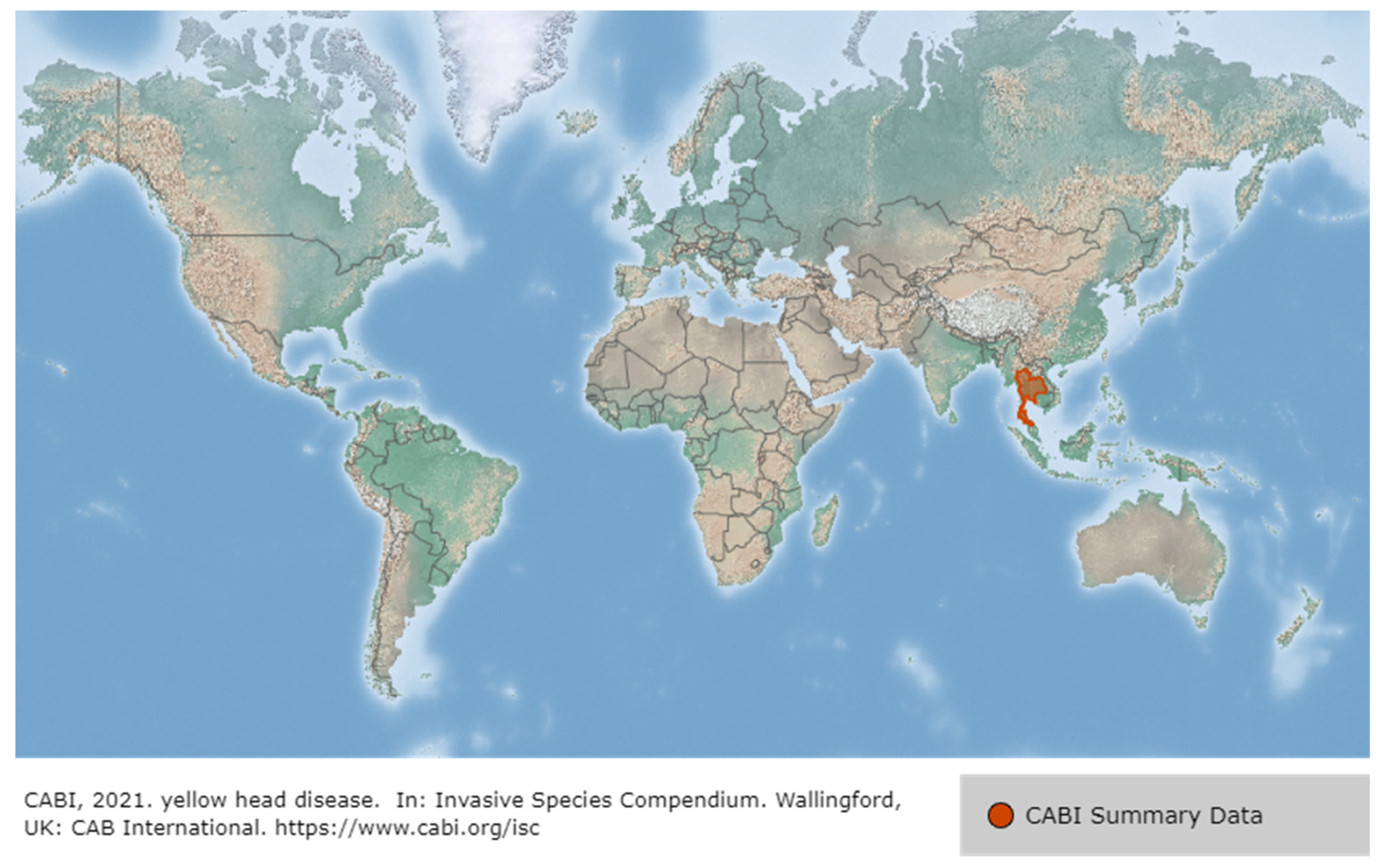
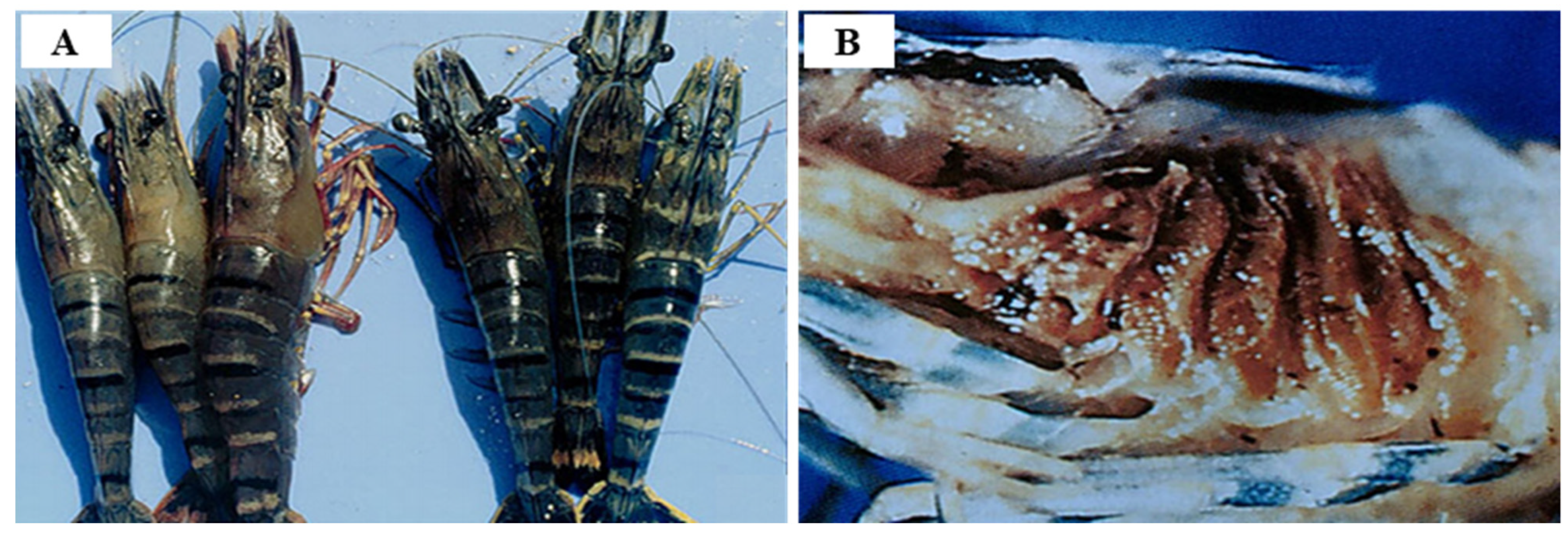
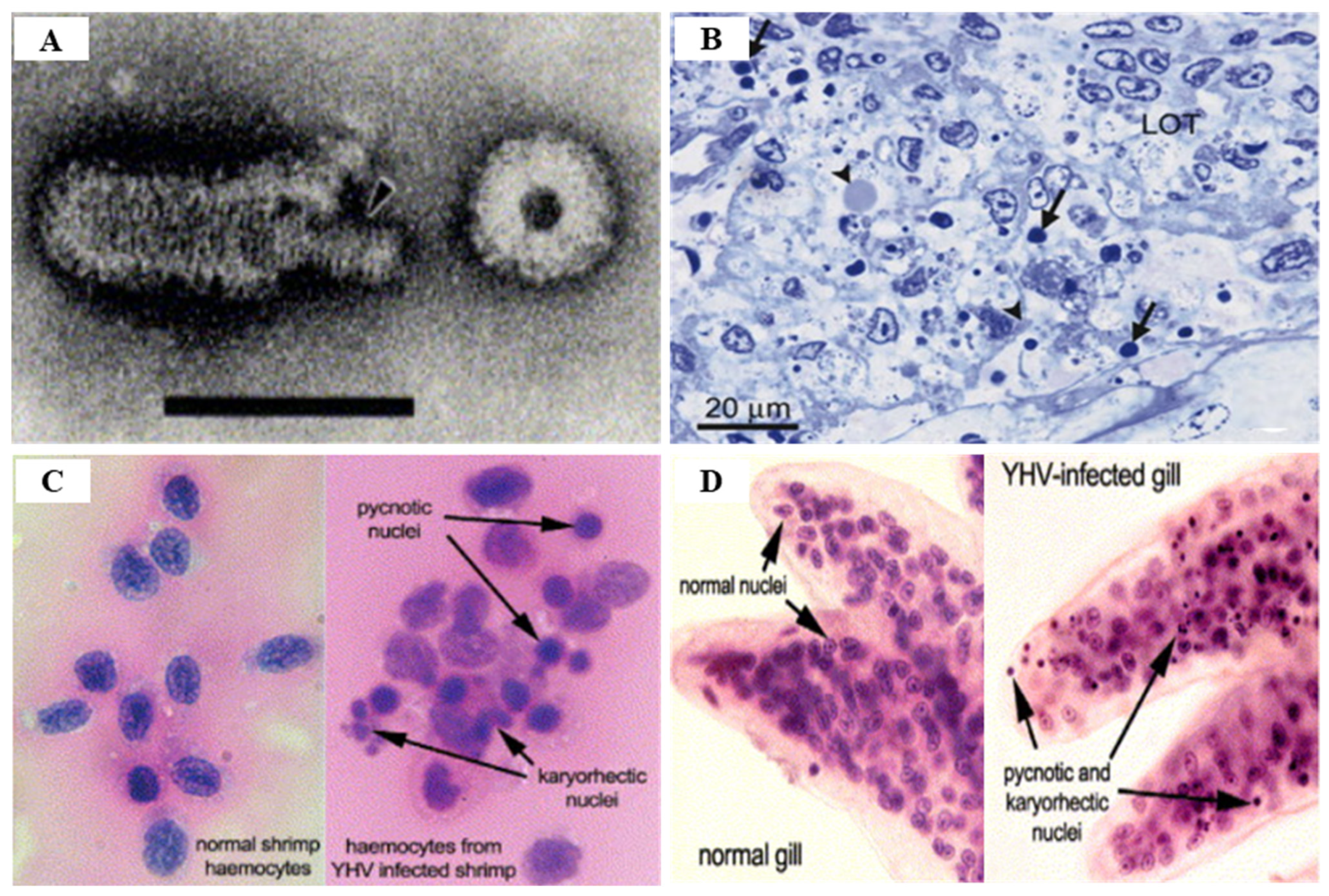
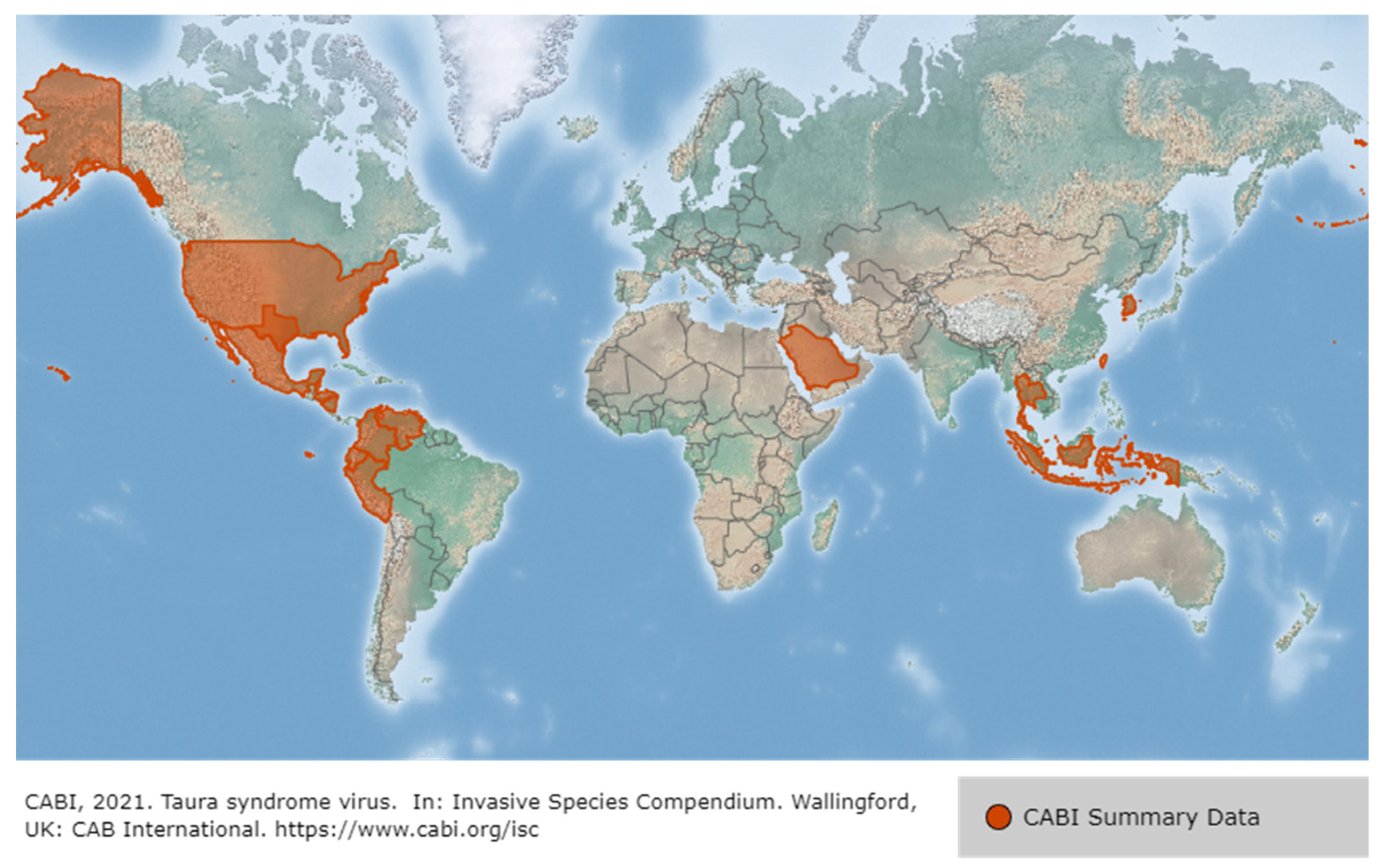
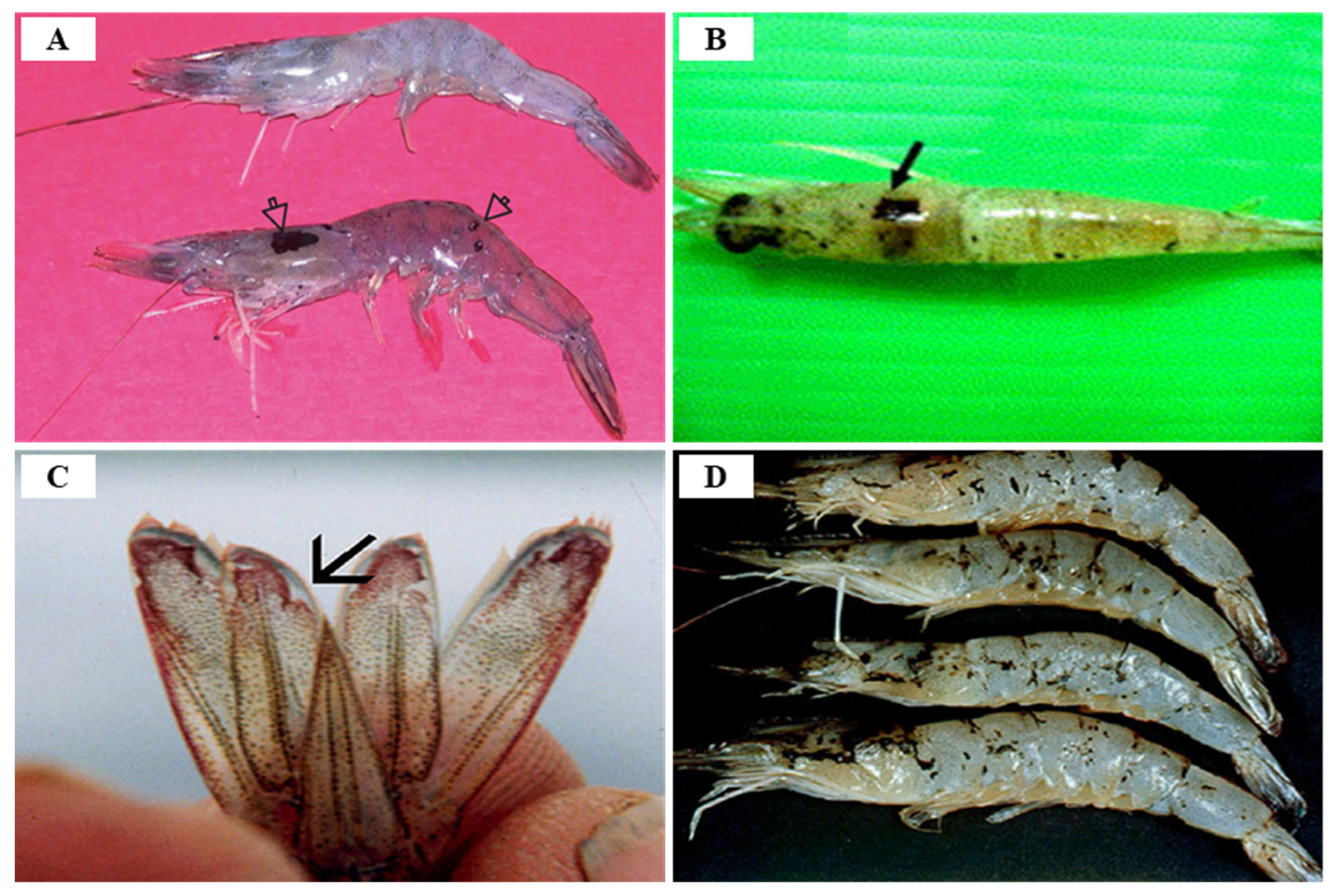
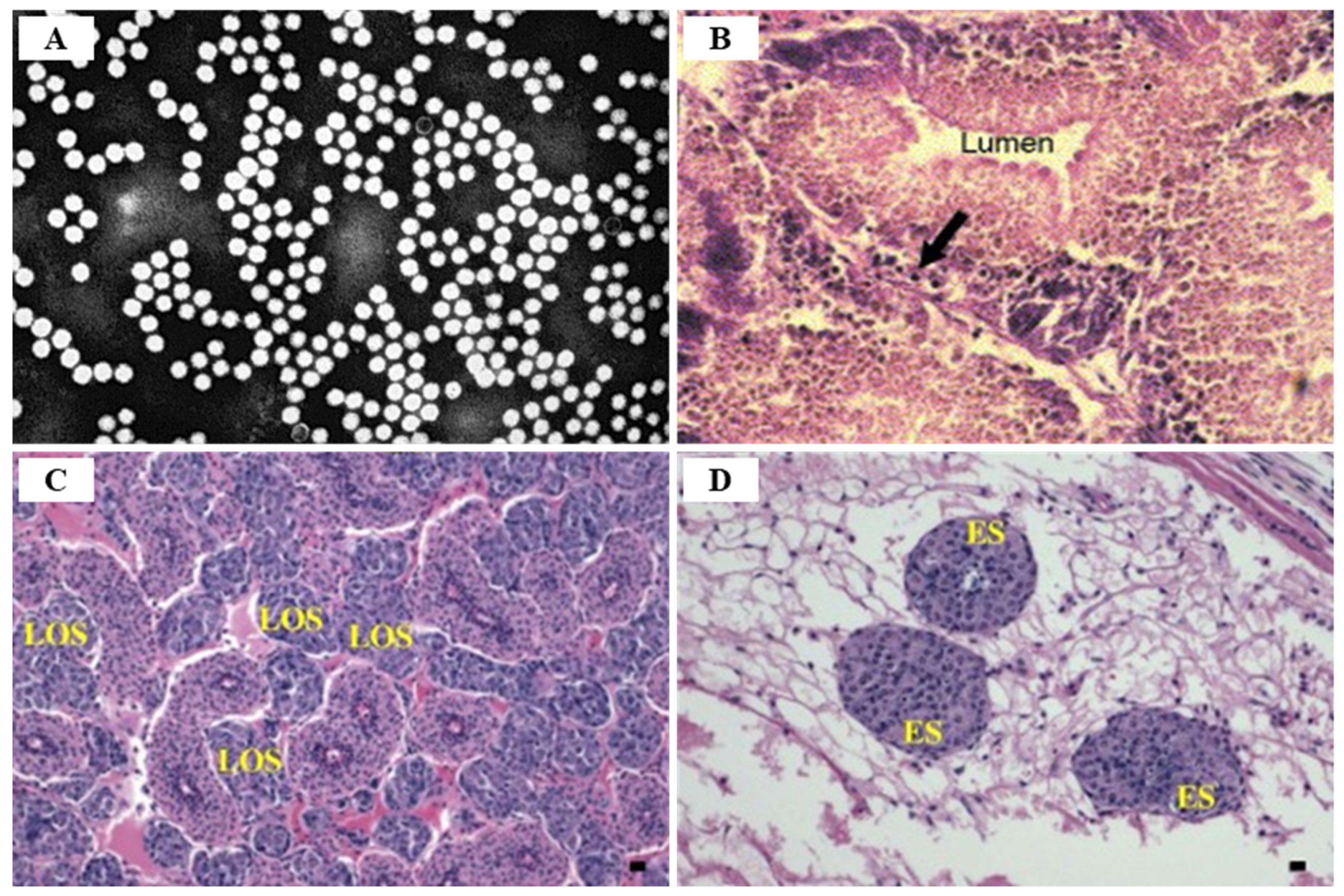
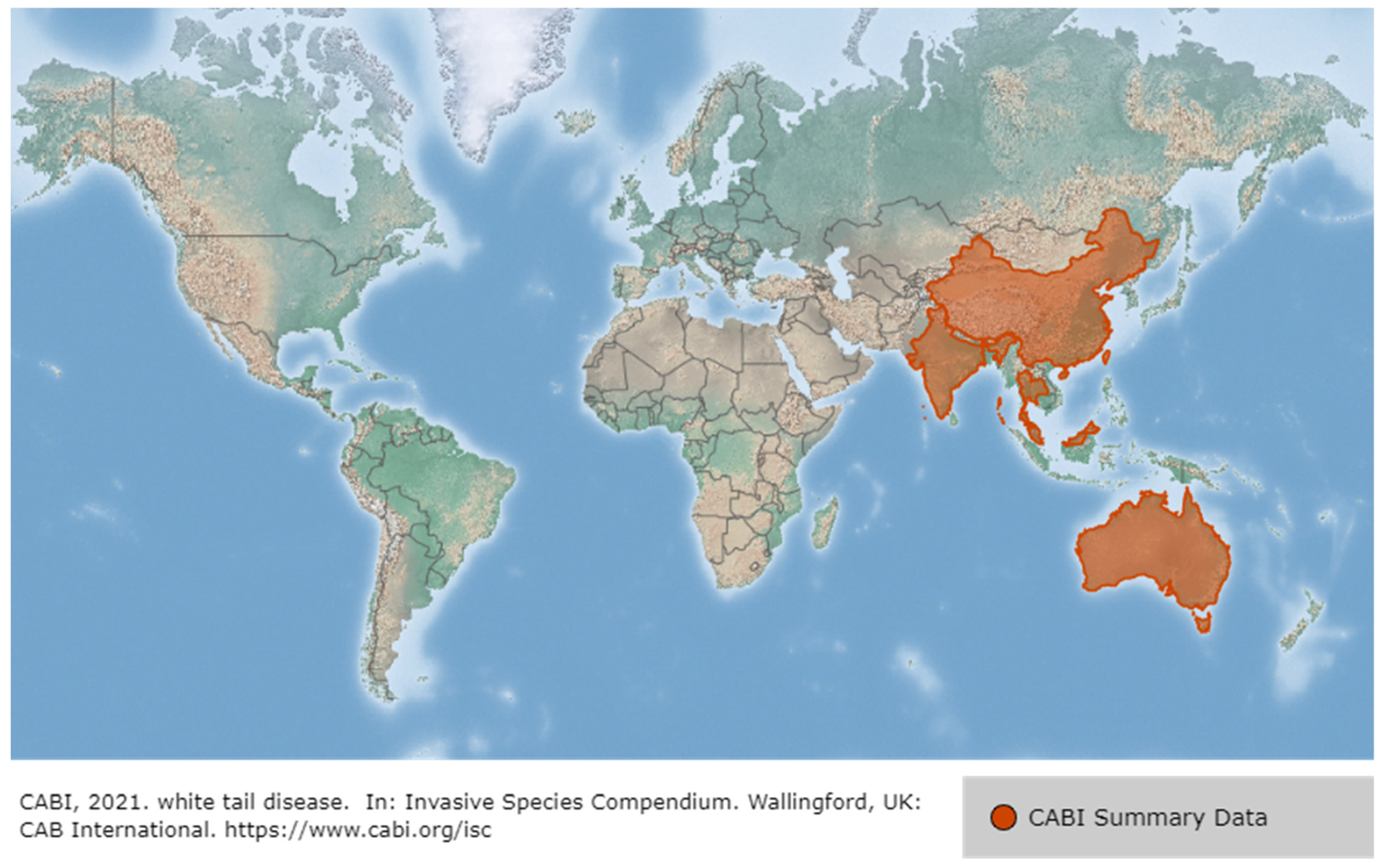
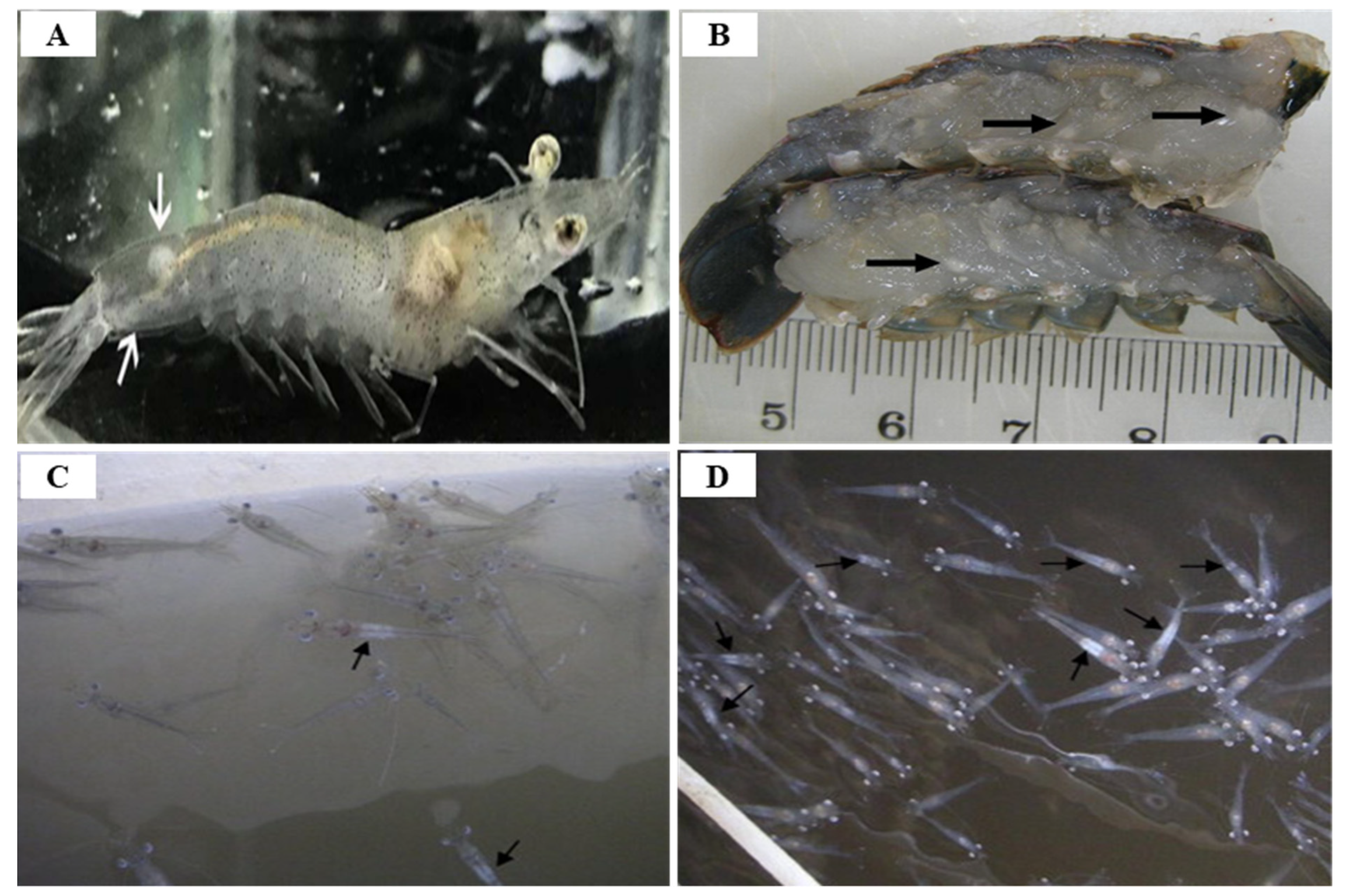
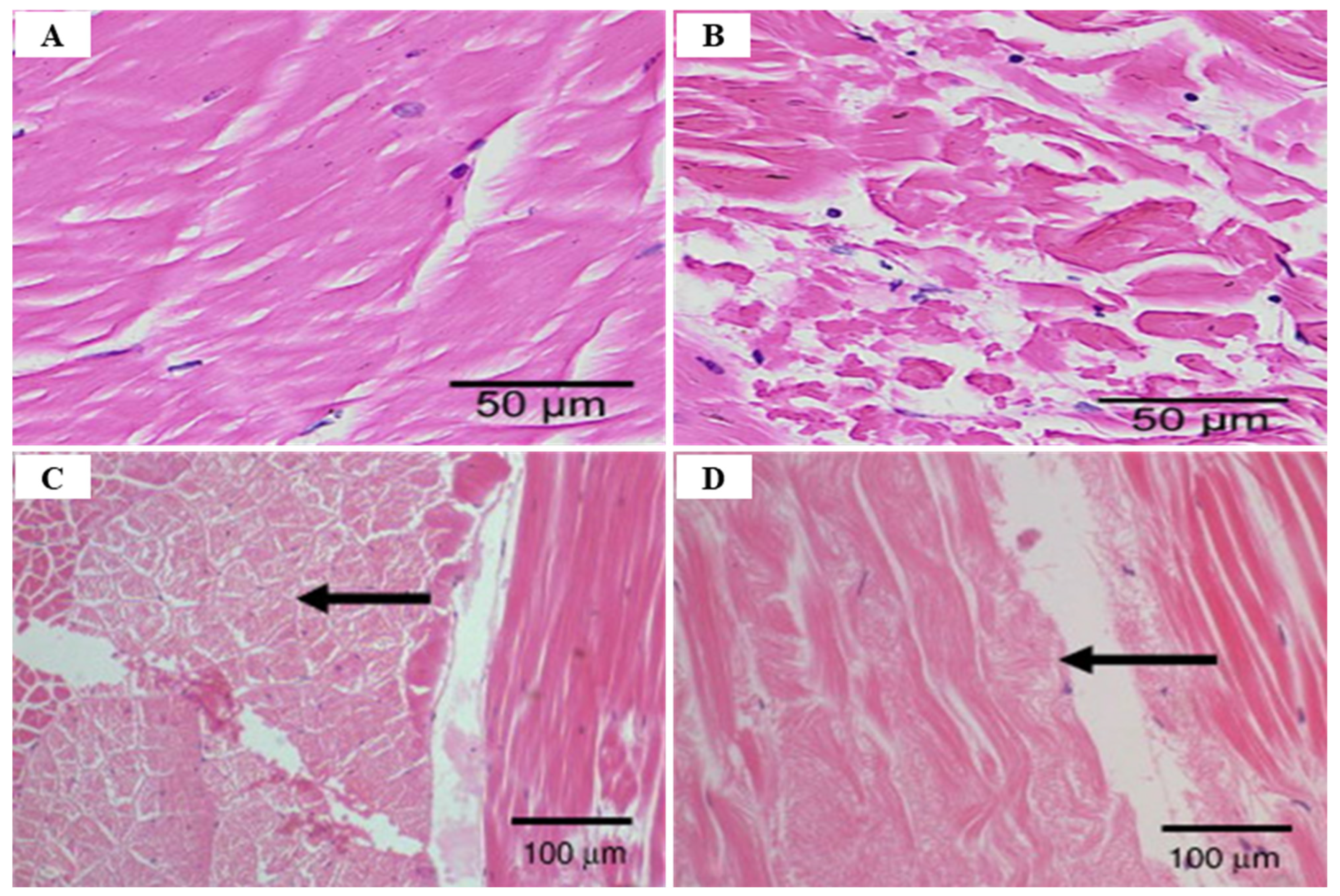
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Yu, Y.-B.; Choi, J.-H.; Jo, A.-H.; Hong, S.-M.; Kang, J.-C.; Kim, J.-H. Viral Shrimp Diseases Listed by the OIE: A Review. Viruses 2022, 14, 585. https://doi.org/10.3390/v14030585
Lee D, Yu Y-B, Choi J-H, Jo A-H, Hong S-M, Kang J-C, Kim J-H. Viral Shrimp Diseases Listed by the OIE: A Review. Viruses. 2022; 14(3):585. https://doi.org/10.3390/v14030585
Chicago/Turabian StyleLee, Dain, Young-Bin Yu, Jae-Ho Choi, A-Hyun Jo, Su-Min Hong, Ju-Chan Kang, and Jun-Hwan Kim. 2022. "Viral Shrimp Diseases Listed by the OIE: A Review" Viruses 14, no. 3: 585. https://doi.org/10.3390/v14030585
APA StyleLee, D., Yu, Y.-B., Choi, J.-H., Jo, A.-H., Hong, S.-M., Kang, J.-C., & Kim, J.-H. (2022). Viral Shrimp Diseases Listed by the OIE: A Review. Viruses, 14(3), 585. https://doi.org/10.3390/v14030585







