Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein
Abstract
:1. Introduction
2. Results
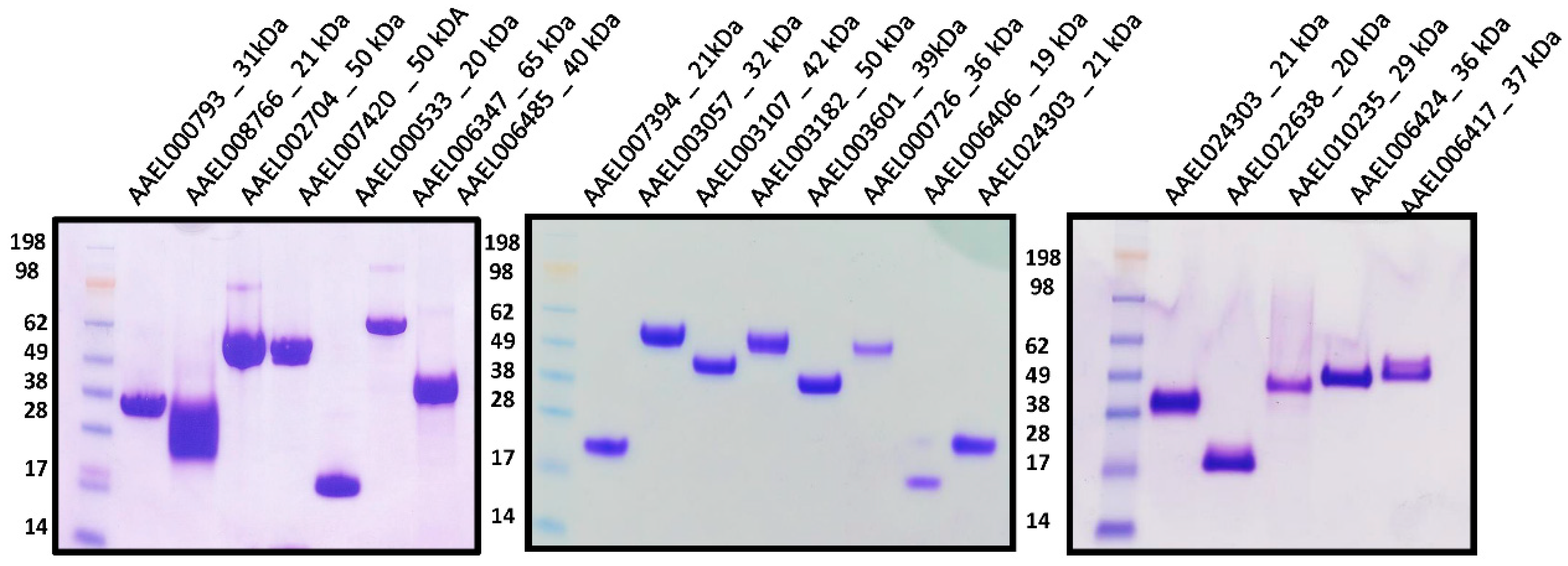
2.1. Expression and Purification of Recombinant Salivary Proteins from Aedes aegypti Mosquitoes
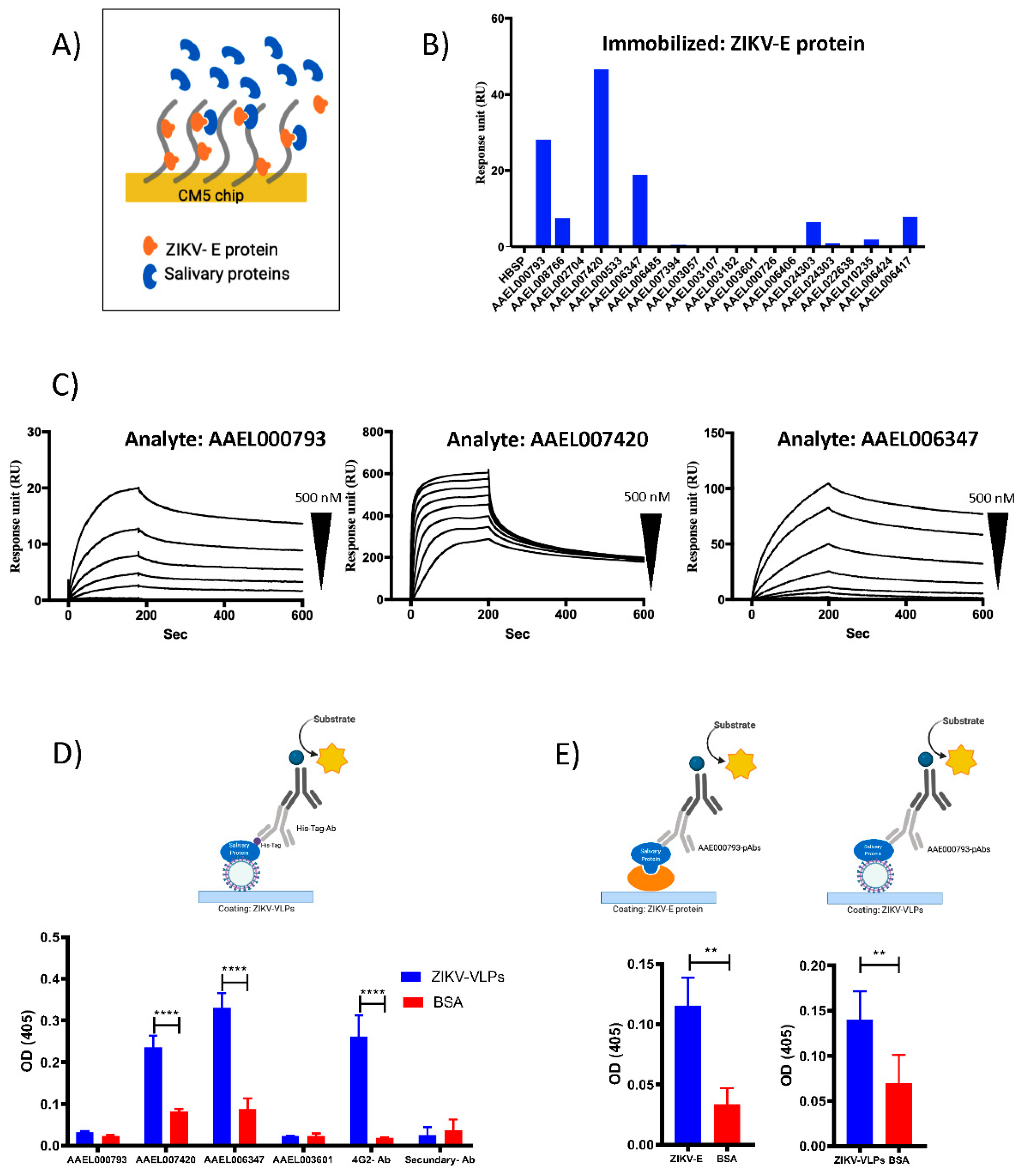
2.2. Recombinant Salivary Proteins from Aedes aegypti Mosquitoes Bind to ZIKV-E and ZIKV-VLPs
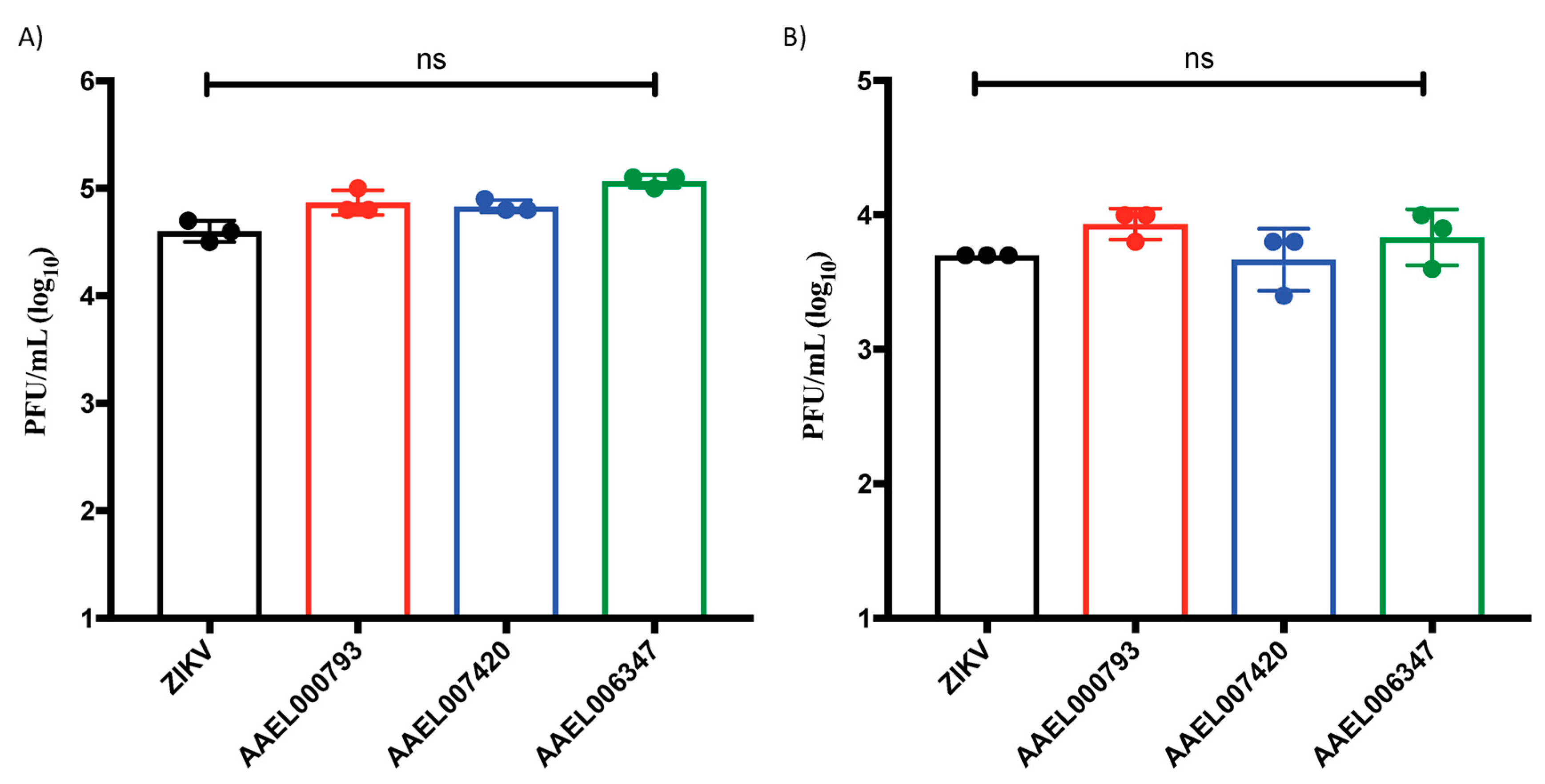
2.3. Proteins That Interact with the ZIKV-E and ZIKV-VLPs Do Not Affect the Viral Replication in In Vitro Cell Cultures of Endothelial Cells and Keratinocytes
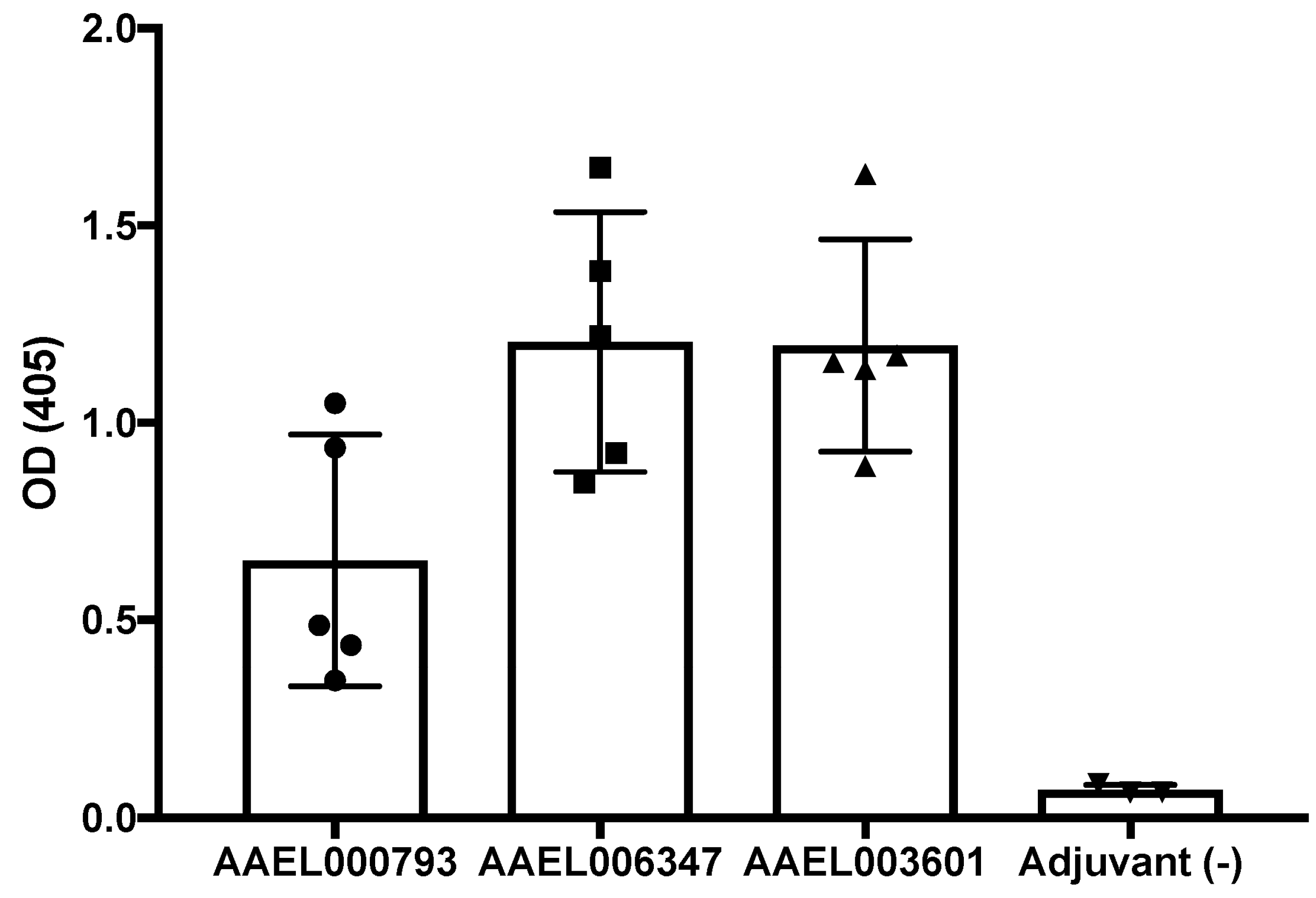
2.4. Production of Polyclonal Antibodies againtst Recombinant Mosquito Salivary Proteins
2.5. Mosquito Salivary Proteins That Interacting with ZIKV-E and ZIKV-VLPs Virus Are Immunogenic in ZIKV and DENV Serum Samples
3. Discussion
4. Materials and Methods
4.1. Protein Purification
4.2. Surface Plasmon Resonance (SPR) Analysis
4.3. Binding ELISA Assays
4.4. Polyclonal Antibodies
4.5. Anti- Recombinant Salivary Protein Antibody Detection in Mice Serum Samples Inmunized with the Salicary Recombinants AAEL000793, AAEL007420 and AAEL006347
4.6. Anti- Recombinant Salivary Protein Antibody Detection in DENV and ZIKV Serum Samples
4.7. Cells and Viruses
4.8. ZIKV Virus Quantification
4.9. Serum Samples from DENV and ZIKV Patients
4.10. Mosquito Salivary Gland Dissections
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Memórias Do Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Mans, B.J.; Arca, B. An insight into the sialome of blood-feeding Nematocera. Insect. Biochem. Mol. Biol. 2010, 40, 767–784. [Google Scholar] [CrossRef] [Green Version]
- Styer, L.M.; Lim, P.Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Mota, J.; Sukupolvi-Petty, S.; Diamond, M.S.; Rico-Hesse, R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012, 86, 7637–7649. [Google Scholar] [CrossRef] [Green Version]
- Le Coupanec, A.; Babin, D.; Fiette, L.; Jouvion, G.; Ave, P.; Misse, D.; Bouloy, M.; Choumet, V. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl. Trop. Dis. 2013, 7, e2237. [Google Scholar] [CrossRef]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef] [Green Version]
- Surasombatpattana, P.; Hamel, R.; Patramool, S.; Luplertlop, N.; Thomas, F.; Despres, P.; Briant, L.; Yssel, H.; Misse, D. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect. Genet. Evol. 2011, 11, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Wichit, S.; Diop, F.; Hamel, R.; Talignani, L.; Ferraris, P.; Cornelie, S.; Liegeois, F.; Thomas, F.; Yssel, H.; Misse, D. Aedes Aegypti saliva enhances chikungunya virus replication in human skin fibroblasts via inhibition of the type I interferon signaling pathway. Infect. Genet. Evol. 2017, 55, 68–70. [Google Scholar] [CrossRef]
- Garcia, M.; Alout, H.; Diop, F.; Damour, A.; Bengue, M.; Weill, M.; Misse, D.; Leveque, N.; Bodet, C. Innate immune response of primary human keratinocytes to west nile virus infection and its modulation by mosquito saliva. Front. Cell. Infect. Microbiol. 2018, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Surasombatpattana, P.; Ekchariyawat, P.; Hamel, R.; Patramool, S.; Thongrungkiat, S.; Denizot, M.; Delaunay, P.; Thomas, F.; Luplertlop, N.; Yssel, H.; et al. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J. Investig. Dermatol. 2014, 134, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Guo, X.; Shen, C.; Hao, X.; Sun, P.; Li, P.; Xu, T.; Hu, C.; Rose, O.; Zhou, H.; et al. Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin-beta receptor. Nat. Immunol. 2018, 19, 342–353. [Google Scholar] [CrossRef]
- Sun, P.; Nie, K.; Zhu, Y.; Liu, Y.; Wu, P.; Liu, Z.; Du, S.; Fan, H.; Chen, C.H.; Zhang, R.; et al. A mosquito salivary protein promotes flavivirus transmission by activation of autophagy. Nat. Commun. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao-Lormeau, V.M. Dengue viruses binding proteins from Aedes aegypti and Aedes polynesiensis salivary glands. Virol. J. 2009, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cime-Castillo, J.; Delannoy, P.; Mendoza-Hernandez, G.; Monroy-Martinez, V.; Harduin-Lepers, A.; Lanz-Mendoza, H.; Hernandez-Hernandez Fde, L.; Zenteno, E.; Cabello-Gutierrez, C.; Ruiz-Ordaz, B.H. Sialic acid expression in the mosquito Aedes aegypti and its possible role in dengue virus-vector interactions. BioMed Res. Int. 2015, 2015, 504187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, M.J.; Londono-Renteria, B.; Troupin, A.; Watson, A.M.; Klimstra, W.B.; Fikrig, E.; Colpitts, T.M. Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLoS Negl. Trop. Dis. 2016, 10, e0004941. [Google Scholar] [CrossRef]
- Conway, M.J.; Watson, A.M.; Colpitts, T.M.; Dragovic, S.M.; Li, Z.; Wang, P.; Feitosa, F.; Shepherd, D.T.; Ryman, K.D.; Klimstra, W.B.; et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J. Virol. 2014, 88, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Londono-Renteria, B.; Cardenas, J.C.; Cardenas, L.D.; Christofferson, R.C.; Chisenhall, D.M.; Wesson, D.M.; McCracken, M.K.; Carvajal, D.; Mores, C.N. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS ONE 2013, 8, e81211. [Google Scholar] [CrossRef]
- Doucoure, S.; Mouchet, F.; Cournil, A.; Le Goff, G.; Cornelie, S.; Roca, Y.; Giraldez, M.G.; Simon, Z.B.; Loayza, R.; Misse, D.; et al. Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: A new biomarker of exposure to Dengue vector bites. Am. J. Trop. Med. Hyg. 2012, 87, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.E.; Oliveira, F.; Coutinho-Abreu, I.V.; Herbert, S.; Meneses, C.; Kamhawi, S.; Baus, H.A.; Han, A.; Czajkowski, L.; Rosas, L.A.; et al. Safety and immunogenicity of a mosquito saliva peptide-based vaccine: A randomised, placebo-controlled, double-blind, phase 1 trial. Lancet 2020, 395, 1998–2007. [Google Scholar] [CrossRef]
- Hastings, A.K.; Uraki, R.; Gaitsch, H.; Dhaliwal, K.; Stanley, S.; Sproch, H.; Williamson, E.; MacNeil, T.; Marin-Lopez, A.; Hwang, J.; et al. Aedes aegypti NeSt1 protein enhances zika virus pathogenesis by activating neutrophils. J. Virol. 2019, 93, e00395-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, H.A.; Singh, S.; Champagne, D.E. Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function. Parasite Immunol. 2004, 26, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.S.; Higgs, S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Kneubehl, A.R.; Spencer Clinton, J.L.; Paust, S.; Rico-Hesse, R. Mosquito saliva alone has profound effects on the human immune system. PLoS Negl. Trop. Dis. 2018, 12, e0006439. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Martin-Martin, I.; Arca, B.; Calvo, E. A deep insight into the sialome of male and female Aedes aegypti mosquitoes. PLoS ONE 2016, 11, e0151400. [Google Scholar] [CrossRef] [Green Version]
- Calvo, E.; Mizurini, D.M.; Sa-Nunes, A.; Ribeiro, J.M.; Andersen, J.F.; Mans, B.J.; Monteiro, R.Q.; Kotsyfakis, M.; Francischetti, I.M. Alboserpin, a factor Xa inhibitor from the mosquito vector of yellow fever, binds heparin and membrane phospholipids and exhibits antithrombotic activity. J. Biol. Chem. 2011, 286, 27998–28010. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.M.; Sarkis, J.J.; Rossignol, P.A.; Spielman, A. Salivary apyrase of Aedes aegypti: Characterization and secretory fate. Comp. Biochem. Physiol. B 1984, 79, 81–86. [Google Scholar] [CrossRef]
- Champagne, D.E.; Smartt, C.T.; Ribeiro, J.M.; James, A.A. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5’-nucleotidase family. Proc. Natl. Acad. Sci. USA 1995, 92, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Daniella, A.; Lefteri, S.R.B.; Pingen, M.; Terry, S.; Beswick, E.F.; Georgiev, G.; Van der Laan, M.; Mastrullo, V.; Campagnolo, P.; Waterhouse, R.; et al. Mosquito saliva sialokinin-dependent enhancement of arbovirus infection through endothelial barrier leakage. bioRxiv 2021. [Google Scholar] [CrossRef]
- Buezo Montero, S.; Gabrieli, P.; Severini, F.; Picci, L.; Di Luca, M.; Forneris, F.; Facchinelli, L.; Ponzi, M.; Lombardo, F.; Arca, B. Analysis in a murine model points to IgG responses against the 34k2 salivary proteins from Aedes albopictus and Aedes aegypti as novel promising candidate markers of host exposure to Aedes mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007806. [Google Scholar] [CrossRef] [Green Version]
- Londono-Renteria, B.; Montiel, J.; Calvo, E.; Tobon-Castano, A.; Valdivia, H.O.; Escobedo-Vargas, K.; Romero, L.; Bosantes, M.; Fisher, M.L.; Conway, M.J.; et al. Antibody responses against anopheles darlingi immunogenic peptides in plasmodium infected humans. Front. Cell. Infect. Microbiol. 2020, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Doucoure, S.; Cornelie, S.; Patramool, S.; Mouchet, F.; Demettre, E.; Seveno, M.; Dehecq, J.S.; Rutee, H.; Herve, J.P.; Favier, F.; et al. First screening of Aedes albopictus immunogenic salivary proteins. Insect. Mol. Biol. 2013, 22, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.L.; Shakeri, H.; Rozo-Lopez, P.; Conway, M.J.; Duggan, N.; Jaberi-Douraki, M.; Colpitts, T.M. Serosurvey of human antibodies recognizing aedes aegypti D7 salivary proteins in Colombia. Front. Public Health 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, J.C.; Drame, P.M.; Luque-Burgos, K.A.; Berrio, J.D.; Entrena-Mutis, E.; Gonzalez, M.U.; Carvajal, D.J.; Gutierrez-Silva, L.Y.; Cardenas, L.D.; Colpitts, T.M.; et al. IgG1 and IgG4 antibodies against Aedes aegypti salivary proteins and risk for dengue infections. PLoS ONE 2019, 14, e0208455. [Google Scholar] [CrossRef] [Green Version]
- Oktarianti, R.; Senjarini, K.; Hayano, T.; Fatchiyah, F. Proteomic analysis of immunogenic proteins from salivary glands of Aedes aegypti. J. Infect. Public Health 2015, 8, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Machain-Williams, C.; Mammen, M.P., Jr.; Zeidner, N.S.; Beaty, B.J.; Prenni, J.E.; Nisalak, A.; Blair, C.D. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol. 2012, 34, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Castanha, P.M.S.; Erdos, G.; Watkins, S.C.; Falo, L.D., Jr.; Marques, E.T.A.; Barratt-Boyes, S.M. Reciprocal immune enhancement of dengue and Zika virus infection in human skin. JCI Insight 2020, 5, e133653. [Google Scholar] [CrossRef]
- Olajiga, O.; Holguin-Rocha, A.F.; Rippee-Brooks, M.; Eppler, M.; Harris, S.L.; Londono-Renteria, B. Vertebrate responses against arthropod salivary proteins and their therapeutic potential. Vaccines 2021, 9, 347. [Google Scholar] [CrossRef]
- Schneider, B.S.; McGee, C.E.; Jordan, J.M.; Stevenson, H.L.; Soong, L.; Higgs, S. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS ONE 2007, 2, e1171. [Google Scholar] [CrossRef] [Green Version]
- Reagan, K.L.; Machain-Williams, C.; Wang, T.; Blair, C.D. Immunization of mice with recombinant mosquito salivary protein D7 enhances mortality from subsequent West Nile virus infection via mosquito bite. PLoS Negl. Trop. Dis. 2012, 6, e1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, M.A.; Glasner, D.R.; Shah, S.; Michlmayr, D.; Kramer, L.D.; Harris, E. Mosquito saliva increases endothelial permeability in the skin, immune cell migration, and dengue pathogenesis during antibody-dependent enhancement. PLoS Pathog. 2016, 12, e1005676. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Protein | ka (1/Ms) | kd (1/s) | KD (nM) |
|---|---|---|---|
| NIH435-3 | 20,400 ± 4667 | 0.0005310 ± 0.00004243 | 26.49 ± 4.101 |
| NIH435-7 | 5,681,850 ± 7,945,264 | 0.003508 ± 0.002630 | 13.18 ± 17.97 |
| NIH435-9 | 75,550 ± 1485 | 0.001057 ± 0.0003019 | 13.99 ± 4.243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Leon, P.C.; Shrivastava, G.; Martin-Martin, I.; Cardenas, J.C.; Londono-Renteria, B.; Calvo, E. Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein. Viruses 2022, 14, 221. https://doi.org/10.3390/v14020221
Valenzuela-Leon PC, Shrivastava G, Martin-Martin I, Cardenas JC, Londono-Renteria B, Calvo E. Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein. Viruses. 2022; 14(2):221. https://doi.org/10.3390/v14020221
Chicago/Turabian StyleValenzuela-Leon, Paola Carolina, Gaurav Shrivastava, Ines Martin-Martin, Jenny C. Cardenas, Berlin Londono-Renteria, and Eric Calvo. 2022. "Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein" Viruses 14, no. 2: 221. https://doi.org/10.3390/v14020221
APA StyleValenzuela-Leon, P. C., Shrivastava, G., Martin-Martin, I., Cardenas, J. C., Londono-Renteria, B., & Calvo, E. (2022). Multiple Salivary Proteins from Aedes aegypti Mosquito Bind to the Zika Virus Envelope Protein. Viruses, 14(2), 221. https://doi.org/10.3390/v14020221








