Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Virus
2.3. Recombinant Vaccine Design
2.4. Animal Cohort
2.5. rSVV-SIV Immunization and SIV Challenge
2.6. Clinical Evaluations
2.7. Peripheral Blood: Lymph Node (LN) and Intestinal Cells Processing Techniques
2.8. Quantitative SIV Plasma Virus Load (VL)
2.9. Quantification of SIV Antigen-Specific IgG Responses
2.10. Quantification of SIVMAC Neutralization Antibody Titers
2.11. Quantification of SVV Vector Antibody Titers
2.12. Binding Antibody Multiplex Assay (BAMA)
2.13. Tissue T-cell Immunophenotyping
2.14. Antigen-Specific Intracellular Cytokine Flow Cytometry Staining and Quantification
2.15. Statistical Analyses
3. Results
3.1. Immunization and SIV Challenge
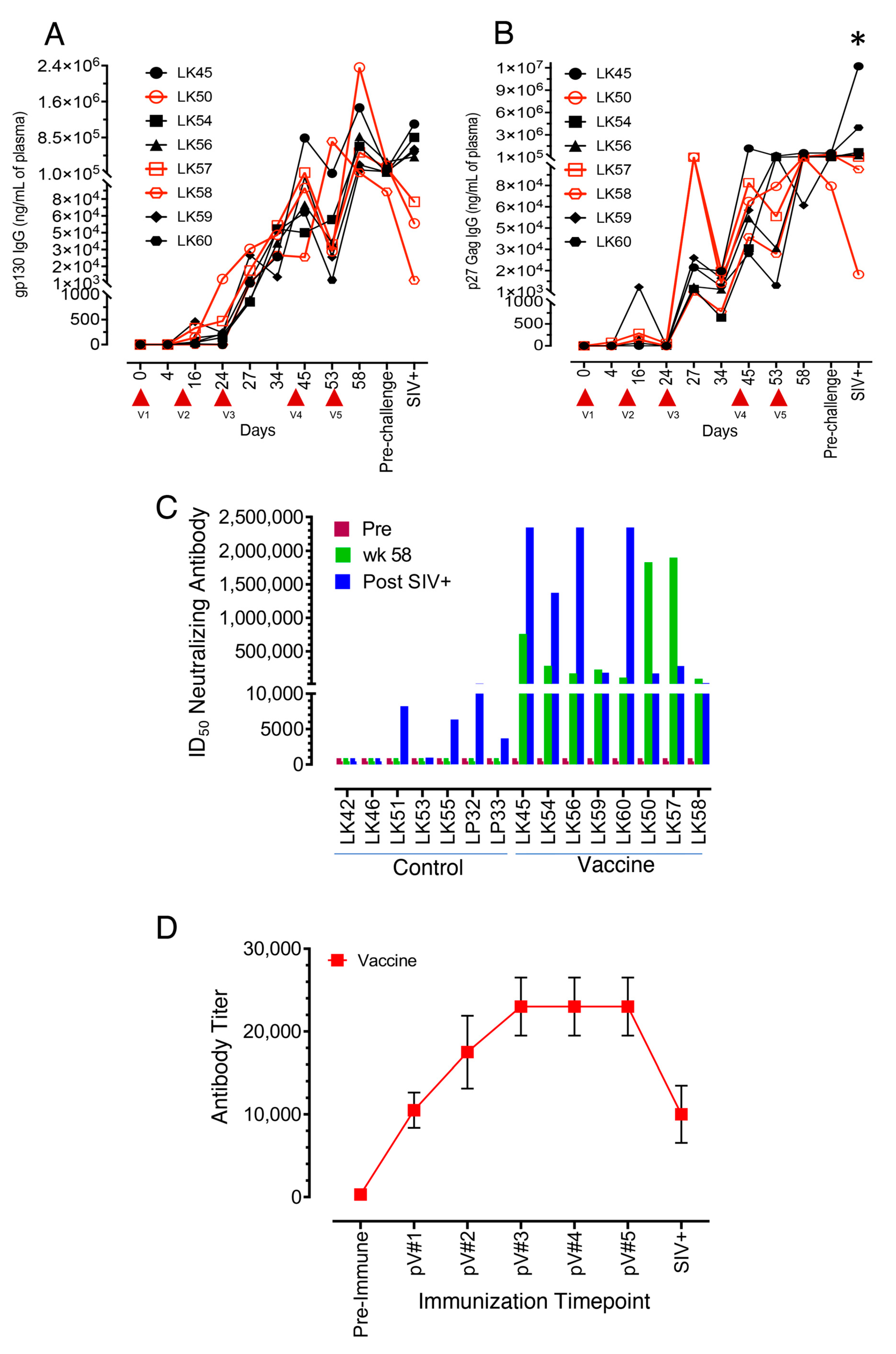
3.2. All Vaccinated Macaques Generated Strong Antigen-Specific IgG Responses after Intramuscular Protein Boosts
3.3. Neutralizing Antibodies to the SIVmac-CX-1 Challenge Virus was Elicited following the Serial Vaccine Regime
3.4. SVV Vector Antibody Responses following Vaccination
3.5. SIV-Gag-, SIV-Env- and gp70 V1/V2 Binding Antibodies Detected in Both VP and VI RMs
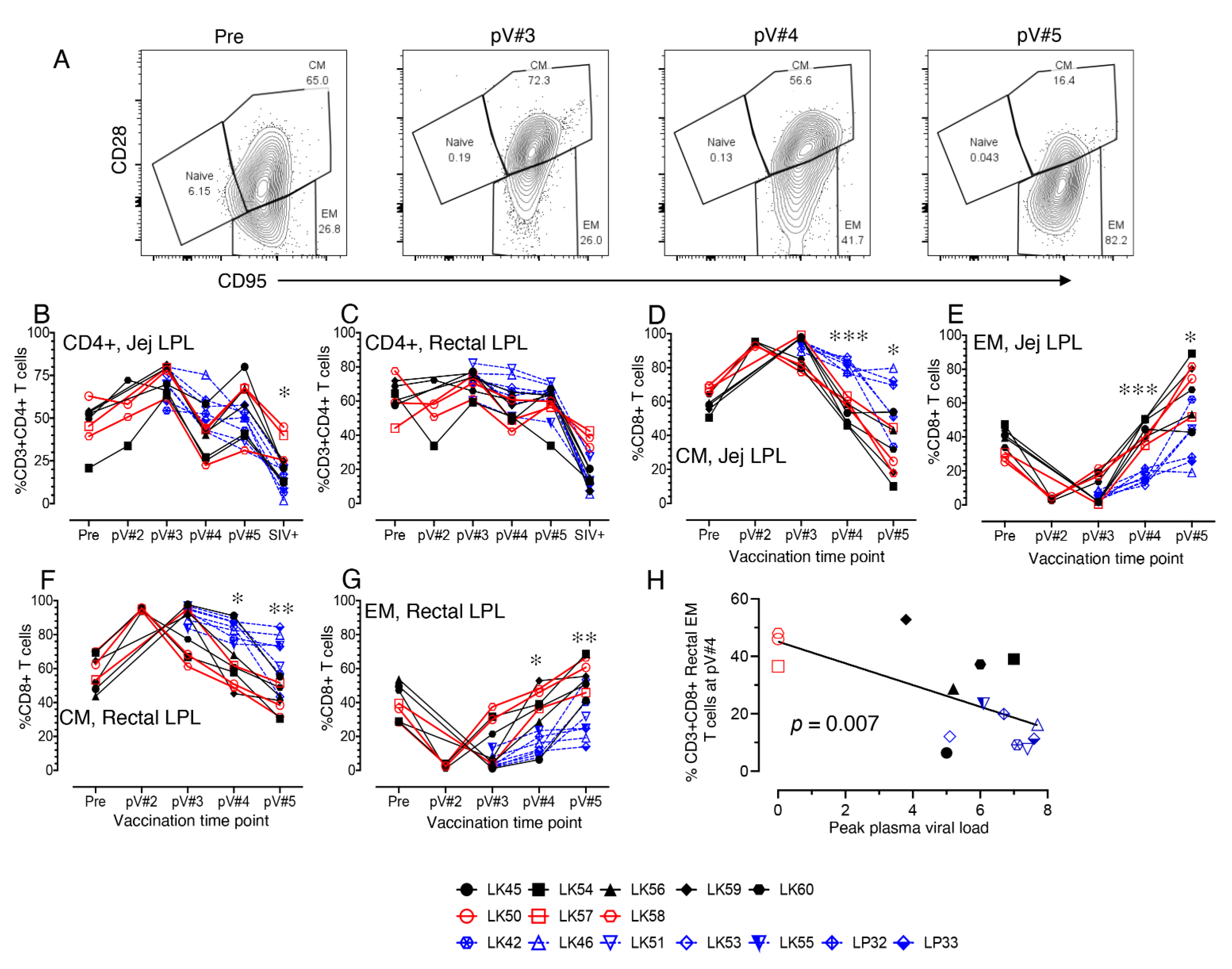
3.6. Impact of Vaccination on the Distribution of Naïve and Memory CD4+ and CD8+ T Cell Populations in PBMC and LN Lymphocytes
3.7. Impact of Vaccination on the Distribution of Total CD4, Naïve and Memory CD4+ and CD8+ T cell Populations in Mucosal Tissues
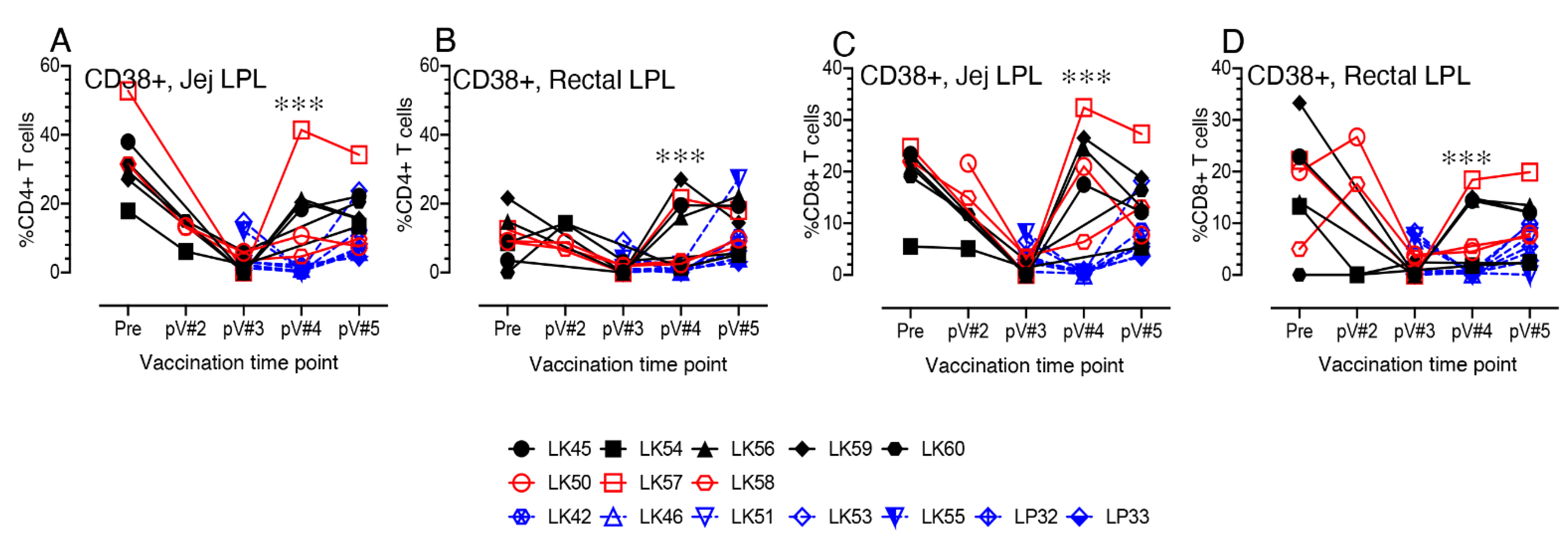
3.8. CD38+ T cell Activation in Vaccinated Macaques
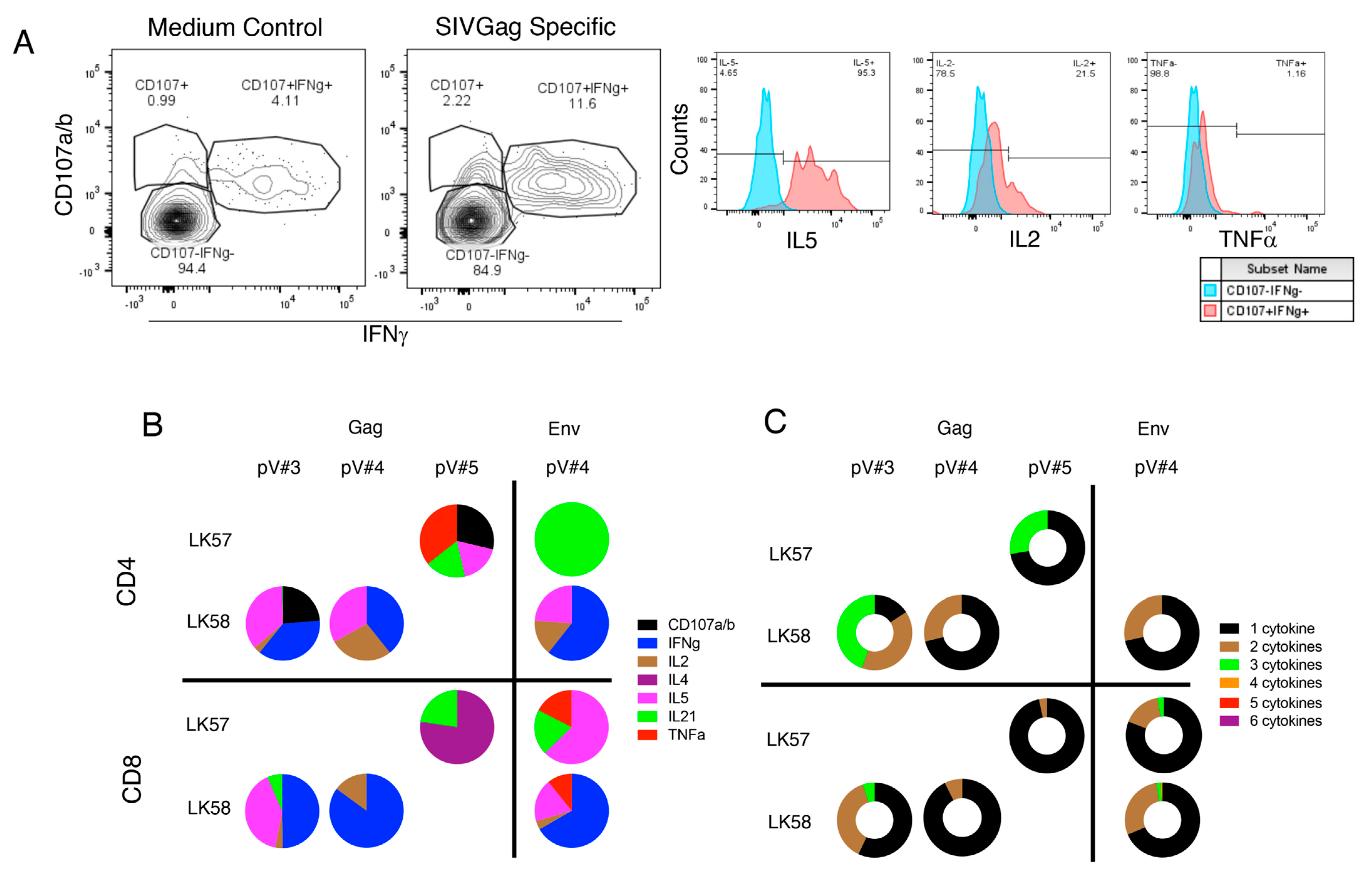
3.9. rSVV-SIV Vaccination Induced Potent and Polyfunctional Cytokine Responses in Lymph Node Tissue in Vaccinated Macaques
3.10. Vaccination Induces Antigen Specific Cytokine Responses in Jejunum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global AIDS Update 2021. UNAIDS: Confronting Inequalities; Lessons for Pandemic Responses from 40 Years of AIDS; UNAIDS: Geneva, Switzerland, 2021.
- Wymant, C.; Bezemer, D.; Blanquart, F.; Ferretti, L.; Gall, A.; Hall, M.; Golubchik, T.; Bakker, M.; Ong, S.H.; Zhao, L.; et al. A highly virulent variant of HIV-1 circulating in the Netherlands. Science 2022, 375, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Danger: UNAIDS Global AIDS Update 2022; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2022.
- Tesoriero, J.M.; Swain, C.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Uruena, J.M.; Davies, M.A.; Sued, O.; Marcos, M.A.; Martinez, E.; Bertagnolio, S.; Alcami, J.; Miro, J.M.; et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Rentsch, C.T.; MacKenna, B.; Schultze, A.; Mehrkar, A.; Bates, C.J.; Eggo, R.M.; Morton, C.E.; Bacon, S.C.J.; Inglesby, P.; et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021, 8, e24–e32. [Google Scholar] [CrossRef] [PubMed]
- Grose, C. Varicella vaccination of children in the United States: Assessment after the first decade 1995–2005. J. Clin. Virol. 2005, 33, 89–95; discussion 96–98. [Google Scholar] [CrossRef] [PubMed]
- Gillet, Y.; Habermehl, P.; Thomas, S.; Eymin, C.; Fiquet, A. Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (M-M-RvaxPro) and a varicella vaccine (VARIVAX) by intramuscular or subcutaneous routes at separate injection sites: A randomised clinical trial. BMC Med. 2009, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; LaRussa, P.S.; Gershon, A.A.; Steinberg, S.P.; Freudigman, K.; Shapiro, E.D. The effectiveness of the varicella vaccine in clinical practice. N. Engl. J. Med. 2001, 344, 955–960. [Google Scholar] [CrossRef]
- Levin, M.J.; Gershon, A.A.; Weinberg, A.; Song, L.Y.; Fentin, T.; Nowak, B.; Pediatric, A.C.T.G.T. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. J. Infect. Dis. 2006, 194, 247–255. [Google Scholar] [CrossRef]
- Son, M.; Shapiro, E.D.; LaRussa, P.; Neu, N.; Michalik, D.E.; Meglin, M.; Jurgrau, A.; Bitar, W.; Vasquez, M.; Flynn, P.; et al. Effectiveness of varicella vaccine in children infected with HIV. J. Infect. Dis. 2010, 201, 1806–1810. [Google Scholar] [CrossRef]
- Bekker, V.; Westerlaken, G.H.; Scherpbier, H.; Alders, S.; Zaaijer, H.; van Baarle, D.; Kuijpers, T. Varicella vaccination in HIV-1-infected children after immune reconstitution. AIDS 2006, 20, 2321–2329. [Google Scholar] [CrossRef]
- Gray, W.L. Recombinant varicella-zoster virus vaccines as platforms for expression of foreign antigens. Adv. Virol. 2013, 2013, 219439. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Hayakawa, Y.; Mori, H.; Namazue, J.; Takamizawa, A.; Yoshida, I.; Yamanishi, K.; Takahashi, M. Development of immunogenic recombinant Oka varicella vaccine expressing hepatitis B virus surface antigen. J. Gen. Virol. 1991, 72, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Sato, H.; Yoshida, Y.; Yamamura, J.I.; Tsurita, M.; Kurokawa, M.; Kageyama, S. Construction of Oka varicella vaccine expressing human immunodeficiency virus env antigen. J. Med. Virol. 2001, 64, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Somboonthum, P.; Yoshii, H.; Okamoto, S.; Koike, M.; Gomi, Y.; Uchiyama, Y.; Takahashi, M.; Yamanishi, K.; Mori, Y. Generation of a recombinant Oka varicella vaccine expressing mumps virus hemagglutinin-neuraminidase protein as a polyvalent live vaccine. Vaccine 2007, 25, 8741–8755. [Google Scholar] [CrossRef] [PubMed]
- Heineman, T.C.; Connelly, B.L.; Bourne, N.; Stanberry, L.R.; Cohen, J. Immunization with recombinant varicella-zoster virus expressing herpes simplex virus type 2 glycoprotein D reduces the severity of genital herpes in guinea pigs. J. Virol. 1995, 69, 8109–8113. [Google Scholar] [CrossRef]
- Heineman, T.C.; Pesnicak, L.; Ali, M.A.; Krogmann, T.; Krudwig, N.; Cohen, J.I. Varicella-zoster virus expressing HSV-2 glycoproteins B and D induces protection against HSV-2 challenge. Vaccine 2004, 22, 2558–2565. [Google Scholar] [CrossRef]
- Matsuura, M.; Somboonthum, P.; Murakami, K.; Ota, M.; Shoji, M.; Kawabata, K.; Mizuguchi, H.; Gomi, Y.; Yamanishi, K.; Mori, Y. Novel polyvalent live vaccine against varicella-zoster and mumps virus infections. Microbiol. Immunol. 2013, 57, 704–714. [Google Scholar] [CrossRef]
- Gray, W.L. Simian varicella: A model for human varicella-zoster virus infections. Rev. Med. Virol. 2004, 14, 363–381. [Google Scholar] [CrossRef]
- Gray, W.L. Simian varicella in old world monkeys. Comp. Med. 2008, 58, 22–30. [Google Scholar]
- Fletcher, T.M., 3rd; Gray, W.L. Simian varicella virus: Characterization of virion and infected cell polypeptides and the antigenic cross-reactivity with varicella-zoster virus. J. Gen. Virol. 1992, 73, 1209–1215. [Google Scholar] [CrossRef]
- Gray, W.L.; Pumphrey, C.Y.; Ruyechan, W.T.; Fletcher, T.M. The simian varicella virus and varicella zoster virus genomes are similar in size and structure. Virology 1992, 186, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Traina-Dorge, V.; Davis, K.A.; Gray, W.L. Recombinant simian varicella viruses induce immune responses to simian immunodeficiency virus (SIV) antigens in immunized vervet monkeys. Virology 2007, 364, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Traina-Dorge, V.; Pahar, B.; Marx, P.; Kissinger, P.; Montefiori, D.; Ou, Y.; Gray, W.L. Recombinant varicella vaccines induce neutralizing antibodies and cellular immune responses to SIV and reduce viral loads in immunized rhesus macaques. Vaccine 2010, 28, 6483–6490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pahar, B.; Gray, W.L.; Phelps, K.; Didier, E.S.; deHaro, E.; Marx, P.A.; Traina-Dorge, V.L. Increased cellular immune responses and CD4+ T-cell proliferation correlate with reduced plasma viral load in SIV challenged recombinant simian varicella virus—Simian immunodeficiency virus (rSVV-SIV) vaccinated rhesus macaques. Virol. J. 2012, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, H.; Li, L.; Omange, R.W.; Hai, Y.; Luo, M. Current advances in HIV vaccine preclinical studies using Macaque models. Vaccine 2019, 37, 3388–3399. [Google Scholar] [CrossRef]
- Gray, W.L.; Williams, R.J.; Chang, R.; Soike, K.F. Experimental simian varicella virus infection of St. Kitts vervet monkeys. J. Med. Primatol. 1998, 27, 177–183. [Google Scholar] [CrossRef]
- Del Prete, G.Q.; Scarlotta, M.; Newman, L.; Reid, C.; Parodi, L.M.; Roser, J.D.; Oswald, K.; Marx, P.A.; Miller, C.J.; Desrosiers, R.C.; et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J. Virol. 2013, 87, 4584–4595. [Google Scholar] [CrossRef]
- Ward, T.M.; Traina-Dorge, V.; Davis, K.A.; Gray, W.L. Recombinant simian varicella viruses expressing respiratory syncytial virus antigens are immunogenic. J. Gen. Virol. 2008, 89, 741–750. [Google Scholar] [CrossRef]
- Knapp, L.A.; Lehmann, E.; Piekarczyk, M.S.; Urvater, J.A.; Watkins, D.I. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 1997, 50, 657–661. [Google Scholar] [CrossRef]
- Heberling, R.L.; Kalter, S.S.; Marx, P.A.; Lowry, J.K.; Rodriguez, A.R. Dot immunobinding assay compared with enzyme-linked immunosorbent assay for rapid and specific detection of retrovirus antibody induced by human or simian acquired immunodeficiency syndrome. J. Clin. Microbiol. 1988, 26, 765–767. [Google Scholar] [CrossRef]
- Ou, Y.; Davis, K.A.; Traina-Dorge, V.; Gray, W.L. Simian varicella virus expresses a latency-associated transcript that is antisense to open reading frame 61 (ICP0) mRNA in neural ganglia of latently infected monkeys. J. Virol. 2007, 81, 8149–8156. [Google Scholar] [CrossRef] [PubMed]
- Kenway-Lynch, C.S.; Das, A.; Lackner, A.A.; Pahar, B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 2014, 88, 9442–9457. [Google Scholar] [CrossRef] [PubMed]
- Kenway-Lynch, C.S.; Das, A.; Pan, D.; Lackner, A.A.; Pahar, B. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T Cells during Acute Simian Immunodeficiency Virus Infection. J. Virol. 2013, 87, 11916–11923. [Google Scholar] [CrossRef]
- Pan, D.; Das, A.; Srivastav, S.K.; Traina-Dorge, V.; Didier, P.J.; Pahar, B. Lack of T-cell-mediated IL-2 and TNFalpha production is linked to decreased CD58 expression in intestinal tissue during acute simian immunodeficiency virus infection. J. Gen. Virol. 2019, 100, 26–34. [Google Scholar] [CrossRef]
- Kumar, V.; Torben, W.; Mansfield, J.; Alvarez, X.; Vande Stouwe, C.; Li, J.; Byrareddy, S.N.; Didier, P.J.; Pahar, B.; Molina, P.E.; et al. Cannabinoid Attenuation of Intestinal Inflammation in Chronic SIV-Infected Rhesus Macaques Involves T Cell Modulation and Differential Expression of Micro-RNAs and Pro-inflammatory Genes. Front. Immunol. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.M.; Molden, J.; Busman-Sahay, K.; Abdulhaqq, S.; Wu, H.L.; Weber, W.C.; Bateman, K.B.; Reed, J.S.; Northrup, M.; Maier, N.; et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020, 16, e1008339. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Kenway-Lynch, C.S.; Marx, P.; Srivastav, S.K.; LaBranche, C.; Montefiori, D.C.; Das, A. Breadth and magnitude of antigen-specific antibody responses in the control of plasma viremia in simian immunodeficiency virus infected macaques. Virol. J. 2016, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009, 485, 395–405. [Google Scholar]
- Gray, W.L.; Wichman, G.; Das, A.; Traina-Dorge, V. An enzyme-linked immunosorbent assay (ELISA) to determine Simian Varicella Virus antibody titers in infected rhesus monkeys (Macaca mulatta). J. Med. Primatol. 2022, 51, 20–26. [Google Scholar] [CrossRef]
- Perez, L.G.; Martinez, D.R.; deCamp, A.C.; Pinter, A.; Berman, P.W.; Francis, D.; Sinangil, F.; Lee, C.; Greene, K.; Gao, H.; et al. V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLoS ONE 2017, 12, e0180720. [Google Scholar] [CrossRef]
- Gorini, G.; Fourati, S.; Vaccari, M.; Rahman, M.A.; Gordon, S.N.; Brown, D.R.; Law, L.; Chang, J.; Green, R.; Barrenas, F.; et al. Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition. PLoS Pathog. 2020, 16, e1008377. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Wang, X.; Dufour, J.; Lackner, A.A.; Veazey, R.S. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3). Virology 2007, 363, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Gordon, S.; Franchini, G.; Berzofsky, J.A. Nonhuman primate models for HIV/AIDS vaccine development. Curr. Protoc. Immunol. 2013, 102, 12–14. [Google Scholar] [CrossRef]
- Moffat, J.F.; Zerboni, L.; Kinchington, P.R.; Grose, C.; Kaneshima, H.; Arvin, A.M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 1998, 72, 965–974. [Google Scholar] [CrossRef]
- Kinchington, P.R.; Ling, P.; Pensiero, M.; Moss, B.; Ruyechan, W.T.; Hay, J. The glycoprotein products of varicella-zoster virus gene 14 and their defective accumulation in a vaccine strain (Oka). J. Virol. 1990, 64, 4540–4548. [Google Scholar] [CrossRef]
- Grinfeld, E.; Ross, A.; Forster, T.; Ghazal, P.; Kennedy, P.G. Genome-wide reduction in transcriptomal profiles of varicella-zoster virus vaccine strains compared with parental Oka strain using long oligonucleotide microarrays. Virus Genes 2009, 38, 19–29. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Gray, G.E.; Huang, Y.; Grunenberg, N.; Laher, F.; Roux, S.; Andersen-Nissen, E.; De Rosa, S.C.; Flach, B.; Randhawa, A.K.; Jensen, R.; et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl. Med. 2019, 11, eaax1880. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Welten, S.P.; Redeker, A.; Franken, K.L.; Benedict, C.A.; Yagita, H.; Wensveen, F.M.; Borst, J.; Melief, C.J.; van Lier, R.A.; van Gisbergen, K.P.; et al. CD27-CD70 costimulation controls T cell immunity during acute and persistent cytomegalovirus infection. J. Virol. 2013, 87, 6851–6865. [Google Scholar] [CrossRef]
- Welten, S.P.M.; Redeker, A.; Toes, R.E.M.; Arens, R. Viral Persistence Induces Antibody Inflation without Altering Antibody Avidity. J. Virol. 2016, 90, 4402–4411. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Lackner, A.A.; Veazey, R.S. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 2006, 36, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Das, A.; Lackner, A.A.; Veazey, R.S.; Pahar, B. Intestinal double-positive CD4+CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood 2008, 112, 4981–4990. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.; Paiardini, M.; Pandrea, I.; Lederman, M.M.; Sodora, D.L. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Investig. 2007, 117, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.N.; Klatt, N.R.; Bosinger, S.E.; Brenchley, J.M.; Milush, J.M.; Engram, J.C.; Dunham, R.M.; Paiardini, M.; Klucking, S.; Danesh, A.; et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 2007, 179, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Pandrea, I.V.; Gautam, R.; Ribeiro, R.M.; Brenchley, J.M.; Butler, I.F.; Pattison, M.; Rasmussen, T.; Marx, P.A.; Silvestri, G.; Lackner, A.A.; et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 2007, 179, 3035–3046. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef]
- Panagioti, E.; Klenerman, P.; Lee, L.N.; van der Burg, S.H.; Arens, R. Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections. Front. Immunol. 2018, 9, 276. [Google Scholar] [CrossRef]




| Macaque | Antigen # | Tissue | % of CD3+CD4+ T cells | % of CD3+CD8+ T cells | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD107a/b | IFNγ | IL2 | IL4 | IL5 | IL21 | TNFα | CD107a/b | IFNγ | IL2 | IL4 | IL5 | IL21 | TNFα | |||
| LK50 | Gag | LN | 2.99 | |||||||||||||
| LK57 | Gag | LN | 0.53 | 0.09 | 1.62 | 0.24 | 0.27 | 0.22 | 0.54 | 3.62 | 0.15 | 0.74 | ||||
| LK58 | Gag | LN | 0.36 | 0.34 | 1.04 | |||||||||||
| LK54 | Gag | LN | 0.48 | |||||||||||||
| LK56 | Gag | LN | 1.75 | 0.44 | 0.57 | 0.54 | 1.49 | 1.26 | 0.32 | 0.95 | 1.49 | 0.50 | ||||
| LK60 | Gag | LN | 0.63 | 0.99 | ||||||||||||
| LK50 | Env | LN | 0.19 | 0.22 | 1.61 | 0.14 | ||||||||||
| LK57 | Env | LN | 0.52 | 0.12 | 0.12 | 0.09 | 0.29 | 0.12 | 0.22 | 1.91 | 0.17 | 0.48 | ||||
| LK58 | Env | LN | 0.54 | 0.05 | ||||||||||||
| LK54 | Env | LN | 0.52 | 0.10 | ||||||||||||
| LK56 | Env | LN | 0.74 | 0.14 | 0.33 | 0.37 | 2.55 | 0.11 | 0.17 | |||||||
| LK50 | Gag | PBMC | 0.06 | 0.45 | 0.15 | |||||||||||
| LK58 | Gag | PBMC | 0.05 | |||||||||||||
| LK45 | Gag | PBMC | 0.18 | 1.58 | 4.37 | |||||||||||
| LK54 | Gag | PBMC | 0.45 | |||||||||||||
| LK56 | Gag | PBMC | 0.51 | 3.14 | ||||||||||||
| LK59 | Gag | PBMC | 0.09 | 1.50 | 0.11 | 1.73 | 1.12 | 0.46 | 0.33 | 0.65 | 3.44 | 0.75 | ||||
| LK60 | Gag | PBMC | 0.24 | 0.17 | ||||||||||||
| LK50 | Env | PBMC | 0.52 | 0.17 | ||||||||||||
| LK57 | Env | PBMC | 1.41 | 0.16 | 0.11 | 0.54 | ||||||||||
| LK58 | Env | PBMC | 1.54 | |||||||||||||
| LK45 | Env | PBMC | 0.50 | |||||||||||||
| LK54 | Env | PBMC | 0.32 | 1.22 | 1.12 | |||||||||||
| LK59 | Env | PBMC | 0.30 | 0.38 | 1.30 | 0.25 | 0.35 | 0.37 | 1.96 | 0.37 | 0.35 | |||||
| LK60 | Env | PBMC | 0.09 | 0.08 | 0.09 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahar, B.; Gray, W.; Fahlberg, M.; Grasperge, B.; Hunter, M.; Das, A.; Mabee, C.; Aye, P.P.; Schiro, F.; Hensley, K.; et al. Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques. Viruses 2022, 14, 2819. https://doi.org/10.3390/v14122819
Pahar B, Gray W, Fahlberg M, Grasperge B, Hunter M, Das A, Mabee C, Aye PP, Schiro F, Hensley K, et al. Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques. Viruses. 2022; 14(12):2819. https://doi.org/10.3390/v14122819
Chicago/Turabian StylePahar, Bapi, Wayne Gray, Marissa Fahlberg, Brooke Grasperge, Meredith Hunter, Arpita Das, Christopher Mabee, Pyone Pyone Aye, Faith Schiro, Krystle Hensley, and et al. 2022. "Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques" Viruses 14, no. 12: 2819. https://doi.org/10.3390/v14122819
APA StylePahar, B., Gray, W., Fahlberg, M., Grasperge, B., Hunter, M., Das, A., Mabee, C., Aye, P. P., Schiro, F., Hensley, K., Ratnayake, A., Goff, K., LaBranche, C., Shen, X., Tomaras, G. D., DeMarco, C. T., Montefiori, D., Kissinger, P., Marx, P. A., & Traina-Dorge, V. (2022). Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques. Viruses, 14(12), 2819. https://doi.org/10.3390/v14122819





