Previous Infection with SARS-CoV-2 Correlates with Increased Protective Humoral Responses after a Single Dose of an Inactivated COVID-19 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment and Clinical Sample Collection
2.2. Detection of SARS-CoV2-Specific Antibodies
2.3. PRNT
2.4. Statistical Analysis
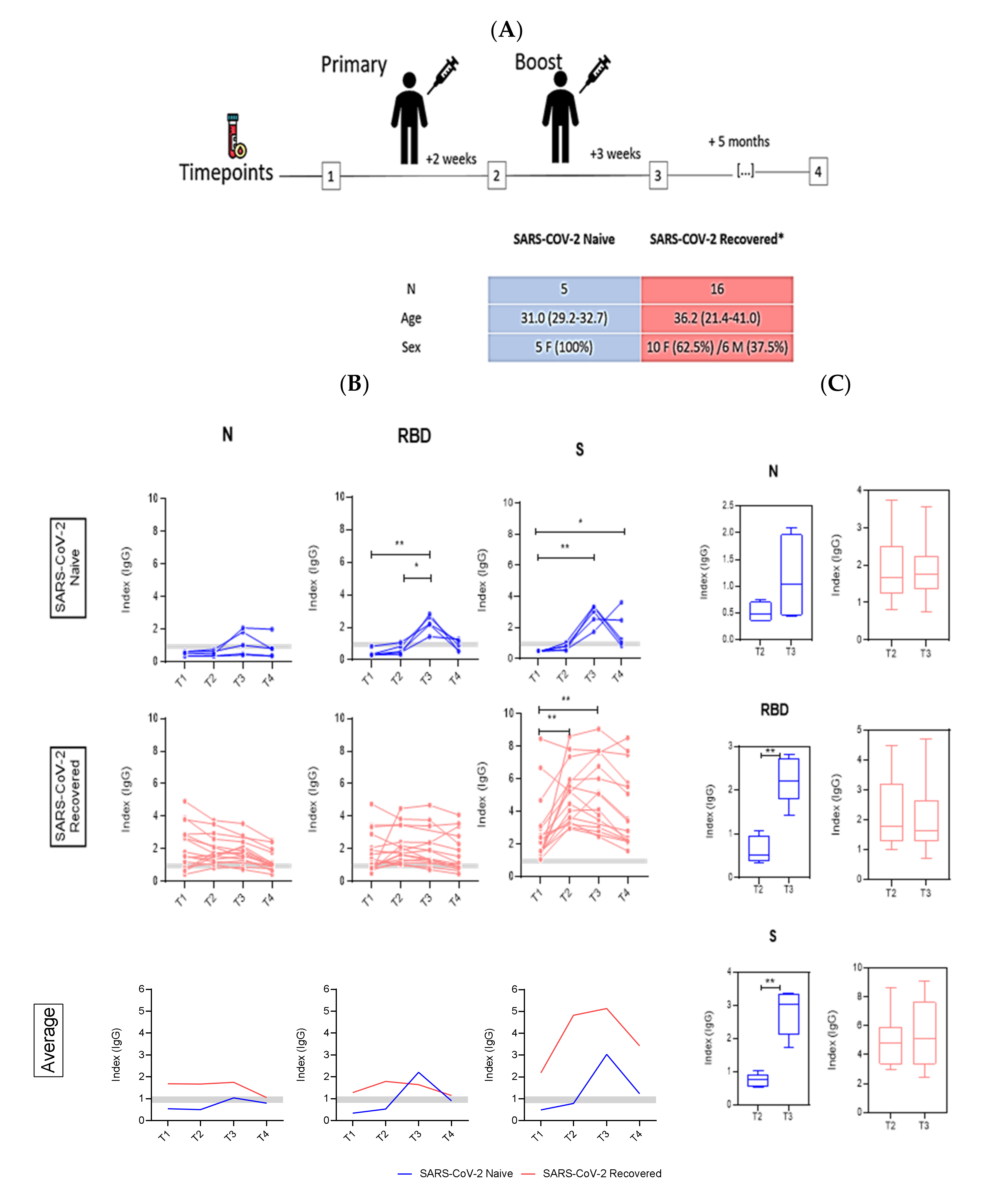
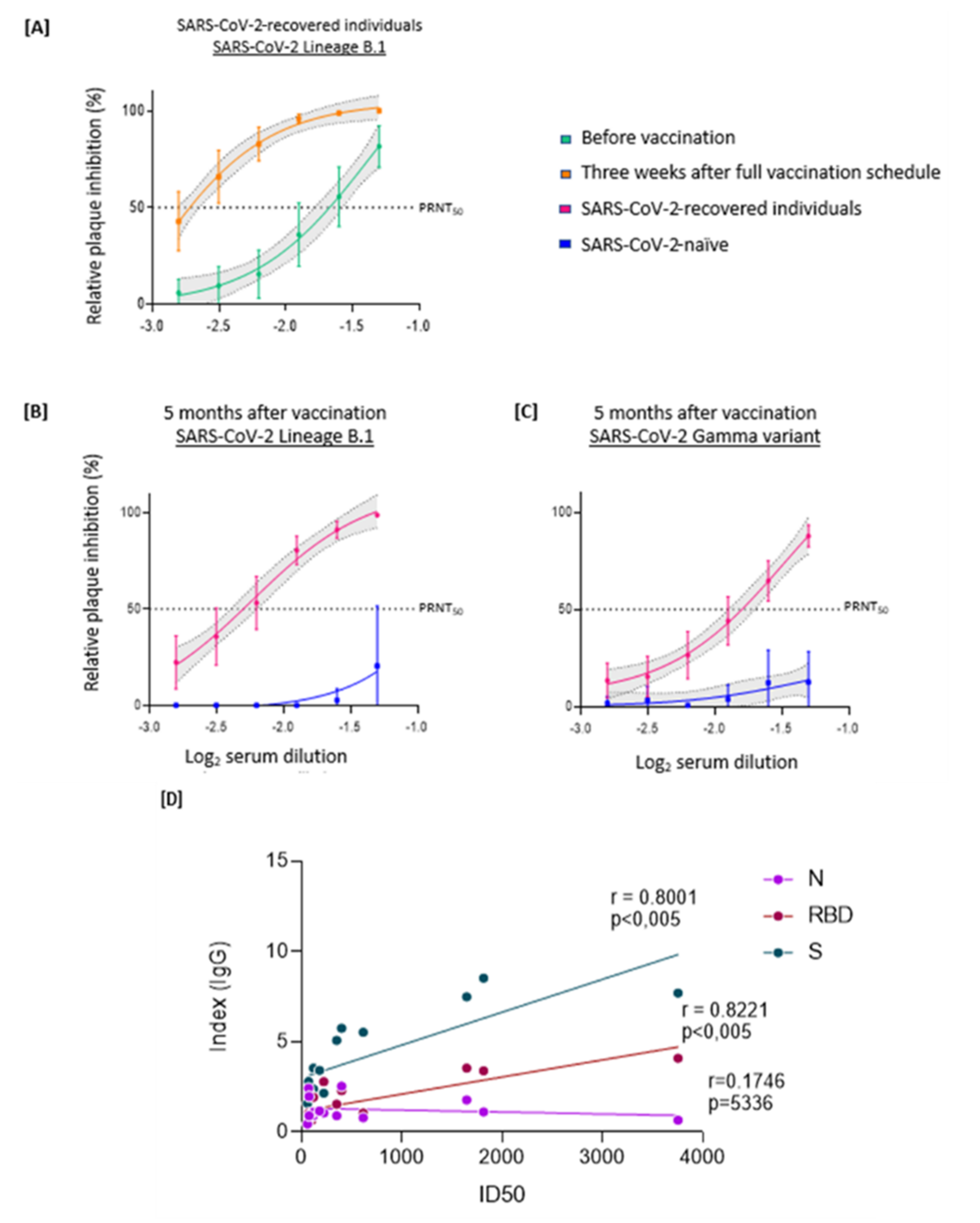
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scobie, H.M.; Johnson, A.G.; Suthar, A.B.; Severson, R.; Alden, N.B.; Balter, S.; Bertolino, D.; Blythe, D.; Brady, S.; Cadwell, B.; et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status—13 U.S. Jurisdictions, April 4–July 17, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Padma, T.V. COVID vaccines to reach poorest countries in 2023—Despite recent pledges. Nature 2021, 595, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Bravo, C.; Torres-Carranza, D.; Sanchez-Trujillo, L.; Gómez-Lahoz, A.M.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; Bujan, J.; et al. An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times. Vaccines 2021, 9, 433. [Google Scholar] [CrossRef]
- Mallapaty, S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature 2021, 594, 161–162. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; Villela, E.F.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Who Coronavirus Dashboard. Available online:https://covid19.who.int/ (accessed on 27 September 2021).
- Stamatatos, L.; Czartoski, J.; Wan, Y.-H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Samanovic, M.I.; Cornelius, A.R.; Gray-Gaillard, S.L.; Allen, J.R.; Karmacharya, T.; Wilson, J.P.; Wesley Hyman, S.; Tuen, M.; Koralov, S.B.; Mulligan, M.J.; et al. Robust immune responses are observed after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. Sci. Transl. Med. 2021, 7, eabi8961. [Google Scholar] [CrossRef] [PubMed]
- Brochot, E.; Demey, B.; Handala, L.; François, C.; Duverlie, G.; Castelain, S. Comparison of different serological assays for SARS-CoV-2 in real life. J. Clin. Virol. 2020, 130, 104569. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Bagno, F.F.; Sérgio, S.A.R.; Figueiredo, M.M.; Godoi, L.C.; Andrade LA, F.; Salazar, N.C.; Soares, C.P.; Aguiar, A.; Almeida, F.J.; da Silva, E.D.; et al. Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Souza, W.M.; Amorim, M.R.; Sesti-Costa, R.; Coimbra, L.D.; Brunetti, N.S.; A Toledo-Teixeira, D.; de Souza, G.F.; Muraro, S.P.; Parise, P.L.; Barbosa, P.P.; et al. Neutralisation of SARS-CoV-2 lineage P.1 by antibodies elicited through natural SARS-CoV-2 infection or vaccination with an inactivated SARS-CoV-2 vaccine: An immunological study. Lancet Microbe 2021, 2, e527–e535. [Google Scholar] [CrossRef]
- Meyer, B.; Drosten, C.; Müller, M.A. Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Res. 2014, 194, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.S.; Yuki, E.F.N.; Pedrosa, T.; Fusco, S.R.G.; Rojo, P.T.; Pereira, R.M.R.; Shinjo, S.K.; Andrade, D.C.O.; et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: A phase 4 trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Steel, K.J.A.; Hemmings, O.; O’Bryne, A.; Kouphou, N.; Pickering, S.; Galao, R.; et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Mc Chan, J.; Chik, T.S.; et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25, 2000421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, S.L.; Cheng, S.M.; Leung, J.N.; Leung, K.; Lee, C.-K.; Peiris, J.M.; Wu, J.T. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Eurosurveillance 2022, 27, 2101197. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagno, F.F.; Andrade, L.A.F.; Sérgio, S.A.R.; Parise, P.L.; Toledo-Teixeira, D.A.; Gazzinelli, R.T.; Fernandes, A.P.S.M.; Teixeira, S.M.R.; Granja, F.; Proença-Módena, J.L.; et al. Previous Infection with SARS-CoV-2 Correlates with Increased Protective Humoral Responses after a Single Dose of an Inactivated COVID-19 Vaccine. Viruses 2022, 14, 510. https://doi.org/10.3390/v14030510
Bagno FF, Andrade LAF, Sérgio SAR, Parise PL, Toledo-Teixeira DA, Gazzinelli RT, Fernandes APSM, Teixeira SMR, Granja F, Proença-Módena JL, et al. Previous Infection with SARS-CoV-2 Correlates with Increased Protective Humoral Responses after a Single Dose of an Inactivated COVID-19 Vaccine. Viruses. 2022; 14(3):510. https://doi.org/10.3390/v14030510
Chicago/Turabian StyleBagno, Flávia F., Luis A. F. Andrade, Sarah A. R. Sérgio, Pierina L. Parise, Daniel A. Toledo-Teixeira, Ricardo T. Gazzinelli, Ana P. S. M. Fernandes, Santuza M. R. Teixeira, Fabiana Granja, José L. Proença-Módena, and et al. 2022. "Previous Infection with SARS-CoV-2 Correlates with Increased Protective Humoral Responses after a Single Dose of an Inactivated COVID-19 Vaccine" Viruses 14, no. 3: 510. https://doi.org/10.3390/v14030510
APA StyleBagno, F. F., Andrade, L. A. F., Sérgio, S. A. R., Parise, P. L., Toledo-Teixeira, D. A., Gazzinelli, R. T., Fernandes, A. P. S. M., Teixeira, S. M. R., Granja, F., Proença-Módena, J. L., & da Fonseca, F. G. (2022). Previous Infection with SARS-CoV-2 Correlates with Increased Protective Humoral Responses after a Single Dose of an Inactivated COVID-19 Vaccine. Viruses, 14(3), 510. https://doi.org/10.3390/v14030510







