Expression Profile and Biological Role of Immune Checkpoints in Disease Progression of HIV/SIV Infection
Abstract
1. Introduction
2. HIV Infection Upregulates the Expression of Immune Checkpoints in T Cells
| IC Marker | Healthy Controls | HIV-Infected Individuals | Long-Term Nonprogressors | Viremic Individuals | ART-Treated HIV-Infected Individuals | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | CD4+ T Cells | CD8+ T Cells | |
| PD-1 | 5–20% [9,10,11,12] RM: 12% [29] | 5–23% [9,10,12,19,21,23,30] | 10% [10] | 10% [10] | 6–21% [9,11,16,31] | 11–20% [9,31] | 14–50% [9,11,12,14,15,16,31] RM: 6% [29] | 21–50% [9,12,14,15,19,20,21,23,30,31] | 10–35% [9,11,12,15,17,18] RM: 10% [29] | 10–30% [9,11,12,15,18,21,23,30] |
| TIGIT | 10–18% [10,11,32] | 30% [10,32,33] RM: 20% [32] | 20% [10] | 50% [10] RM: 40% [32] | 18–19% [11,32] | 50–57% [32,33] | 21–30% [11,32] | 65–70% [32,33] | 17–20% [11,17,32] | 45–60% [32,33] |
| CTLA-4 | 1–6% [11,12,34,35] RM: 4% [29] | 1–2% [12,35] | 6%-11% [34,35] | 1% [35] | 3–7% [11,16,31] | 0.67% [31] | 2–10% [11,12,16,31] RM: 2% [29] | 0.37, 10–12% [12] | 1–8% [11,12,34] RM: 1% [29] | 5% [12] |
| LAG-3 | 6% [36] RM: 9% [36] | 5–7% [30,36] RM: 9% [36] | N/A | N/A | 0.065% [31] | 0.047% [31] | 0.021,14–48% [14,31,36] RM: 12% [36] | 0.004,8–32% [14,30,31,36] RM: 15% [36] | 12% [17] | 4% [30] |
| Tim-3 | 3–15% [10,37] RM: 1% [38] | 5–29% [10,37] RM: 5% [38] | 5% [10] | 5% [10] | 22% [37] | 30% [37] | 30–41% [14,31,37] RM: 2% [38] | 14–59% [14,20,37] RM: 5% [38] | 0.8% [17] | N/A |
| CD160 | 3% [10] | 1–10% [10,21,30,39] | 12% [10] | 20% [10] | N/A | N/A | N/A | 15–40% [21,30,39] | 1.1% [17] | 10–16% [21,30] |
| 2B4 | 3% [10] | 40–57% [10,21,30] | 10% [10] | 65% [10] | 5% [31] | 70% [31] | 10% [31] | 75–85% [21,30,31] | 9.5% [17] | 60–70% [21,30] |
| BTLA | 85% [24] | 69% [24] | N/A | N/A | 75% [24] | 42% [24] | 60% [24] | 21% [24] | N/A | N/A |
| VISTA | 3% [10] | 5% [10] | 8% [10] | 10% [10] | N/A | N/A | N/A | N/A | N/A | N/A |
3. Immune Checkpoints Are Associated with Progression of Disease in HIV/SIV Infection
3.1. Correlation between ICs from CD28 Superfamily and Progression of Disease in HIV/SIV Infection
3.2. Correlation between ICs from CD4 Family Members and Disease Progression in HIV Infection
3.3. Correlation between TIGIT and Disease Progression in HIV/SIV Infection
3.4. Correlation between Tim-3 and Disease Progression in HIV/SIV Infection
3.5. Correlation between CD160 and Disease Progression in HIV/SIV Infection
3.6. Correlation between Co-Expression of Checkpoint Markers and Disease Progression in HIV Infection
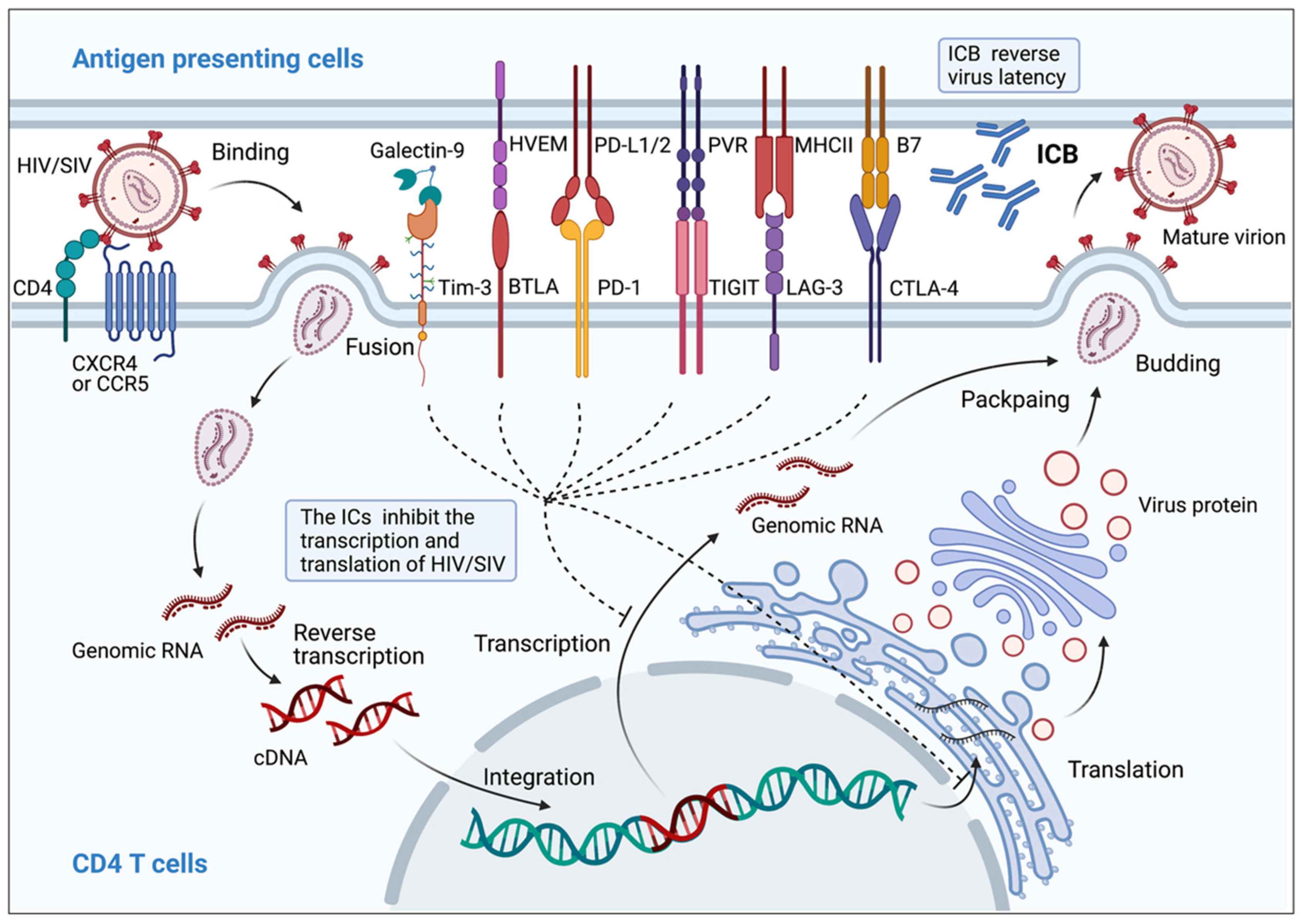
3.7. Correlation between Immune Checkpoint Markers and HIV/SIV Latency
| IC Marker | Function Mediated by HIV/SIV Infection | |||
|---|---|---|---|---|
| CD4+ T Cell | CD8+ T Cell | |||
| PD-1 | Proliferation |
| Proliferation | |
| Cytokine secretion |
| Cytokine secretion | ||
| Virus reservoirs |
| |||
| TIGIT | Virus reservoirs | Proliferation | ||
| Cytokine secretion |
| |||
| CTLA-4 | Proliferation |
| Cytokine secretion |
|
| Cytokine secretion |
| |||
| Virus reservoirs | ||||
| LAG-3 | Proliferation |
| Proliferation |
|
| Cytokine secretion |
| Cytokine secretion |
| |
| Virus reservoirs | ||||
| Tim-3 | Proliferation | Proliferation | ||
| Cytokine secretion |
| Cytokine secretion | ||
| Virus reservoirs | ||||
| CD160 | N/A | Proliferation | ||
| Cytokine secretion | ||||
| 2B4 | Cytokine secretion |
| Proliferation |
|
| Cytokine secretion |
| |||
| BTLA | Cytokine secretion |
| N/A | |
| Virus reservoirs |
| |||
| VISTA | Cytokine secretion |
| Cytokine secretion |
|
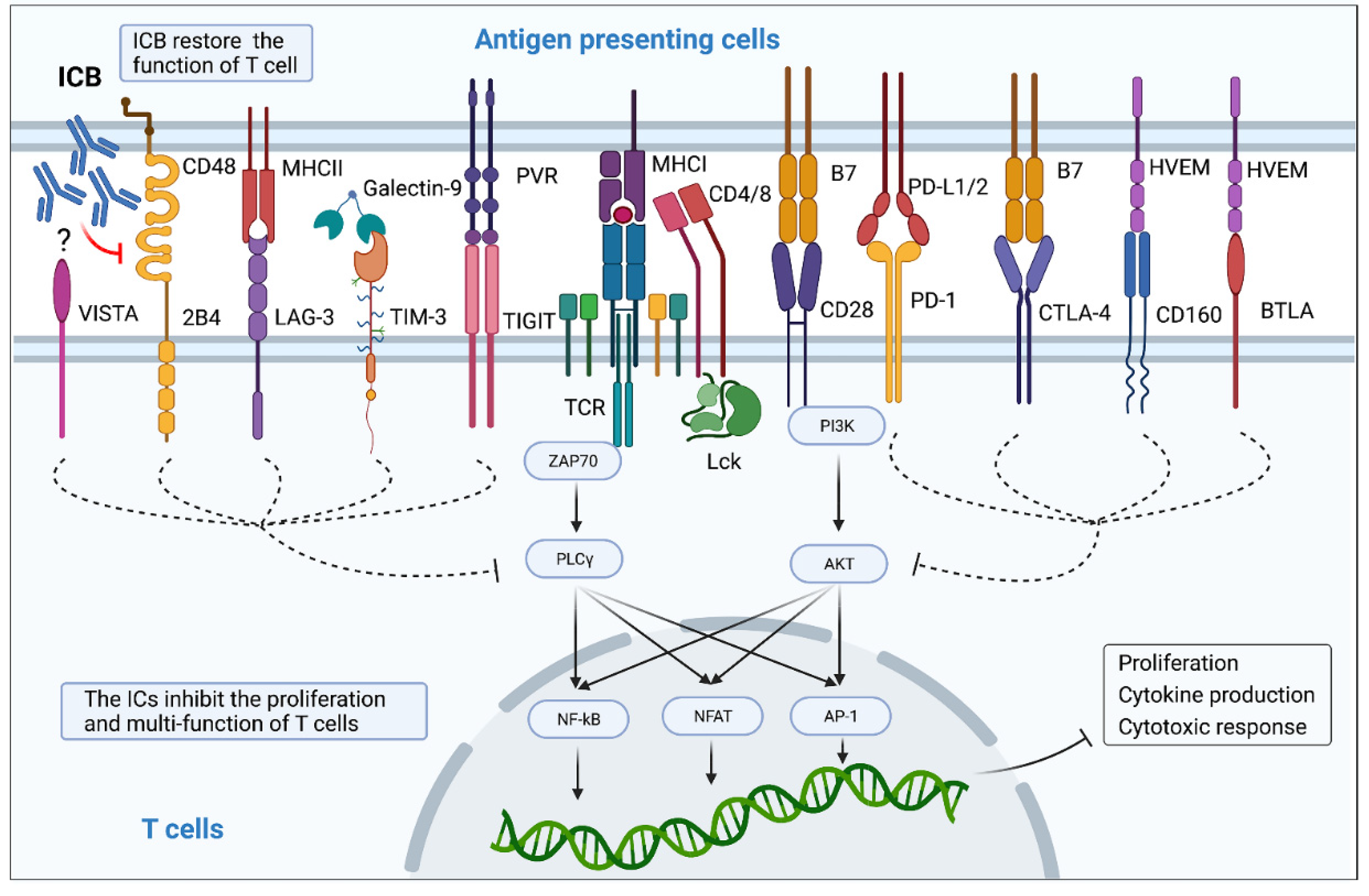
4. Therapeutic Effects of Immune Checkpoint Blockers on HIV/SIV Infection
4.1. Immune Checkpoint Blockers Confer Partial Protection against Progression of HIV Infection
| Reference | IC blocker | Target | Objective | Treatment | Outcomes |
|---|---|---|---|---|---|
| Oscar Blanch-Lombarte [78] | Pembrolizumab | PD-1 | ART HIV-1-infected individual with metastatic melanoma | Pembrolizumab (2 mg/kg/3 weeks) |
|
| Vanessa A Evans [67] | Nivolumab | PD-1 | ART HIV-infected individual with metastatic melanoma | Single intravenous infusion of nivolumab (3 mg/kg) |
|
| Jillian S.Y. Lau [79] | Nivolumab Ipilimumab | PD-1 CTLA-4 | ART HIV-infected individual with metastatic melanoma | Ipilimumab (1 mg/kg/3 weeks) and Nivolumab (3 mg/kg/3 weeks) |
|
| Fiona Wightman [77] | Ipilimumab | CTLA-4 | ART HIV-infected individual with metastatic melanoma | Ipilimumab (3 mg/kg, four doses/3 week) |
|
| A Guihot [80] | Nivolumab | PD-1 | ART HIV-infected individual with NSCLC | Nivolumab (15 injections/14 days) |
|
| M Hentrich [81] | Nivolumab | PD-1 | ART HIV-infected individual with NSCLC | Chemoradiotherapy and surgical resection Nivolumab (3 mg/kg) |
|
| Brennan McCullar [82] | Nivolumab | PD-1 | ART HIV-infected individual with NSCLC | One cycle of carboplatin/paclitaxel Definitive chemo-radiation with cisplatin and etoposide Start nivolumab |
|
| Gwenaëlle Le Garff [83] | Nivolumab | PD-1 | ART HIV-infected individual with NSCLC | Decompressive radiotherapy Six cisplatin/gemcitabine and four Taxotere chemotherapy treatments Start nivolumab |
|
| E P Scully [84] | Nivolumab Pembrolizumab | PD-1 | ART HIV-1-infected individuals with malignancies | Nivolumab (participant 1 with head and neck SCC, standard dosing, for 18 months) Nivolumab (participant 2 with head and neck SCC, four doses) Pembrolizumab (participant 3 with squamous cell carcinoma of the skin) |
|
| Neil J Shah [85] | Nivolumab, Pembrolizumab, Atezolizumab, Durvalumab and Avelumab | PD-1/PD-L1 | HIV-infected individuals with advanced-stage cancers | Anti-PD-(L)1 monotherapy or anti-PD-(L)1 monotherapy combined with chemotherapy |
|
| Thomas A. Rasmussen [86] | Nivolumab Ipilimumab | PD-1 CTLA-4 | ART HIV-infected individual with advanced malignancies | Nivolumab (240 mg every 2 weeks) in combination with ipilimumab (1 mg/kg every 6 weeks) |
|
| Cynthia L Gay [89] | BMS-936559 | PD-L1 | ART HIV-1-infected adults | Single infusions of BMS-936559 (0.3 mg/kg) |
|
| Elizabeth Colston [88] | Ipilimumab | CTLA-4 | Chronic HIV-1 -infected individuals | Ipilimumab, 0.1, 1, or 3 mg/kg, two doses every 28 days; or 5 mg/kg, four doses every 28 days |
|
4.2. Immune Checkpoint Blockers Confer Partial Protection against Progression of SIV Infection
| Reference | IC Blocker | Target | Objective | Treatment | Outcomes |
|---|---|---|---|---|---|
| Vijayakumar Velu [96] | Humanized mouse anti-human PD-1 Ab (clone EH12-1540) | PD-1 | SIV251/SIVmac239-infected Indian rhesus macaques | Anti-PD-1 Ab (3 mg/kg) in early chronic phase and in late chronic phase on days 0, 3, 7, and 10 |
|
| Adam C Finnefrock [107] | Anti-human PD-1 Ab (clone 1B8) | PD-1 | SIVmac239-infected rhesus macaques | Therapeutic model: single infusion of anti-PD-1 Ab 1B8 (5 mg/kg) to chronic SIV-infected macaques before or during ART Prophylactic model: anti-PD-1 Ab 1B8 (5 mg/kg) to naive macaques immunized with an SIV-Gag adenovirus vector vaccine |
|
| Ravi Dyavar Shetty [97] | Mouse anti- human PD-1 Ab | PD-1 | SIV-infected rhesus macaques | Anti-PD-1 Ab (3 mg/kg) at either 10 or 90 weeks after SIV infection on 0, 3, 7, and 10 days |
|
| Praveen K Amancha [99] | Recombinant macaque PD-1 fused to macaque Ig-Fc (rPD-1-Fc) | PD-1 | SIVmac239-infected rhesus macaques | rPD-1-Fc (50 mg/kg) alone or in combination with ART during the early chronic phase |
|
| Geetha H Mylvagana [98] | Primatized anti–PD-1 Ab (clone EH12- 2132/2133) | PD-1 | Chronic SIVmac251-infected rhesus macaques | Stage I: anti-PD-1 Ab (3 mg/kg/dose, 5 doses) between 24 and 30 weeks after infection on days 0, 3, 7, 10, and 14. Stage II: RMs again treated with anti-PD-1 Ab (10 mg/kg/dose, three monthly, 3 doses) at 26–30 weeks following ART initiation |
|
| Diego A Vargas- Inchaustegui [102] | B7-DC-Ig fusion protein | PD-1 | Chronic SIVmac251- infected rhesus macaques | ART plus B7-DC-Ig (10 mg/kg, weekly, 11 weeks), then B7-DC-Ig alone for 12 weeks |
|
| Elena Bekerman [110] | Human/rhesus chimeric anti- PD-1 antibody | PD-1 | ART SIVmac251-infected rhesus macaques | Anti-PD-1 chimeric Ab (10 mg/kg, every other week, four doses) with or without TLR7 agonist vesatolimod (0.15 mg/kg, every other week, 10 doses) |
|
| Sheikh Abdul Rahman [108] | Primatized anti–PD-1 antibody (clone EH12) | PD-1 | Chronical SIVmac239-infected rhesus macaques | Immunized with a CD40L plus TLR7 agonist–adjuvanted DNA/MVA SIV239 vaccine (DNA vaccine: 1 mg/333 µL, 600 µL/dose, at weeks 38 and 42 MVA vaccine: 1 mL/dose, at weeks 46 and 60) during ART. Received anti–PD-1 treatment on days 0, 3, 7, 10, and 14, starting 10 days before the initiation of ART (3 mg/kg) and on week 38–44 starting with the first DNA prime during ART (10 mg/kg, 3 doses, every 3 weeks) |
|
| ChunxiuWu [109] | Anti-PD-1 antibody (GB226) | PD-1 | ChronicallySIV-infected macaque | Anti-PD-1 antibody injection (20 mg/kg) every 2 weeks from 1 to 7 weeks and rAd5-SIVgpe (1011vp in 1 mL PBS) at weeks 0 and 4 post ART discontinuation; ART treatment begins at week 3 before the initial vaccination |
|
| Enxiang Pan [106] | Genolimzumab | PD-1 | Chinese rhesus monkeys | Genolimzumab injection (20 mg/kg, every two weeks) at weeks −1, 1, 3, 5, and 7 and rAd5-SIVgpe (1011vp) injection at week 0 and 4; at week 42 after the initial vaccination, animals were challenged with repeated low-dose SIVmac239 |
|
| Ping Che [101] | Avelumab | PD-L1 | ART SIVmac239-infected rhesus macaques | Avelumab (20 mg/kg, weekly, for 24 weeks) and rhIL-15 (10 µg/kg, daily, continuous infusion for 10 days, two cycles), then, ART was discontinued and avelumab treatment continued until completion of the 24-week treatment |
|
| Amanda L Gill [100] | Avelumab | PD-L1 | ART SIVmac239-infected rhesus macaques | Avelumab (20 mg/kg, weekly) At week 24, all treatments were discontinued |
|
| Anna Hryniewicz [103] | MDX-010 | CTLA-4 | ART SIVmac251-infected rhesus macaques | Administered MDX-010 (10 mg/kg/injection) after ART initiation at weeks 5 and 8. |
|
| Todd Bradley [105] | Ipilimumab | CTLA-4 | Cynomolgus macaques | Immunized with recombinant CH505 HIV Env gp120 (100 µg every 4 weeks) and ipilimumab (10 mg/kg) during the immunization 1–3 |
|
| Justin Harper [104] | Nivolumab Ipilimumab | PD-1 CTLA-4 | SIVmac239-infected rhesus macaques | Weekly nivolumab and ipilimumab over four weeks during ART, then, ART was interrupted two weeks afterwards with a seven-month follow-up |
|
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Weng, C.H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef]
- Chen, H.; Moussa, M.; Catalfamo, M. The Role of Immunomodulatory Receptors in the Pathogenesis of HIV Infection: A Therapeutic Opportunity for HIV Cure? Front. Immunol. 2020, 11, 1223. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, Z.; Wang, X.; Fu, J.L.; Yao, J.; Jiao, Y.; Chen, L.; Zhang, H.; Wei, J.; Jin, L.; et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 2007, 109, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.; Dunsmore, G.; Koleva, P.; Xu, L.; Houston, S.; Elahi, S. Galectin-9 and VISTA Expression Define Terminally Exhausted T Cells in HIV-1 Infection. J. Immunol. 2020, 204, 2474–2491. [Google Scholar] [CrossRef]
- Noyan, K.; Nguyen, S.; Betts, M.R.; Sonnerborg, A.; Buggert, M. Human Immunodeficiency Virus Type-1 Elite Controllers Maintain Low Co-Expression of Inhibitory Receptors on CD4+ T Cells. Front. Immunol. 2018, 9, 19. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakamura, H.; Koga, M.; Koibuchi, T.; Fujii, T.; Miura, T.; Iwamoto, A.; Kawana-Tachikawa, A. Imbalanced production of cytokines by T cells associates with the activation/exhaustion status of memory T cells in chronic HIV type 1 infection. AIDS Res. Hum. Retrovir. 2012, 28, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, C.; Song, H.; Ma, L.L.; Chen, S.F.; Wu, M.J.; Zhang, H.; Xu, J.C.; Xu, P. Temporal and spatial characterization of negative regulatory T cells in HIV-infected/AIDS patients raises new diagnostic markers and therapeutic strategies. J. Clin. Lab. Anal. 2021, 35, e23831. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.; Hoffmann, M.; Pace, M.; Williams, J.P.; Thornhill, J.; Hamlyn, E.; Meyerowitz, J.; Willberg, C.; Koelsch, K.K.; Robinson, N.; et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat. Commun. 2015, 6, 8495. [Google Scholar] [CrossRef]
- Macatangay, B.J.C.; Gandhi, R.T.; Jones, R.B.; McMahon, D.K.; Lalama, C.M.; Bosch, R.J.; Cyktor, J.C.; Thomas, A.S.; Borowski, L.; Riddler, S.A.; et al. T cells with high PD-1 expression are associated with lower HIV-specific immune responses despite long-term antiretroviral therapy. AIDS 2020, 34, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Whittall, T.; Peters, B.; Rahman, D.; Kingsley, C.I.; Vaughan, R.; Lehner, T. Immunogenic and tolerogenic signatures in human immunodeficiency virus (HIV)-infected controllers compared with progressors and a conversion strategy of virus control. Clin. Exp. Immunol. 2011, 166, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, R.; Bakeman, W.; Lawani, M.B.; Khoury, G.; Hartogensis, W.; DaFonseca, S.; Killian, M.; Epling, L.; Hoh, R.; Sinclair, E.; et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog. 2016, 12, e1005761. [Google Scholar] [CrossRef]
- Khoury, G.; Fromentin, R.; Solomon, A.; Hartogensis, W.; Killian, M.; Hoh, R.; Somsouk, M.; Hunt, P.W.; Girling, V.; Sinclair, E.; et al. Human Immunodeficiency Virus Persistence and T-Cell Activation in Blood, Rectal, and Lymph Node Tissue in Human Immunodeficiency Virus-Infected Individuals Receiving Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 911–919. [Google Scholar] [CrossRef]
- Petrovas, C.; Casazza, J.P.; Brenchley, J.M.; Price, D.A.; Gostick, E.; Adams, W.C.; Precopio, M.L.; Schacker, T.; Roederer, M.; Douek, D.C.; et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006, 203, 2281–2292. [Google Scholar] [CrossRef]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016, 12, e1005661. [Google Scholar] [CrossRef]
- Jensen, S.S.; Fomsgaard, A.; Larsen, T.K.; Tingstedt, J.L.; Gerstoft, J.; Kronborg, G.; Pedersen, C.; Karlsson, I. Initiation of Antiretroviral Therapy (ART) at Different Stages of HIV-1 Disease Is Not Associated with the Proportion of Exhausted CD8+ T Cells. PLoS ONE 2015, 10, e0139573. [Google Scholar] [CrossRef] [PubMed]
- Tailor, J.; Foldi, J.; Generoso, M.; McCarty, B.; Alankar, A.; Kilberg, M.; Mwamzuka, M.; Marshed, F.; Ahmed, A.; Liu, M.; et al. Disease Progression in Children with Perinatal HIV Correlates with Increased PD-1+ CD8 T Cells that Coexpress Multiple Immune Checkpoints. J. Infect. Dis. 2021, 224, 1785–1795. [Google Scholar]
- Li, J.; Huang, H.H.; Tu, B.; Zhou, M.J.; Hu, W.; Fu, Y.L.; Li, X.Y.; Yang, T.; Song, J.W.; Fan, X.; et al. Reversal of the CD8(+) T-Cell Exhaustion Induced by Chronic HIV-1 Infection Through Combined Blockade of the Adenosine and PD-1 Pathways. Front. Immunol. 2021, 12, 687296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Lu, J.; Zhang, S.; Gu, L.; Fu, J.; Jin, L.; Li, H.; Zhao, M.; Zhang, J.; et al. B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J. Infect. Dis. 2011, 203, 1668–1678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muthumani, K.; Choo, A.Y.; Shedlock, D.J.; Laddy, D.J.; Sundaram, S.G.; Hirao, L.; Wu, L.; Thieu, K.P.; Chung, C.W.; Lankaraman, K.M.; et al. Human immunodeficiency virus type 1 Nef induces programmed death 1 expression through a p38 mitogen-activated protein kinase-dependent mechanism. J. Virol. 2008, 82, 11536–11544. [Google Scholar] [CrossRef]
- El-Far, M.; Ancuta, P.; Routy, J.P.; Zhang, Y.; Bakeman, W.; Bordi, R.; DaFonseca, S.; Said, E.A.; Gosselin, A.; Tep, T.S.; et al. Nef promotes evasion of human immunodeficiency virus type 1-infected cells from the CTLA-4-mediated inhibition of T-cell activation. J. Gen. Virol. 2015, 96, 1463–1477. [Google Scholar] [CrossRef][Green Version]
- Prevost, J.; Edgar, C.R.; Richard, J.; Trothen, S.M.; Jacob, R.A.; Mumby, M.J.; Pickering, S.; Dube, M.; Kaufmann, D.E.; Kirchhoff, F.; et al. HIV-1 Vpu Downregulates Tim-3 from the Surface of Infected CD4(+) T Cells. J. Virol. 2020, 94, e01999-19. [Google Scholar] [CrossRef]
- Jacob, R.A.; Edgar, C.R.; Prevost, J.; Trothen, S.M.; Lurie, A.; Mumby, M.J.; Galbraith, A.; Kirchhoff, F.; Haeryfar, S.M.M.; Finzi, A.; et al. The HIV-1 accessory protein Nef increases surface expression of the checkpoint receptor Tim-3 in infected CD4(+) T cells. J. Biol. Chem. 2021, 297, 101042. [Google Scholar] [CrossRef]
- Hoang, T.N.; Harper, J.L.; Pino, M.; Wang, H.; Micci, L.; King, C.T.; McGary, C.S.; McBrien, J.B.; Cervasi, B.; Silvestri, G.; et al. Bone Marrow-Derived CD4(+) T Cells Are Depleted in Simian Immunodeficiency Virus-Infected Macaques and Contribute to the Size of the Replication-Competent Reservoir. J. Virol. 2019, 93, e01344-18. [Google Scholar] [CrossRef]
- Yamamoto, T.; Price, D.A.; Casazza, J.P.; Ferrari, G.; Nason, M.; Chattopadhyay, P.K.; Roederer, M.; Gostick, E.; Katsikis, P.D.; Douek, D.C.; et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 2011, 117, 4805–4815. [Google Scholar] [CrossRef]
- Teigler, J.E.; Zelinskyy, G.; Eller, M.A.; Bolton, D.L.; Marovich, M.; Gordon, A.D.; Alrubayyi, A.; Alter, G.; Robb, M.L.; Martin, J.N.; et al. Differential Inhibitory Receptor Expression on T Cells Delineates Functional Capacities in Chronic Viral Infection. J. Virol. 2017, 91, e01263-17. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, J.; Scharf, L.; Frederiksen, J.; Naji, A.; Ljunggren, H.G.; Sonnerborg, A.; Lund, O.; Reyes-Teran, G.; Hecht, F.M.; Deeks, S.G.; et al. Perturbed CD8(+) T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci. Rep. 2017, 7, 40354. [Google Scholar] [CrossRef]
- Leng, Q.; Bentwich, Z.; Magen, E.; Kalinkovich, A.; Borkow, G. CTLA-4 upregulation during HIV infection: Association with anergy and possible target for therapeutic intervention. AIDS 2002, 16, 519–529. [Google Scholar] [CrossRef]
- Steiner, K.; Waase, I.; Rau, T.; Dietrich, M.; Fleischer, B.; Broker, B.M. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 1999, 115, 451–457. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef]
- Jones, R.B.; Ndhlovu, L.C.; Barbour, J.D.; Sheth, P.M.; Jha, A.R.; Long, B.R.; Wong, J.C.; Satkunarajah, M.; Schweneker, M.; Chapman, J.M.; et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008, 205, 2763–2779. [Google Scholar] [CrossRef]
- Fujita, T.; Burwitz, B.J.; Chew, G.M.; Reed, J.S.; Pathak, R.; Seger, E.; Clayton, K.L.; Rini, J.M.; Ostrowski, M.A.; Ishii, N.; et al. Expansion of dysfunctional Tim-3-expressing effector memory CD8+ T cells during simian immunodeficiency virus infection in rhesus macaques. J. Immunol. 2014, 193, 5576–5583. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Xu, J.; Qiu, C.; Zhu, L.; Qiu, C.; Fu, W.; Wang, Y.; Ye, L.; Fu, Y.X.; et al. CD160 Plays a Protective Role During Chronic Infection by Enhancing Both Functionalities and Proliferative Capacity of CD8+ T Cells. Front. Immunol. 2020, 11, 2188. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Cockerham, L.R.; Jain, V.; Sinclair, E.; Glidden, D.V.; Hartogenesis, W.; Hatano, H.; Hunt, P.W.; Martin, J.N.; Pilcher, C.D.; Sekaly, R.; et al. Programmed death-1 expression on CD4(+) and CD8(+) T cells in treated and untreated HIV disease. AIDS 2014, 28, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Rallon, N.; Garcia, M.; Garcia-Samaniego, J.; Cabello, A.; Alvarez, B.; Restrepo, C.; Nistal, S.; Gorgolas, M.; Benito, J.M. Expression of PD-1 and Tim-3 markers of T-cell exhaustion is associated with CD4 dynamics during the course of untreated and treated HIV infection. PLoS ONE 2018, 13, e0193829. [Google Scholar] [CrossRef] [PubMed]
- Foldi, J.; Kozhaya, L.; McCarty, B.; Mwamzuka, M.; Marshed, F.; Ilmet, T.; Kilberg, M.; Kravietz, A.; Ahmed, A.; Borkowsky, W.; et al. HIV-Infected Children Have Elevated Levels of PD-1+ Memory CD4 T Cells With Low Proliferative Capacity and High Inflammatory Cytokine Effector Functions. J. Infect. Dis. 2017, 216, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Kassu, A.; Marcus, R.A.; D’Souza, M.B.; Kelly-McKnight, E.A.; Golden-Mason, L.; Akkina, R.; Fontenot, A.P.; Wilson, C.C.; Palmer, B.E. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J. Immunol. 2010, 185, 3007–3018. [Google Scholar] [CrossRef]
- Grabmeier-Pfistershammer, K.; Stecher, C.; Zettl, M.; Rosskopf, S.; Rieger, A.; Zlabinger, G.J.; Steinberger, P. Antibodies targeting BTLA or TIM-3 enhance HIV-1 specific T cell responses in combination with PD-1 blockade. Clin. Immunol. 2017, 183, 167–173. [Google Scholar] [CrossRef]
- Chew, G.M.; Padua, A.J.P.; Chow, D.C.; Souza, S.A.; Clements, D.M.; Corley, M.J.; Pang, A.P.S.; Alejandria, M.M.; Gerschenson, M.; Shikuma, C.M.; et al. Effects of Brief Adjunctive Metformin Therapy in Virologically Suppressed HIV-Infected Adults on Polyfunctional HIV-Specific CD8 T Cell Responses to PD-L1 Blockade. AIDS Res. Hum. Retrovir. 2021, 37, 24–33. [Google Scholar] [CrossRef]
- Petrovas, C.; Price, D.A.; Mattapallil, J.; Ambrozak, D.R.; Geldmacher, C.; Cecchinato, V.; Vaccari, M.; Tryniszewska, E.; Gostick, E.; Roederer, M.; et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 2007, 110, 928–936. [Google Scholar] [CrossRef]
- Onlamoon, N.; Rogers, K.; Mayne, A.E.; Pattanapanyasat, K.; Mori, K.; Villinger, F.; Ansari, A.A. Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection. Immunology 2008, 124, 277–293. [Google Scholar] [CrossRef]
- Kaufmann, D.E.; Kavanagh, D.G.; Pereyra, F.; Zaunders, J.J.; Mackey, E.W.; Miura, T.; Palmer, S.; Brockman, M.; Rathod, A.; Piechocka-Trocha, A.; et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007, 8, 1246–1254. [Google Scholar] [CrossRef]
- Blazkova, J.; Huiting, E.D.; Boddapati, A.K.; Shi, V.; Whitehead, E.J.; Justement, J.S.; Nordstrom, J.L.; Moir, S.; Lack, J.; Chun, T.W. Correlation Between TIGIT Expression on CD8+ T Cells and Higher Cytotoxic Capacity. J. Infect. Dis. 2021, 224, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.N.; Zhu, M.L.; Chen, Y.H.; Fu, Y.J.; Zhang, T.W.; Jiang, Y.J.; Chu, Z.X.; Shang, H. Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common gamma -chain cytokines. Biomed. Res. Int. 2015, 2015, 916936. [Google Scholar] [CrossRef] [PubMed]
- Tendeiro, R.; Foxall, R.B.; Baptista, A.P.; Pinto, F.; Soares, R.S.; Cavaleiro, R.; Valadas, E.; Gomes, P.; Victorino, R.M.; Sousa, A.E. PD-1 and its ligand PD-L1 are progressively up-regulated on CD4 and CD8 T-cells in HIV-2 infection irrespective of the presence of viremia. AIDS 2012, 26, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Sakhdari, A.; Mujib, S.; Vali, B.; Yue, F.Y.; MacParland, S.; Clayton, K.; Jones, R.B.; Liu, J.; Lee, E.Y.; Benko, E.; et al. Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS ONE 2012, 7, e40146. [Google Scholar] [CrossRef]
- Amancha, P.K.; Hong, J.J.; Ansari, A.A.; Villinger, F. Up-regulation of Tim-3 on T cells during acute simian immunodeficiency virus infection and on antigen specific responders. AIDS 2015, 29, 531–536. [Google Scholar] [CrossRef][Green Version]
- Peretz, Y.; He, Z.; Shi, Y.; Yassine-Diab, B.; Goulet, J.P.; Bordi, R.; Filali-Mouhim, A.; Loubert, J.B.; El-Far, M.; Dupuy, F.P.; et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog. 2012, 8, e1002840. [Google Scholar] [CrossRef]
- Pombo, C.; Wherry, E.J.; Gostick, E.; Price, D.A.; Betts, M.R. Elevated Expression of CD160 and 2B4 Defines a Cytolytic HIV-Specific CD8+ T-Cell Population in Elite Controllers. J. Infect. Dis. 2015, 212, 1376–1386. [Google Scholar] [CrossRef]
- Martin, G.E.; Pace, M.; Shearer, F.M.; Zilber, E.; Hurst, J.; Meyerowitz, J.; Thornhill, J.P.; Lwanga, J.; Brown, H.; Robinson, N.; et al. Levels of Human Immunodeficiency Virus DNA Are Determined Before ART Initiation and Linked to CD8 T-Cell Activation and Memory Expansion. J. Infect. Dis. 2020, 221, 1135–1145. [Google Scholar] [CrossRef]
- Pardons, M.; Baxter, A.E.; Massanella, M.; Pagliuzza, A.; Fromentin, R.; Dufour, C.; Leyre, L.; Routy, J.P.; Kaufmann, D.E.; Chomont, N. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019, 15, e1007619. [Google Scholar] [CrossRef]
- Rinaldi, S.; de Armas, L.; Dominguez-Rodriguez, S.; Pallikkuth, S.; Dinh, V.; Pan, L.; Grtner, K.; Pahwa, R.; Cotugno, N.; Rojo, P.; et al. T cell immune discriminants of HIV reservoir size in a pediatric cohort of perinatally infected individuals. PLoS Pathog. 2021, 17, e1009533. [Google Scholar] [CrossRef]
- Banga, R.; Procopio, F.A.; Noto, A.; Pollakis, G.; Cavassini, M.; Ohmiti, K.; Corpataux, J.M.; de Leval, L.; Pantaleo, G.; Perreau, M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 2016, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Levy, Y. Therapeutic vaccines and immunological intervention in HIV infection: A paradigm change. Curr. Opin. HIV AIDS 2016, 11, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Boyer, Z.; Palmer, S. Targeting Immune Checkpoint Molecules to Eliminate Latent HIV. Front. Immunol. 2018, 9, 2339. [Google Scholar] [CrossRef] [PubMed]
- McGary, C.S.; Deleage, C.; Harper, J.; Micci, L.; Ribeiro, S.P.; Paganini, S.; Kuri-Cervantes, L.; Benne, C.; Ryan, E.S.; Balderas, R.; et al. CTLA-4(+)PD-1(-) Memory CD4(+) T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 2017, 47, 776–788.e5. [Google Scholar] [CrossRef]
- Xue, J.; Chong, H.; Zhu, Y.; Zhang, J.; Tong, L.; Lu, J.; Chen, T.; Cong, Z.; Wei, Q.; He, Y. Efficient treatment and pre-exposure prophylaxis in rhesus macaques by an HIV fusion-inhibitory lipopeptide. Cell 2022, 185, 131–144.e18. [Google Scholar] [CrossRef]
- Llewellyn, G.N.; Seclen, E.; Wietgrefe, S.; Liu, S.; Chateau, M.; Pei, H.; Perkey, K.; Marsden, M.D.; Hinkley, S.J.; Paschon, D.E.; et al. Humanized Mouse Model of HIV-1 Latency with Enrichment of Latent Virus in PD-1(+) and TIGIT(+) CD4 T Cells. J. Virol. 2019, 93, e02086-18. [Google Scholar] [CrossRef]
- Evans, V.A.; van der Sluis, R.M.; Solomon, A.; Dantanarayana, A.; McNeil, C.; Garsia, R.; Palmer, S.; Fromentin, R.; Chomont, N.; Sekaly, R.P.; et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS 2018, 32, 1491–1497. [Google Scholar] [CrossRef]
- Van der Sluis, R.M.; Kumar, N.A.; Pascoe, R.D.; Zerbato, J.M.; Evans, V.A.; Dantanarayana, A.I.; Anderson, J.L.; Sekaly, R.P.; Fromentin, R.; Chomont, N.; et al. Combination Immune Checkpoint Blockade to Reverse HIV Latency. J. Immunol. 2020, 204, 1242–1254. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat. Commun. 2019, 10, 814. [Google Scholar] [CrossRef]
- Bui, J.K.; Cyktor, J.C.; Fyne, E.; Campellone, S.; Mason, S.W.; Mellors, J.W. Blockade of the PD-1 axis alone is not sufficient to activate HIV-1 virion production from CD4+ T cells of individuals on suppressive ART. PLoS ONE 2019, 14, e0211112. [Google Scholar] [CrossRef]
- Cook, M.R.; Kim, C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019, 5, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Tapia Rico, G.; Chan, M.M.; Loo, K.F. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat. Rev. 2020, 86, 102011. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.P. Immune checkpoint inhibitors in people living with HIV: What about anti-HIV effects? AIDS 2020, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Guaitoli, G.; Baldessari, C.; Maur, M.; Mussini, C.; Meschiari, M.; Barbieri, F.; Cascinu, S.; Bertolini, F. Treating cancer with immunotherapy in HIV-positive patients: A challenging reality. Crit. Rev. Oncol. Hematol. 2020, 145, 102836. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, J.M.; Purves, H.V.; Lewin, S.R.; Rasmussen, T.A. Between a shock and a hard place: Challenges and developments in HIV latency reversal. Curr. Opin. Virol. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Yoo, S.; Mesplede, T. Combination therapies currently under investigation in phase I and phase II clinical trials for HIV-1. Expert Opin. Investig. Drugs 2020, 29, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Wightman, F.; Solomon, A.; Kumar, S.S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; McNeil, C.; Garsia, R.; Lewin, S.R. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 2015, 29, 504–506. [Google Scholar] [CrossRef]
- Blanch-Lombarte, O.; Galvez, C.; Revollo, B.; Jimenez-Moyano, E.; Llibre, J.M.; Manzano, J.L.; Boada, A.; Dalmau, J.; Daniel, E.S.; Clotet, B.; et al. Enhancement of Antiviral CD8(+) T-Cell Responses and Complete Remission of Metastatic Melanoma in an HIV-1-Infected Subject Treated with Pembrolizumab. J. Clin. Med. 2019, 8, 2089. [Google Scholar] [CrossRef]
- Lau, J.S.Y.; McMahon, J.H.; Gubser, C.; Solomon, A.; Chiu, C.Y.H.; Dantanarayana, A.; Chea, S.; Tennakoon, S.; Zerbato, J.M.; Garlick, J.; et al. The impact of immune checkpoint therapy on the latent reservoir in HIV-infected individuals with cancer on antiretroviral therapy. AIDS 2021, 35, 1631–1636. [Google Scholar] [CrossRef]
- Guihot, A.; Marcelin, A.G.; Massiani, M.A.; Samri, A.; Soulie, C.; Autran, B.; Spano, J.P. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann. Oncol. 2018, 29, 517–518. [Google Scholar] [CrossRef]
- Hentrich, M.; Schipek-Voigt, K.; Jager, H.; Schulz, S.; Schmid, P.; Stotzer, O.; Bojko, P. Nivolumab in HIV-related non-small-cell lung cancer. Ann. Oncol. 2017, 28, 2890. [Google Scholar] [CrossRef] [PubMed]
- McCullar, B.; Alloway, T.; Martin, M. Durable complete response to nivolumab in a patient with HIV and metastatic non-small cell lung cancer. J. Thorac. Dis. 2017, 9, E540–E542. [Google Scholar] [CrossRef] [PubMed]
- McLean, F.E.; Gathogo, E.; Goodall, D.; Jones, R.; Kinloch, S.; Post, F.A. Alemtuzumab induction therapy in HIV-positive renal transplant recipients. AIDS 2017, 31, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.P.; Rutishauser, R.L.; Simoneau, C.R.; Delagreverie, H.; Euler, Z.; Thanh, C.; Li, J.Z.; Hartig, H.; Bakkour, S.; Busch, M.; et al. Inconsistent HIV reservoir dynamics and immune responses following anti-PD-1 therapy in cancer patients with HIV infection. Ann. Oncol. 2018, 29, 2141–2142. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Al-Shbool, G.; Blackburn, M.; Cook, M.; Belouali, A.; Liu, S.V.; Madhavan, S.; He, A.R.; Atkins, M.B.; Gibney, G.T.; et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J. Immunother. Cancer 2019, 7, 353. [Google Scholar] [CrossRef]
- Rasmussen, T.A.; Rajdev, L.; Rhodes, A.; Dantanarayana, A.; Tennakoon, S.; Chea, S.; Spelman, T.; Lensing, S.; Rutishauser, R.; Bakkour, S.; et al. Impact of anti-PD-1 and anti-CTLA-4 on the HIV reservoir in people living with HIV with cancer on antiretroviral therapy: The AIDS Malignancy Consortium-095 study. Clin. Infect. Dis. 2021, 73, e1973–e1981. [Google Scholar] [CrossRef] [PubMed]
- Chughlay, M.F.; Njuguna, C.; Cohen, K.; Maartens, G. Acute interstitial nephritis caused by lopinavir/ritonavir in a surgeon receiving antiretroviral postexposure prophylaxis. AIDS 2015, 29, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Colston, E.; Grasela, D.; Gardiner, D.; Bucy, R.P.; Vakkalagadda, B.; Korman, A.J.; Lowy, I. An open-label, multiple ascending dose study of the anti-CTLA-4 antibody ipilimumab in viremic HIV patients. PLoS ONE 2018, 13, e0198158. [Google Scholar] [CrossRef]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef]
- Anti-PD-1 Therapy OK for Most with HIV. Cancer Discov. 2018, 8, 130–131.
- Gonzalez-Cao, M.; Moran, T.; Dalmau, J.; Garcia-Corbacho, J.; Bracht, J.W.P.; Bernabe, R.; Juan, O.; de Castro, J.; Blanco, R.; Drozdowskyj, A.; et al. Assessment of the Feasibility and Safety of Durvalumab for Treatment of Solid Tumors in Patients With HIV-1 Infection: The Phase 2 DURVAST Study. JAMA Oncol. 2020, 6, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Spano, J.P.; Veyri, M.; Gobert, A.; Guihot, A.; Perre, P.; Kerjouan, M.; Brosseau, S.; Cloarec, N.; Montaudie, H.; Helissey, C.; et al. Immunotherapy for cancer in people living with HIV: Safety with an efficacy signal from the series in real life experience. AIDS 2019, 33, F13–F19. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Junior, P.N.A.; Galanina, N.; Kurzrock, R. Remembering the forgotten child: The role of immune checkpoint inhibition in patients with human immunod eficiency virus and cancer. J. Immunother. Cancer 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Lurain, K.; Ramaswami, R.; Mangusan, R.; Widell, A.; Ekwede, I.; George, J.; Ambinder, R.; Cheever, M.; Gulley, J.L.; Goncalves, P.H.; et al. Use of pembrolizumab with or without pomalidomide in HIV-associated non-Hodgkin’s lymphoma. J. Immunother. Cancer 2021, 9, e002097. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; Bosch, R.J.; McKhann, A.; Moseley, K.F.; Wimbish, C.L.; Hendrickx, S.M.; Messer, M.; Furlong, M.; Campbell, D.M.; Jennings, C.; et al. Suspected Immune-Related Adverse Events With an Anti-PD-1 Inhibitor in Otherwise Healthy People with HIV. J. Acquir. Immune. Defic. Syndr. 2021, 87, e234–e236. [Google Scholar] [CrossRef] [PubMed]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009, 458, 206–210. [Google Scholar] [CrossRef]
- Dyavar Shetty, R.; Velu, V.; Titanji, K.; Bosinger, S.E.; Freeman, G.J.; Silvestri, G.; Amara, R.R. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J. Clin. Investig. 2012, 122, 1712–1716. [Google Scholar] [CrossRef]
- Mylvaganam, G.H.; Chea, L.S.; Tharp, G.K.; Hicks, S.; Velu, V.; Iyer, S.S.; Deleage, C.; Estes, J.D.; Bosinger, S.E.; Freeman, G.J.; et al. Combination anti-PD-1 and antiretroviral therapy provides therapeutic benefit against SIV. JCI Insight 2018, 3, e122940. [Google Scholar] [CrossRef]
- Amancha, P.K.; Hong, J.J.; Rogers, K.; Ansari, A.A.; Villinger, F. In vivo blockade of the programmed cell death-1 pathway using soluble recombinant PD-1-Fc enhances CD4+ and CD8+ T cell responses but has limited clinical benefit. J Immunol. 2013, 191, 6060–6070. [Google Scholar] [CrossRef]
- Gill, A.L.; Green, S.A.; Abdullah, S.; Le Saout, C.; Pittaluga, S.; Chen, H.; Turnier, R.; Lifson, J.; Godin, S.; Qin, J.; et al. Programed death-1/programed death-ligand 1 expression in lymph nodes of HIV infected patients: Results of a pilot safety study in rhesus macaques using anti-programed death-ligand 1 (Avelumab). AIDS 2016, 30, 2487–2493. [Google Scholar] [CrossRef]
- Chen, P.; Chen, H.; Moussa, M.; Cheng, J.; Li, T.; Qin, J.; Lifson, J.D.; Sneller, M.C.; Krymskaya, L.; Godin, S.; et al. Recombinant Human Interleukin-15 and Anti-PD-L1 Combination Therapy Expands a CXCR3+PD1-/low CD8 T-Cell Subset in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Infect. Dis. 2020, 221, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Inchaustegui, D.A.; Xiao, P.; Hogg, A.E.; Demberg, T.; McKinnon, K.; Venzon, D.; Brocca-Cofano, E.; Dipasquale, J.; Lee, E.M.; Hudacik, L.; et al. Immune targeting of PD-1(hi) expressing cells during and after antiretroviral therapy in SIV-infected rhesus macaques. Virology 2013, 447, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, A.; Boasso, A.; Edghill-Smith, Y.; Vaccari, M.; Fuchs, D.; Venzon, D.; Nacsa, J.; Betts, M.R.; Tsai, W.P.; Heraud, J.M.; et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 2006, 108, 3834–3842. [Google Scholar] [CrossRef]
- Harper, J.; Gordon, S.; Chan, C.N.; Wang, H.; Lindemuth, E.; Galardi, C.; Falcinelli, S.D.; Raines, S.L.M.; Read, J.L.; Nguyen, K.; et al. CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat. Med. 2020, 26, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.; Kuraoka, M.; Yeh, C.H.; Tian, M.; Chen, H.; Cain, D.W.; Chen, X.; Cheng, C.; Ellebedy, A.H.; Parks, R.; et al. Immune checkpoint modulation enhances HIV-1 antibody induction. Nat. Commun. 2020, 11, 948. [Google Scholar] [CrossRef]
- Pan, E.; Feng, F.; Li, P.; Yang, Q.; Ma, X.; Wu, C.; Zhao, J.; Yan, H.; Chen, R.; Chen, L.; et al. Immune Protection of SIV Challenge by PD-1 Blockade During Vaccination in Rhesus Monkeys. Front. Immunol. 2018, 9, 2415. [Google Scholar] [CrossRef] [PubMed]
- Finnefrock, A.C.; Tang, A.; Li, F.; Freed, D.C.; Feng, M.; Cox, K.S.; Sykes, K.J.; Guare, J.P.; Miller, M.D.; Olsen, D.B.; et al. PD-1 blockade in rhesus macaques: Impact on chronic infection and prophylactic vaccination. J. Immunol. 2009, 182, 980–987. [Google Scholar] [CrossRef]
- Rahman, S.A.; Yagnik, B.; Bally, A.P.; Morrow, K.N.; Wang, S.; Vanderford, T.H.; Freeman, G.J.; Ahmed, R.; Amara, R.R. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8(+) T cells in lymphoid tissue. Sci. Immunol. 2021, 6, eabh3034. [Google Scholar] [CrossRef]
- Wu, C.; He, Y.; Zhao, J.; Luo, K.; Wen, Z.; Zhang, Y.; Li, M.; Cui, Y.; Liu, Z.; Wang, C.; et al. Exacerbated AIDS Progression by PD-1 Blockade during Therapeutic Vaccination in Chronically Simian Immunodeficiency Virus-Infected Rhesus Macaques after Interruption of Antiretroviral Therapy. J. Virol. 2022, 96, e0178521. [Google Scholar] [CrossRef]
- Bekerman, E.; Hesselgesser, J.; Carr, B.; Nagel, M.; Hung, M.; Wang, A.; Stapleton, L.; von Gegerfelt, A.; Elyard, H.A.; Lifson, J.D.; et al. PD-1 Blockade and TLR7 Activation Lack Therapeutic Benefit in Chronic Simian Immunodeficiency Virus-Infected Macaques on Antiretroviral Therapy. Antimicrob. Agents Chemother. 2019, 63, e01163-19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xue, J. Expression Profile and Biological Role of Immune Checkpoints in Disease Progression of HIV/SIV Infection. Viruses 2022, 14, 581. https://doi.org/10.3390/v14030581
Sun Y, Xue J. Expression Profile and Biological Role of Immune Checkpoints in Disease Progression of HIV/SIV Infection. Viruses. 2022; 14(3):581. https://doi.org/10.3390/v14030581
Chicago/Turabian StyleSun, Yuting, and Jing Xue. 2022. "Expression Profile and Biological Role of Immune Checkpoints in Disease Progression of HIV/SIV Infection" Viruses 14, no. 3: 581. https://doi.org/10.3390/v14030581
APA StyleSun, Y., & Xue, J. (2022). Expression Profile and Biological Role of Immune Checkpoints in Disease Progression of HIV/SIV Infection. Viruses, 14(3), 581. https://doi.org/10.3390/v14030581





