Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19
Abstract
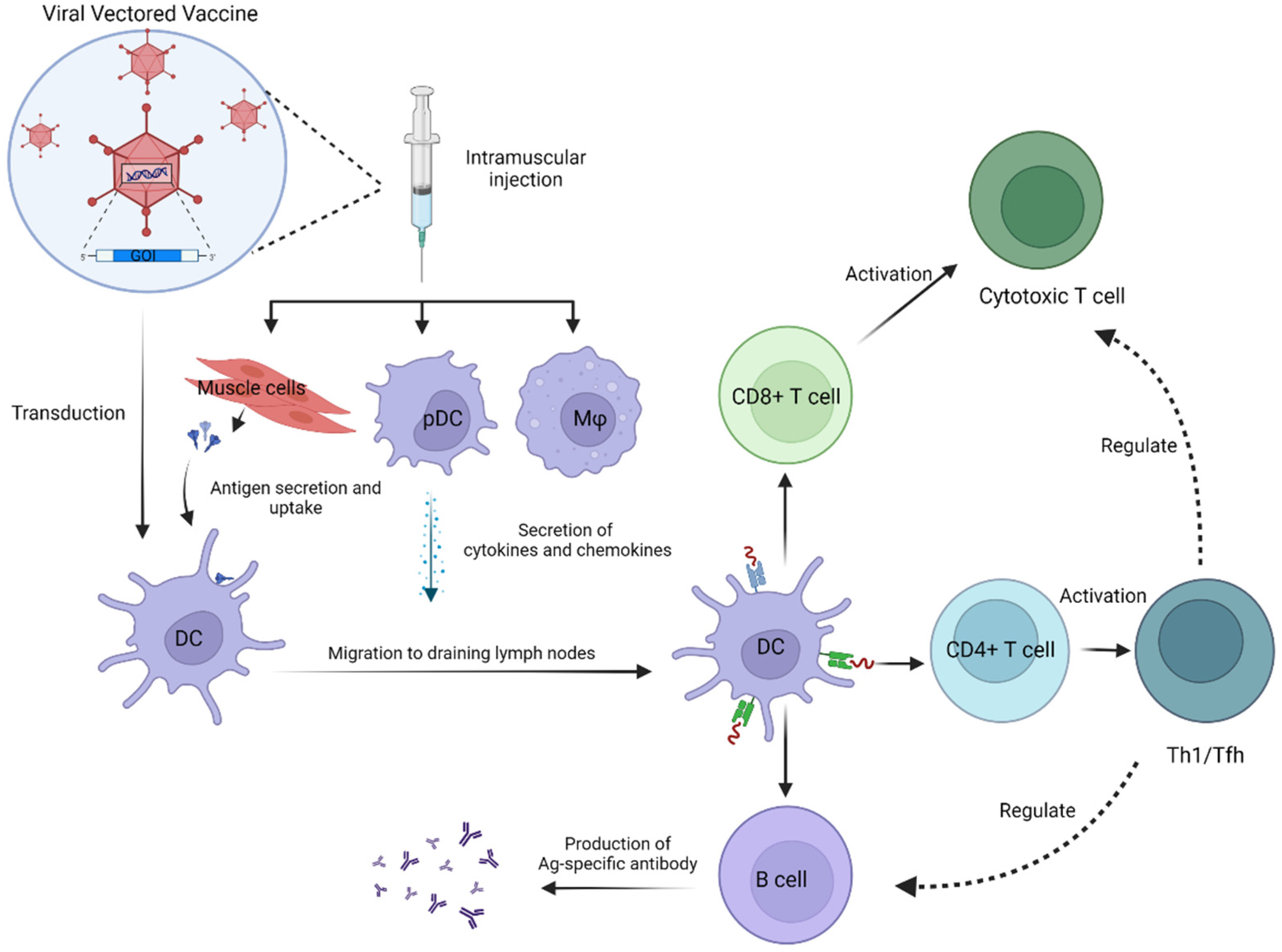
:1. Introduction
2. Immunogenicity
2.1. Humoral Immunity
2.1.1. Adenoviral Vector
2.1.2. Alternative Viral Vectors
| Vaccine | Model | Regimen | Route of Administration | Humoral Immune Response | Cellular Immune Response | Reference |
|---|---|---|---|---|---|---|
| Ad26.COV2.S | Hamster | Single dose of 1 × 1010 vp | IM | Median ELISA titer: 4470 (week 4) Median NAb titer: 359 (week 4) RBD-binding Ab and neutralizing Ab titer > sham | N/A | [10] |
| Mouse | Single dose of 1 × 1010 vp | IM | Binding Ab and neutralizing Ab titer: S.PP > S > sham | Th1-biased response | [17] | |
| Rhesus macaques | Single dose of 1 × 1011 vp | IM | Median NAb titer: 408 (week 4) Median NAb titers 4-fold higher than convalescent titers of macaques and humans | Th1-biased response | [11] | |
| Human | High dose: 1 × 1011 vp Low dose: 5 × 1010 vp Cohort 1a: 18–55 years of age Group 1: placebo/placebo Group 2: low dose/placebo Group 3: low dose/low dose Group 4: high dose/placebo Group 5: high dose/high dose Cohort 3: ≥65 years of age Group 1: placebo Group 2: low dose Group 3: high dose | IM | Binding Ab (ELISA units/ml) (GMC): Cohort 1a Baseline: all below LOQ (<53) Day 29: <53 (Group 1) 478 (Group 2) 586 (Group 3) 625 (Group 4) 788 (Group 5) Day 57: <53 (Group 1) 660 (Group 2) 754 (Group 3) 873 (Group 4) 1100 (Group 5) Day 71: <53 (Group 1) 600 (Group 2) 1677 (Group 3) 951 (Group 4) 2292 (Group 5) Cohort 3 Baseline: all below LOQ (<53) Day 15: <53 (Group 1) 122 (Group 2) 141 (Group 3) Day 29: <53 (Group 1) 312 (Group 2) 350 (Group 3) Neutralizing Ab (IC50) (GMT): Cohort 1a Baseline: all below LOQ (<58) Day 29: <58 (Group 1) 224 (Group 2) 224 (Group 3) 215 (Group 4) 354 (Group 5) Day 57: <58 (Group 1) 310 (Group 2) 288 (Group 3) 370 (Group 4) 488 (Group 5) Day 71: <58 (Group 1) 321 (Group 2) 827 (Group 3) 388 (Group 4) 1266 (Group 5) Cohort 3 Baseline: all below LOQ (<58) Day 15: <58 (Group 1) 212 (Group 2) 172 (Group 3) Day 29: <58 (Group 1) 277 (Group 2) 212 (Group 3) | CD4+ Th1 cells Cohort 1a Day 15: 0% (Placebo) 76% (low dose) 83% (high dose) Cohort 3 Day 15: 0% (Placebo) 60% (low dose) 67% (high dose) CD8+ T cells Cohort 1a Day 15: 0% (placebo) 51% (low dose) 64% (high dose) Cohort 3 0% (placebo) 36% (low dose) 24% (high dose) | [20] | |
| Ad5-nCoV | Mouse | High dose: 5 × 109 vp Middle dose: 5 × 108 vp Low dose: 5 × 107 vp | IM or IN | IM animals: IgG peaked at day 28 NAb titers peaked at week 8 IN animals: IgG peaked week 4 to week 8 NAb titers peaked at week 6 High-dose IN produced higher IgG titers compared to high-dose IM at week 6 and week 8 IM led to a higher ratio of IgG2a to IgG1 compared to IN High-dose IN produced significantly higher NAb titers compared to high-dose IM from week 4 to week 8 Both IM and IN induced S-specific IgG in the trachea-lung but only IN induced S-specific IgA | Middle-dose IM and IN animals showed IFN-γ, TNF-α, and IL-2 responses in splenic CD4+ or CD8+ T cells at week 2 (IM > IN) At week 10, IM induced dose-dependent cellular immunity while IN did not | [22] |
| Human | For AI: High dose: 2 × 1010 vp Low dose: 1 × 1010 vp (day 0 prime + day 28 boost) For IM+AI: 5 × 1010 vp IM on day 0 + 2 × 1010 vp AI on day 28 For IM: 5 × 1010 vp (one dose) or 10 × 1010 vp (two doses) on day 0 | AI, IM, or both | At day 28 after the last vaccination Neutralizing Ab (GMT): AI high dose: 107 AI low dose: 105 IM+AI: 396 IM one dose: 95 IM two dose: 180 RBD-binding IgG (GMC): AI high dose: 261 AI low dose: 289 IM+AI: 2013 IM one dose: 915 IM two dose: 1190 | IFN-γ responses peaked by day 14 after initial immunization for IM and AI vaccinees Aerosol immunization with 1/5 of the IM dose engendered similar IFN-γ responses to that of IM immunizations A boost immunization at day 28 significantly augmented IFN-γ response in the IM+AI group and AI (low dose) group S-specific IFN-γ, IL-2, and TNF-α (but no IL-4) were detected in supernatants of PBMCs 14 days after the first immunization (Th1-biased response) | [32] | |
| hAd5 S-Fusion + N-ETSD | Mouse | 1 × 1010 vp (SC) 1 × 109 vp (IN) | Combinations of prime and boost through SC and IN | Stronger IgG2a, IgG2b, NAb, and N-specific antibody responses compared to hAd5-S-WT IN prime + IN boost produced similar or better humoral immunity compared to SC prime + SC or IN boost IN + SC prime-only immunization induced similar or better humoral immunity compared to those with a boost | SC prime + SC or IN boost induced stronger T cell responses compared to IN prime + IN boost | [25] |
| Ad5-S-nb2 | Mouse and rhesus macaques | For mouse: 1 × 109 vp IM 5 × 109 vp IM 1 × 109 vp IN 5 × 109 vp IN For macaque: 1 × 1011 vp IM 5 × 1010 vp IN 1 × 1010 vp IM | IM or IN | In mice: 5 × 109 vp IM induced IgGs by day 6 (continued to increase until day 28); 1 × 109 vp IM yielded a lower magnitude of IgG response IN induced weaker IgG responses by day 11 but increased to a similar level by day 28 compared with IM IN induced stronger S-specific IgAs in bronchoalveolar lavage fluids compared to IM In macaques: 1 × 1011 vp IM elicited S-specific IgG by day 12 Higher-dose immunizations led to higher IgG titers by day 24 After day 18: IgG continued to increase in IM animals but remained stable in IN animals IM induced 1–2 logs higher serum IgG titers than IN | In both mice and macaques: IM induced stronger systemic cellular immunity than IN | [31] |
| rAd5 Based (CoroVaxG.3) | Mouse | Single dose of 109 or 1010 vp | IM | Induction of S-specific IgG 2 weeks after the single immunization A single immunization of either 109 or 1010 vp induced durable antibody responses for at least 140 days Mouse sera could neutralize pseudo-viruses that expressed D614G, B.1.117, P.1, B.1.617.2 Spikes | Potent IFN-γ-T cell response as early as 2 weeks post- vaccination Durable IFN-γ-T cell response that sustained at a stable level for at least 140 days By day 140, vaccinated animals had central- memory T cells in splenocytes | [62] |
| Ad5-SARS-CoV-2 spike | Mouse | Prime: 106 PFU (LD) 109 PFU (SD) Boost: 109 PFU | IM | Antibody responses: LD/SD > SD/SD More protracted prime-boost intervals led to better antibody responses | CD8+ T cell response: LD/SD > SD/SD CD4+ T cell response: LD/SD > SD/SD | [23] |
| ChAdOx1 nCoV-19 (AZD1222) | Human | Prime: 5 × 1010 vp (SD) Boost: SD or 2.5 × 1010 vp | IM | S-specific IgG (GMT): Day 28: 35,990 Day 56: 25,667 (SD/LD) Day 56: 44,485 (SD/SD) Median NAb titers: Day 28: 451 Day 56: 253 (SD/LD) Day 56: 424 (SD/SD) | IFN-γ-T cell response peaked by day 14 after the initial immunization A boost immunization did not enhance T cell immunity | [63] |
| Ferret | Prime or prime boost: 2.5 × 1010 vp per dose | IM or IN | Prime-only: IM elicited higher NAbs than IN Prime boost: Both IM and IN induced significantly higher NAb titers 7 days after boost | 5 days after SARS-CoV-2 challenge, IM prime-boost animals displayed significantly higher levels of IFN-γ-secreting cells relative to IM prime-only animals | [39] | |
| Mouse and pig | For mouse: prime or prime boost: 108 IU For pig: prime or prime boost: 109 IU | IM | Mice: Prime-boost animals had stronger binding antibody responses than prime-only animals Pigs: Prime-boost animals had significantly stronger NAb response than prime-only animals (>1-log increase in titer) | Mice: CD4+ and CD8+ T cell responses were similar, irrespective of regimen Pigs: Prime-boost animals exhibited stronger IFN-γ responses relative to prime-only animals 2 weeks post-boost | [37] | |
| Rhesus macaque | Prime or prime boost: 2.5 × 1010 vp | IM | S-specific antibodies significantly increased after boost All prime-boost animals exhibited IgM antibodies Endpoint IgG titers Prime: 400-6400 Prime boost: 400-19,200 NAb titers Prime: 5–40 Prime boost: 10–160 | Prime boost elicited similar levels of IFN-γ-T response compared with prime-only | [38] | |
| ChAd-SARS-CoV-2-S | Hamster | Single dose of 1010 vp | IM or IN | A single immunization elicited robust S-specific and RBD-specific SARS-CoV-2- neutral izing antibodies IN induced significantly higher antibody levels compared to IM | N/A | [41] |
| ChAd-SARS-CoV-2-S | Rhesus macaque | Single dose of 1011 vp | IN | Day 21: induction of S-specific and RBD-specific binding antibodies Day 21: low levels of IgA present All vaccinated animals developed NAbs NAb titers increased by 10-fold 7 days after challenge compared to 7 days before challenge | All vaccinated animals developed T cell immunity toward the S protein of SARS-CoV-2 | [64] |
| Methyltransferase-defective VSV-based SARS-CoV-2 vaccine (rVSV-D1762A-S) | Mouse and hamster | Mice: 106 PFU Hamsters: 106 PFU | IM | IFNAR1-/-mice (immunocompromised): Developed RBD-specific antibodies that continued to increase from week 2 to week 10 BALB/c mice (immunocompetent): Developed strong NAb responses Hamsters Developed higher levels of NAbs at weeks 4 and 6 compared to convalescent plasma from 10 recovered COVID-19 patients | Th1-biased response | [49] |
| VSV-based SARS-CoV-2 vaccine (VSV-SARS2-EBOV) | Rhesus macaque | Single dose of 1 × 107 PFU | IM or IN | IM and IN both elicited robust NAb responses | IM elicited stronger cellular immunity compared to IN | [65] |
| Sputnik V (rAd26-S + rAd5-S) | Human | Prime: rAd26-S: Boost: rAd5-S Formulations: frozen or lyophilized | IM | RBD-specific IgG titers Day 42: 14,703 (frozen) Day 42: l1,143 (lyophilized) NAbs (100% seroconversion) Day 42: 4925 (frozen) Day 42: 4595 (lyophilized) | Cellular response Day 28: 2.5% CD4+ (frozen) Day 28: 1.3% CD8+ (frozen) Day 28: 1.3% CD4+ (lyophilized) Day 28: 1.1% CD8+ (lyophilized) | [35] |
| Sad23L-nCoV-S-CaP | Mouse | Prime or prime boost: 107 PFU | IM | Elicited strong S-specific antibody responses The boost immunization induced titers of: 105.01 anti-S1 binding Ab 104.77 anti-S2 binding Ab 103.04 pseudo-virus NAb | 1466.16 SFCs/106 cells (IFN-γ T cell response to S peptides) | [44,46] |
| GRAd-COV2 | Mouse and macaque | Mice: Single dose of 1 × 109 vp Macaques: Single dose of 5 × 1010 vp | IM | Mice: S- and RBD-specific antibodies rapidly rose post-vaccination and increased over time Induction of functional antibodies capable of inhibiting pseudo-type virus Macaques: RBD- and S-specific antibodies peaked between week 2 and week 4 and persisted until at least week 10 Peak NAb titers: 1580–4635 (IC50) NAb titers at week 10 were similar or higher than convalescent titers from recovered COVID-19 patients | Mice: Th1-biased response Macaques: Potent IFN-γ T cell response by week 2 (700–3500 SFC/106 PBMCs) and by week 8 (400–2500 SFC/106 PBMCs) Induction of both CD4+ and CD8+ T cells | [45] |
| Oncolytic virus (OV-spike) | Mouse | Prime boost (IV): 1 × 106 PFU or 5 × 105 PFU Prime boost (IP): 1 × 106 PFU or 2 × 105 PFU | IV or IP | BALB/c mice: Peak antibody production on day 28 50% of vaccinated mice showed high levels of antibody on day 70 C57BL/6 mice: Peak antibody production on day 21 S-specific antibodies detected as early as day 7 after the first immunization | Induction of CD4+ and CD8+ T cell immunity (ELISPOT: approximately 100 IFNγ+ SFC/ 3 × 105 splenocytes) | [59]. |
| rMeV-preS | Rat, mouse, and hamster | Rats: Day 0: 4 × 105 PFU (SC) Day 28: 2 × 106 PFU Mice: Prime or prime boost Day 0: 8 × 105 PFU (half SC and half IN) Week 4: 8 × 105 PFU Hamsters: Day 0: 8 × 105 PFU (SC and IN) Week 3: 8 × 105 PFU | IN and/or SC | Rats: All vaccinated animals developed S-specific antibodies by week 4 Mice: Prime-boost significantly augmented S-specific antibodies by week 7 compared to prime-only Hamsters: Vaccinated animals developed higher NAb titers at weeks 4 and 6 than those found in sera of 6 convalescent COVID-19 patients | Mice: Th1-biased response | [51] |
| YF-S0 | Hamster, mouse, and macaque | Hamsters: Day 0: 103 PFU (IP) Day 7: boost Mice: Day 0: 400 PFU (IP) Day 7: boost Macaques: Day 0: 105 PFU (SC) Day 7: boost | IP or SC | Hamsters Log-transformed GMT: IgG: 3.5 NAb: 2.2 Mice Log-transformed GMT: IgG: 4.0 NAb: 3.0 Macaques Log-transformed GMT: NAb: 2.6 (day 14) NAb: 2.5 (day 21) | In Mice: Th1-biassed response (ELISPOT: <500 SFC/106 splenocytes) | [55] |
2.2. Cellular Immunity
2.3. Innate Immunity
2.4. Immune Correlates of Protection
3. Durability and Breadth
4. Boosters
5. Challenges
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic: Diseases at a Glance. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 29 December 2021).
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Pinschewer, D.D. Virally vectored vaccine delivery: Medical needs, mechanisms, advantages and challenges. Swiss Med. Wkly. 2017, 147, w14465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, S.J.; Heeney, J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker and Landscape. 2022. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 25 January 2022).
- Lundstrom, K. Self-Replicating RNA Viruses for Vaccine Development against Infectious Diseases and Cancer. Vaccines 2021, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors for COVID-19 Vaccine Development. Viruses 2021, 13, 317. [Google Scholar] [CrossRef]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Tostanoski, L.H.; Wegmann, F.; Martinot, A.J.; Loos, C.; McMahan, K.; Mercado, N.B.; Yu, J.; Chan, C.N.; Bondoc, S.; Starke, C.E.; et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med. 2020, 26, 1694–1700. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef]
- Mutua, G.; Anzala, O.; Luhn, K.; Robinson, C.; Bockstal, V.; Anumendem, D.; Douoguih, M. Safety and Immunogenicity of a 2-Dose Heterologous Vaccine Regimen With Ad26.ZEBOV and MVA-BN-Filo Ebola Vaccines: 12-Month Data From a Phase 1 Randomized Clinical Trial in Nairobi, Kenya. J. Infect. Dis. 2019, 220, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef]
- Salisch, N.C.; Stephenson, K.E.; Williams, K.; Cox, F.; van der Fits, L.; Heerwegh, D.; Truyers, C.; Habets, M.N.; Kanjilal, D.G.; Larocca, R.A.; et al. A Double-Blind, Randomized, Placebo-Controlled Phase 1 Study of Ad26.ZIKV.001, an Ad26-Vectored Anti–Zika Virus Vaccine. Ann. Intern. Med. 2021, 174, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine with an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged ≥60 Years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [CrossRef] [PubMed]
- van der Lubbe, J.E.M.; Rosendahl Huber, S.K.; Vijayan, A.; Dekking, L.; van Huizen, E.; Vreugdenhil, J.; Choi, Y.; Baert, M.R.M.; Feddes-de Boer, K.; Izquierdo Gil, A.; et al. Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease. NPJ Vaccines 2021, 6, 39. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski Lisa, H.; Peter, L.; Mercado Noe, B.; McMahan, K.; Mahrokhian Shant, H.; Nkolola Joseph, P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S.; et al. Low-dose Ad26.COV2.S protection against SARS-CoV-2 challenge in rhesus macaques. Cell 2021, 184, 3467–3473.e11. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Sanchez, S.; Palacio, N.; Dangi, T.; Ciucci, T.; Penaloza-MacMaster, P. Fractionating a COVID-19 Ad5-vectored vaccine improves virus-specific immunity. Sci. Immunol. 2021, 6, eabi8635. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Balint, J.; Dinkins, K.; Adisetiyo, H.; Morimoto, B.; et al. Intranasal plus subcutaneous prime vaccination with a dual antigen COVID-19 vaccine elicits T-cell and antibody responses in mice. Sci. Rep. 2021, 11, 14917. [Google Scholar] [CrossRef] [PubMed]
- Gabitzsch, E.; Safrit, J.T.; Verma, M.; Rice, A.; Sieling, P.; Zakin, L.; Shin, A.; Morimoto, B.; Adisetiyo, H.; Wong, R.; et al. Dual-Antigen COVID-19 Vaccine Subcutaneous Prime Delivery with Oral Boosts Protects NHP Against SARS-CoV-2 Challenge. Front. Immunol. 2021, 12, 729837. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: A randomised, double-blind, placebo-controlled, phase 2b trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021. [Google Scholar] [CrossRef]
- Kim, E.; Weisel, F.J.; Balmert, S.C.; Khan, M.S.; Huang, S.; Erdos, G.; Kenniston, T.W.; Carey, C.D.; Joachim, S.M.; Conter, L.J.; et al. A single subcutaneous or intranasal immunization with adenovirus-based SARS-CoV-2 vaccine induces robust humoral and cellular immune responses in mice. Eur. J. Immunol. 2021, 51, 1774–1784. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet. Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health. Eur. 2021, 11, 100241. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Graham, S.P.; McLean, R.K.; Spencer, A.J.; Belij-Rammerstorfer, S.; Wright, D.; Ulaszewska, M.; Edwards, J.C.; Hayes, J.W.P.; Martini, V.; Thakur, N.; et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 2020, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Marsh, G.A.; McAuley, A.J.; Au, G.G.; Riddell, S.; Layton, D.; Singanallur, N.B.; Layton, R.; Payne, J.; Durr, P.A.; Bender, H.; et al. ChAdOx1 nCoV-19 (AZD1222) vaccine candidate significantly reduces SARS-CoV-2 shedding in ferrets. NPJ Vaccines 2021, 6, 67. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Bricker, T.L.; Darling, T.L.; Hassan, A.O.; Harastani, H.H.; Soung, A.; Jiang, X.; Dai, Y.N.; Zhao, H.; Adams, L.J.; Holtzman, M.J.; et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021, 36, 109400. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e113. [Google Scholar] [CrossRef]
- Hassan, A.O.; Shrihari, S.; Gorman, M.J.; Ying, B.; Yaun, D.; Raju, S.; Chen, R.E.; Dmitriev, I.P.; Kashentseva, E.; Adams, L.J.; et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021, 36, 109452. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, P.; Zou, P.; Wang, C.; Liu, B.; Wu, C.; Li, T.; Zhang, L.; Zhang, Y.; Li, C. A Self-Biomineralized Novel Adenovirus Vectored COVID-19 Vaccine for Boosting Immunization of Mice. Virol. Sin. 2021, 36, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Raggioli, A.; Gentile, M.; Battella, S.; Lahm, A.; Sommella, A.; Contino, A.M.; Urbanowicz, R.A.; Scala, R.; Barra, F.; et al. Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, P.; Liu, B.; Yang, C.; Liang, C.; Wang, Q.; Zhang, L.; Tang, X.; Li, J.; Hou, S.; et al. Prime-boost vaccination of mice and rhesus macaques with two novel adenovirus vectored COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, D.C.; Kurup, D.; Wirblich, C.; Ronk, A.J.; Mire, C.; Kuzmina, N.; Shaik, N.; Periasamy, S.; Hyde, M.A.; Williams, J.M.; et al. A single dose of replication-competent VSV-vectored vaccine expressing SARS-CoV-2 S1 protects against virus replication in a hamster model of severe COVID-19. NPJ Vaccines 2021, 6, 91. [Google Scholar] [CrossRef]
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Dravid, P.; Li, A.; Zeng, C.; Kc, M.; Trivedi, S.; Sharma, H.; Chaiwatpongsakorn, S.; Zani, A.; et al. A Methyltransferase-Defective Vesicular Stomatitis Virus-Based SARS-CoV-2 Vaccine Candidate Provides Complete Protection against SARS-CoV-2 Infection in Hamsters. J. Virol. 2021, 95, e0059221. [Google Scholar] [CrossRef]
- Case, J.B.; Rothlauf, P.W.; Chen, R.E.; Kafai, N.M.; Fox, J.M.; Smith, B.K.; Shrihari, S.; McCune, B.T.; Harvey, I.B.; Keeler, S.P.; et al. Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host Microbe 2020, 28, 465–474.e4. [Google Scholar] [CrossRef]
- Lu, M.; Dravid, P.; Zhang, Y.; Trivedi, S.; Li, A.; Harder, O.; Kc, M.; Chaiwatpongsakorn, S.; Zani, A.; Kenney, A.; et al. A safe and highly efficacious measles virus-based vaccine expressing SARS-CoV-2 stabilized prefusion spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2026153118. [Google Scholar] [CrossRef]
- Frantz, P.N.; Barinov, A.; Ruffie, C.; Combredet, C.; Najburg, V.; de Melo, G.D.; Larrous, F.; Kergoat, L.; Teeravechyan, S.; Jongkaewwattana, A.; et al. A live measles-vectored COVID-19 vaccine induces strong immunity and protection from SARS-CoV-2 challenge in mice and hamsters. Nat. Commun. 2021, 12, 6277. [Google Scholar] [CrossRef]
- Merck and IAVI Discontinue Development of COVID-19 Vaccine Candidate V590. Available online: https://www.iavi.org/news-resources/press-releases/2021/merck-and-iavi-discontinue-development-of-covid-19-vaccine-candidate-v590 (accessed on 25 January 2022).
- Merck Discontinues Development of SARS-CoV-2/COVID-19 Vaccine Candidates; Continues Development of Two Investigational Therapeutic Candidates. Available online: https://www.merck.com/news/merck-discontinues-development-of-sars-cov-2-covid-19-vaccine-candidates-continues-development-of-two-investigational-therapeutic-candidates/ (accessed on 25 January 2022).
- Sanchez-Felipe, L.; Vercruysse, T.; Sharma, S.; Ma, J.; Lemmens, V.; Van Looveren, D.; Arkalagud Javarappa, M.P.; Boudewijns, R.; Malengier-Devlies, B.; Liesenborghs, L.; et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 2021, 590, 320–325. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Li, K.; Rowe, D.K.; Diaz, M.C.H.; Griffin, E.F.; Beavis, A.C.; Johnson, S.K.; Padykula, I.; Jones, C.A.; Briggs, K.; et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 2021, 7, eabi5246. [Google Scholar] [CrossRef] [PubMed]
- Kurup, D.; Malherbe, D.C.; Wirblich, C.; Lambert, R.; Ronk, A.J.; Zabihi Diba, L.; Bukreyev, A.; Schnell, M.J. Inactivated rabies virus vectored SARS-CoV-2 vaccine prevents disease in a Syrian hamster model. PLoS Pathog. 2021, 17, e1009383. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schafer, A.; Dinnon, K.H., 3rd; Garcia-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, W.; Tian, L.; Rao, Y.; Qin, C.; Jaramillo, S.A.; Settles, E.W.; Ma, S.; Zhang, J.; Yu, K.; et al. Dual roles of a novel oncolytic viral vector-based SARS-CoV-2 vaccine: Preventing COVID-19 and treating tumor progression. Biorxiv Prepr. Serv. Biol. 2021. [Google Scholar] [CrossRef]
- Liu, R.; Americo, J.L.; Cotter, C.A.; Earl, P.L.; Erez, N.; Peng, C.; Moss, B. One or two injections of MVA-vectored vaccine shields hACE2 transgenic mice from SARS-CoV-2 upper and lower respiratory tract infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2026785118. [Google Scholar] [CrossRef]
- Tscherne, A.; Schwarz, J.H.; Rohde, C.; Kupke, A.; Kalodimou, G.; Limpinsel, L.; Okba, N.M.A.; Bosnjak, B.; Sandrock, I.; Odak, I.; et al. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2026207118. [Google Scholar] [CrossRef]
- Lopez, M.V.; Vinzon, S.E.; Cafferata, E.G.A.; Nunez, F.J.; Soto, A.; Sanchez-Lamas, M.; Afonso, M.J.; Aguilar-Cortes, D.; Rios, G.D.; Maricato, J.T.; et al. A Single Dose of a Hybrid hAdV5-Based Anti-COVID-19 Vaccine Induces a Long-Lasting Immune Response and Broad Coverage against VOC. Vaccines 2021, 9, 1106. [Google Scholar] [CrossRef]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Furuyama, W.; Shifflett, K.; Pinski, A.N.; Griffin, A.J.; Feldmann, F.; Okumura, A.; Gourdine, T.; Jankeel, A.; Lovaglio, J.; Hanley, P.W.; et al. Rapid protection from COVID-19 in nonhuman primates vaccinated intramuscularly but not intranasally with a single dose of a recombinant vaccine. Biorxiv Prepr. Serv. Biol. 2021. [Google Scholar] [CrossRef]
- Solforosi, L.; Kuipers, H.; Jongeneelen, M.; Rosendahl Huber, S.K.; van der Lubbe, J.E.M.; Dekking, L.; Czapska-Casey, D.N.; Izquierdo Gil, A.; Baert, M.R.M.; Drijver, J.; et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021, 218, e20202756. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Stephenson, K.E.; Sadoff, J.; Yu, J.; Chang, A.; Gebre, M.; McMahan, K.; Liu, J.; Chandrashekar, A.; Patel, S.; et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 385, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Silva-Cayetano, A.; Foster, W.S.; Innocentin, S.; Belij-Rammerstorfer, S.; Spencer, A.J.; Burton, O.T.; Fra-Bido, S.; Le Lee, J.; Thakur, N.; Conceicao, C.; et al. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med 2021, 2, 243–262.e8. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Yang, Y. Innate Immune Response to Adenoviral Vectors Is Mediated by both Toll-Like Receptor-Dependent and -Independent Pathways. J. Virol. 2007, 81, 3170–3180. [Google Scholar] [CrossRef] [Green Version]
- Fejer, G.; Freudenberg, M.; Greber, U.F.; Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. 2011, 1, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Netea, M.G. Trained Innate Immunity, Epigenetics, and Covid-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, e145157. [Google Scholar] [CrossRef] [PubMed]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of trained immunity by influenza vaccination—Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Manry, J.; Michailidis, E.; Hoffmann, H.-H.; Eto, S.; Garcia-Prat, M.; et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 2021, 6, eabl4340. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen Lindsey, B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Krammer, F. Correlates of protection from SARS-CoV-2 infection. Lancet 2021, 397, 1421–1423. [Google Scholar] [CrossRef]
- Bradburne, A.F.; Bynoe, M.L.; Tyrrell, D.A. Effects of a “New” Human Respiratory Virus in Volunteers. Br. Med. J. 1967, 3, 767. [Google Scholar] [CrossRef] [Green Version]
- Barrow, G.I.; Higgins, P.G.; Al-Nakib, W.; Smith, A.P.; Wenham, R.B.M.; Tyrrell, D.A.J. The effect of intranasal nedocromil sodium on viral upper respiratory tract infections in human volunteers. Clin. Exp. Allergy 1990, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wong, R.; Soo, Y.O.Y.; Wong, W.S.; Lee, C.K.; Ng, M.H.L.; Chan, P.; Wong, K.C.; Leung, C.B.; Cheng, G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.; Perlman, S. T Cell Responses Are Required for Protection from Clinical Disease and for Virus Clearance in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford Katharine, H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome Keith, R.; Bloom Jesse, D.; Greninger Alexander, L.; McAdam Alexander, J. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107–e02120. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Roozendaal, R.; Solforosi, L.; Stieh, D.J.; Serroyen, J.; Straetemans, R.; Dari, A.; Boulton, M.; Wegmann, F.; Rosendahl Huber, S.K.; van der Lubbe, J.E.M.; et al. SARS-CoV-2 binding and neutralizing antibody levels after Ad26.COV2.S vaccination predict durable protection in rhesus macaques. Nat. Commun. 2021, 12, 5877. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2021, 375, 43–50. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Cardenas, V.; Shukarev, G.; Vaissiere, N.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Scheper, G.; Hendriks, J.; et al. Durability of antibody responses elicited by a single dose of Ad26.COV2.S and substantial increase following late boosting. Medrxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Pegu, A.; O’Connell Sarah, E.; Schmidt Stephen, D.; O’Dell, S.; Talana Chloe, A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett Kizzmekia, S.; et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Zabaleta, N.; Dai, W.; Bhatt, U.; Herate, C.; Maisonnasse, P.; Chichester, J.A.; Sanmiguel, J.; Estelien, R.; Michalson, K.T.; Diop, C.; et al. An AAV-based, room-temperature-stable, single-dose COVID-19 vaccine provides durable immunogenicity and protection in non-human primates. Cell Host Microbe 2021, 29, 1437–1453.e1438. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.-H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Tatsis, N.; Fitzgerald, J.C.; Reyes-Sandoval, A.; Harris-McCoy, K.C.; Hensley, S.E.; Zhou, D.; Lin, S.-W.; Bian, A.; Xiang, Z.Q.; Iparraguirre, A.; et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: Implications for their use as vaccines. Blood 2007, 110, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Schmidt, F.; Schaefer-Babajew, D.; Wang, Z.; Lorenzi, J.C.; Flyak, A.I.; DeLaitsch, A.T.; Huey-Tubman, K.E.; et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021, 54, 1853–1868.e7. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Normark, J.; Vikstrom, L.; Gwon, Y.D.; Persson, I.L.; Edin, A.; Bjorsell, T.; Dernstedt, A.; Christ, W.; Tevell, S.; Evander, M.; et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 385, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Groß, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pöhlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2022, 75, 103761. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Rietdijk, W.J.R.; Goorhuis, A.; Postma, D.F.; Visser, L.G.; Geers, D.; Schmitz, K.S.; Garrido, H.M.G.; Koopmans, M.P.G.; Dalm, V.A.S.H.; et al. Immunogenicity and reactogenicity of booster vaccinations after Ad26.COV2.S priming. MedRxiv 2021. [Google Scholar] [CrossRef]
- Kim Huat, N.K.; Er Lim, J.M.; Gill, U.S.; de Alwis, R.; Tan, N.; Nan Toh, J.Z.; Abbott, J.E.; Usai, C.; Ooi, E.E.; Hong Low, J.G.; et al. Differential immunogenicity of homologous versus heterologous boost in Ad26.COV2.S vaccine recipients. MedRxiv 2021. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Nair, M.S.; Chandrashekar, A.; Mohri, H.; Wang, M.; Barouch, D.H.; Huang, Y.; Ho, D.D. Ad26.COV2.S boosts antibody and T-cell responses following BNT162b2 vaccination. Emerg. Microbes Infect. 2021, 10, 2220–2222. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef] [PubMed]
- Reimann, P.; Ulmer, H.; Mutschlechner, B.; Benda, M.; Severgnini, L.; Volgger, A.; Lang, T.; Atzl, M.; Huynh, M.; Gasser, K.; et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br. J. Haematol. 2022, 196, 577–584. [Google Scholar] [CrossRef]
- Khan, K.; Lustig, G.; Bernstein, M.; Archary, D.; Cele, S.; Karim, F.; Smith, M.; Ganga, Y.; Jule, Z.; Reedoy, K.; et al. Immunogenicity of SARS-CoV-2 infection and Ad26.CoV2.S vaccination in people living with HIV. Clin. Infect. Dis. 2021, ciab1008. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef]
- Suzuki-Kouyama, E.; Katayama, K.; Sakurai, F.; Yamaguchi, T.; Kurachi, S.; Kawabata, K.; Nakagawa, S.; Mizuguchi, H. Hexon-specific PEGylated adenovirus vectors utilizing avidin-biotin interaction. Biomaterials 2011, 32, 1724–1730. [Google Scholar] [CrossRef]
- Yotnda, P.; Chen, D.-H.; Chiu, W.; Piedra, P.A.; Davis, A.; Templeton, N.S.; Brenner, M.K. Bilamellar Cationic Liposomes Protect Adenovectors from Preexisting Humoral Immune Responses. Mol. Ther. 2002, 5, 233–241. [Google Scholar] [CrossRef]
- Rodriguez, E.V.C.; Bouazza, F.Z.; Dauby, N.; Mullier, F.; d’Otreppe, S.; Jissendi Tchofo, P.; Bartiaux, M.; Sirjacques, C.; Roman, A.; Hermans, C.; et al. Fatal vaccine-induced immune thrombotic thrombocytopenia (VITT) post Ad26.COV2.S: First documented case outside US. Infection 2021. [Google Scholar] [CrossRef]
- Siegler, J.E.; Klein, P.; Yaghi, S.; Vigilante, N.; Abdalkader, M.; Coutinho, J.M.; Abdul Khalek, F.; Nguyen, T.N. Cerebral Vein Thrombosis With Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke 2021, 52, 3045–3053. [Google Scholar] [CrossRef]
- Gurtler, L.; Seitz, R.; Schramm, W. Cerebral venous thrombosis after COVID-19 vaccination: Is the risk of thrombosis increased by intravascular application of the vaccine? Infection 2021, 49, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, E.R.; Mabbott, N.A. The clinical correlates of vaccine-induced immune thrombotic thrombocytopenia after immunisation with adenovirus vector-based SARS-CoV-2 vaccines. Immunother. Adv. 2021, 1, ltab019. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P.; Ng, A.P.; Linden, M.; Ignjatovic, V.; Farley, A.; Taoudi, S.; Pasricha, S.R.; Torresi, J. Vaccine-induced immune thrombosis and thrombocytopenia syndrome following adenovirus-vectored severe acute respiratory syndrome coronavirus 2 vaccination: A novel hypothesis regarding mechanisms and implications for future vaccine development. Immunol. Cell Biol. 2021, 99, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Volker, U.; et al. Insights in ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Othman, M.; Labelle, A.; Mazzetti, I.; Elbatarny, H.S.; Lillicrap, D. Adenovirus-induced thrombocytopenia: The role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 2006, 109, 2832–2839. [Google Scholar] [CrossRef]
- Roman, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM):Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 12, 653786. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Romano, A.; Bozzao, A.; Salvetti, M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult. Scler. 2021, 13524585211040222. [Google Scholar] [CrossRef]
- Nasuelli, N.A.; De Marchi, F.; Cecchin, M.; De Paoli, I.; Onorato, S.; Pettinaroli, R.; Savoini, G.; Godi, L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 4747–4749. [Google Scholar] [CrossRef]
- Shin, M.; Hyun, C.Y.; Choi, Y.H.; Choi, J.Y.; Lee, K.H.; Cho, Y.S. COVID-19 Vaccination-Associated Lymphadenopathy on FDG PET/CT: Distinctive Features in Adenovirus-Vectored Vaccine. Clin. Nucl. Med. 2021, 46, 814–819. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, A.; Yu, J. Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19. Viruses 2022, 14, 380. https://doi.org/10.3390/v14020380
Chang A, Yu J. Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19. Viruses. 2022; 14(2):380. https://doi.org/10.3390/v14020380
Chicago/Turabian StyleChang, Aiquan, and Jingyou Yu. 2022. "Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19" Viruses 14, no. 2: 380. https://doi.org/10.3390/v14020380
APA StyleChang, A., & Yu, J. (2022). Fighting Fire with Fire: Immunogenicity of Viral Vectored Vaccines against COVID-19. Viruses, 14(2), 380. https://doi.org/10.3390/v14020380






