Kinetics of Glycoprotein-Specific Antibody Response in Patients with Severe Fever with Thrombocytopenia Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Quantitative RT-PCR
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis of Specific Antibodies to NP and Gn
2.4. Cytokine Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
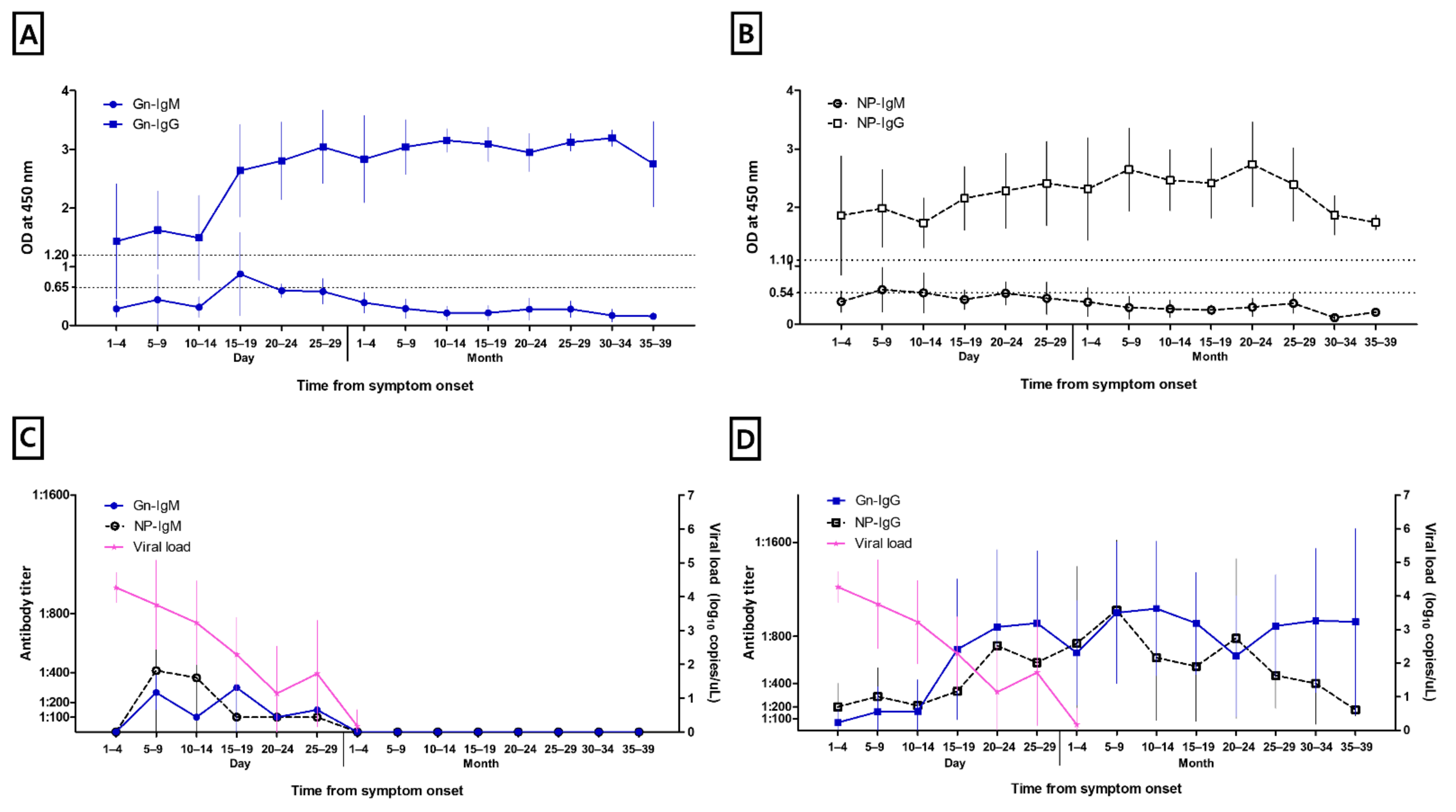
3.2. Anti-Gn and NP Specific IgM and IgG
3.3. Viral Load
3.4. Cytokines and Chemokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in Czhina. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.J.; Park, S.W.; Bae, I.G.; Kim, S.H.; Ryu, S.Y.; Kim, H.A.; Jang, H.C.; Hur, J.; Jun, J.B.; Jung, Y.; et al. Severe Fever with Thrombocytopenia Syndrome in South Korea, 2013–2015. PLoS Negl. Trop. Dis. 2016, 10, e0005264. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, B.; Li, Y.; Du, Y.; Ma, H.; Li, X.; Guo, W.; Xu, B.; Huang, X. Severe fever with thrombocytopenia syndrome: A systematic review and meta-analysis of epidemiology, clinical signs, routine laboratory diagnosis, risk factors, and outcomes. BMC Infect. Dis. 2020, 20, 575. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, C.; Zhan, F.; Wang, X.; Liang, M.; Zhang, Q.; Ding, S.; Guan, X.; Huo, X.; Li, C.; et al. Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J. Infect. Dis. 2012, 206, 1085–1094. [Google Scholar] [CrossRef]
- Song, P.; Zheng, N.; Zhang, L.; Liu, Y.; Chen, T.; Bao, C.; Li, Z.; Yong, W.; Zhang, Y.; Wu, C.; et al. Downregulation of Interferon-β and Inhibition of TLR3 Expression are associated with Fatal Outcome of Severe Fever with Thrombocytopenia Syndrome. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Zheng, N.; Liu, Y.; Tian, C.; Wu, X.; Ma, X.; Chen, D.; Zou, X.; Wang, G.; Wang, H.; et al. Deficient humoral responses and disrupted B-cell immunity are associated with fatal SFTSV infection. Nat. Commun. 2018, 9, 3328. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kühl, A.; Kaup, F.; Soldan, S.S.; González-Scarano, F.; Weber, F.; He, Y.; et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 2013, 87, 4384–4394. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qi, Y.; Liu, C.; Gao, W.; Chen, P.; Fu, L.; Peng, B.; Wang, H.; Jing, Z.; Zhong, G.; et al. Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J. Virol. 2014, 88, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.B.; Cui, N.; Hu, J.G.; Chen, W.W.; Xu, W.; Li, H.; Zhang, X.A.; Ly, H.; Liu, W.; Cao, W.C. Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: A cohort study in China. Vaccine 2015, 33, 1250–1255. [Google Scholar] [CrossRef]
- Wang, G.; Chang, H.; Jia, B.; Liu, Y.; Huang, R.; Wu, W.; Hao, Y.; Yan, X.; Xia, J.; Chen, Y.; et al. Nucleocapsid protein-specific IgM antibody responses in the disease progression of severe fever with thrombocytopenia syndrome. Ticks Tick Borne Dis. 2019, 10, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.H.; Kim, M.J.; Kim, M.C.; Park, S.Y.; Park, S.Y.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Lee, K.H.; et al. Kinetics of Serological Response in Patients with Severe Fever with Thrombocytopenia Syndrome. Viruses 2020, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kong, Q.; Liu, Y.; Li, J.; Bian, T.; Ma, X.; Ye, Y.; Li, J. Time Course of Severe Fever With Thrombocytopenia Syndrome Virus and Antibodies in Patients by Long-Term Follow-Up Study, China. Front. Microbiol. 2021, 12, 744037. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Koo, B.; Jin, C.E.; Kim, M.C.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Shin, Y.; et al. Rapid Diagnosis of Tick-Borne Illnesses by Use of One-Step Isothermal Nucleic Acid Amplification and Bio-Optical Sensor Detection. Clin. Chem. 2018, 64, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.T.; Zhao, L.; Wen, H.L.; Yang, Y.; Yu, H.; Yu, X.J. Neutralizing Antibodies to Severe Fever with Thrombocytopenia Syndrome Virus 4 Years after Hospitalization, China. Emerg. Infect. Dis. 2016, 22, 1985–1987. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhang, L.; Zhang, W.; Chi, Y.; Zeng, X.; Li, X.; Qi, X.; Jin, Q.; Zhang, X.; Huang, M.; et al. Human antibody neutralizes severe Fever with thrombocytopenia syndrome virus, an emerging hemorrhagic Fever virus. Clin. Vaccine Immunol. 2013, 20, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kim, J.; Ko, M.; Chun, J.Y.; Kim, H.; Kim, S.; Min, J.Y.; Park, W.B.; Oh, M.D.; Chung, J. An anti-Gn glycoprotein antibody from a convalescent patient potently inhibits the infection of severe fever with thrombocytopenia syndrome virus. PLoS Pathog. 2019, 15, e1007375. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T. Vaccine Development for Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 627. [Google Scholar] [CrossRef]
- Park, A.; Park, S.J.; Jung, K.L.; Kim, S.M.; Kim, E.H.; Kim, Y.I.; Foo, S.S.; Kim, S.; Kim, S.G.; Yu, K.M.; et al. Molecular Signatures of Inflammatory Profile and B-Cell Function in Patients with Severe Fever with Thrombocytopenia Syndrome. mBio 2021, 12, e02583-20. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Xing, Y.; Li, S.; Kong, L.; Zhang, Y.; Zhang, L.; Liu, N.; Wang, Q.; Wang, S.; et al. Concurrent measurement of dynamic changes in viral load, serum enzymes, T cell subsets, and cytokines in patients with severe fever with thrombocytopenia syndrome. PLoS ONE 2014, 9, e91679. [Google Scholar] [CrossRef]
- Liu, M.M.; Lei, X.Y.; Yu, H.; Zhang, J.Z.; Yu, X.J. Correlation of cytokine level with the severity of severe fever with thrombocytopenia syndrome. Virol. J. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.S.; Kim, M.C.; Kim, J.Y.; Jeon, N.Y.; Ryu, B.H.; Hong, J.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; et al. Kinetics of viral load and cytokines in severe fever with thrombocytopenia syndrome. J. Clin. Virol. 2018, 101, 57–62. [Google Scholar] [CrossRef] [PubMed]


| Days from Symptom Onset | Gn-IgM | Gn-IgG | NP-IgM | NP-IgG |
|---|---|---|---|---|
| Days 1–4 | 0/3 (0) | 1/3 (33.3) | 0/3 (0) | 2/3 (66.7) |
| Days 5–9 | 3/16 (18.8) | 9/16 (56.3) | 6/16 (37.5) | 14/16 (87.5) |
| Days 10–14 | 1/9 (11.1) | 6/9 (66.7) | 4/9 (44.4) | 9/9 (100.0) |
| Days 15–19 | 4/9 (44.4) | 9/9 (100.0) | 2/9 (22.2) | 9/9 (100.0) |
| Days 20–24 | 2/5 (40.0) | 5/5 (100.0) | 2/5 (40.0) | 5/5 (100.0) |
| Days 25–29 | 2/8 (25.0) | 8/8 (100.0) | 2/8 (25.0) | 8/8 (100.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.; Kim, E.; Kwon, B.; Cho, Y.-G.; Bae, S.; Jung, J.; Kim, M.-J.; Chong, Y.-P.; Kim, S.-H.; Lee, S.-O.; et al. Kinetics of Glycoprotein-Specific Antibody Response in Patients with Severe Fever with Thrombocytopenia Syndrome. Viruses 2022, 14, 256. https://doi.org/10.3390/v14020256
Chung H, Kim E, Kwon B, Cho Y-G, Bae S, Jung J, Kim M-J, Chong Y-P, Kim S-H, Lee S-O, et al. Kinetics of Glycoprotein-Specific Antibody Response in Patients with Severe Fever with Thrombocytopenia Syndrome. Viruses. 2022; 14(2):256. https://doi.org/10.3390/v14020256
Chicago/Turabian StyleChung, Hyemin, Eunsil Kim, Bomin Kwon, Yeong-Geon Cho, Seongman Bae, Jiwon Jung, Min-Jae Kim, Yong-Pil Chong, Sung-Han Kim, Sang-Oh Lee, and et al. 2022. "Kinetics of Glycoprotein-Specific Antibody Response in Patients with Severe Fever with Thrombocytopenia Syndrome" Viruses 14, no. 2: 256. https://doi.org/10.3390/v14020256
APA StyleChung, H., Kim, E., Kwon, B., Cho, Y.-G., Bae, S., Jung, J., Kim, M.-J., Chong, Y.-P., Kim, S.-H., Lee, S.-O., Choi, S.-H., Kim, Y.-S., & Korea SFTS Study Group. (2022). Kinetics of Glycoprotein-Specific Antibody Response in Patients with Severe Fever with Thrombocytopenia Syndrome. Viruses, 14(2), 256. https://doi.org/10.3390/v14020256






