Inhibitory Potential of Chromene Derivatives on Structural and Non-Structural Proteins of Dengue Virus
Abstract
1. Introduction
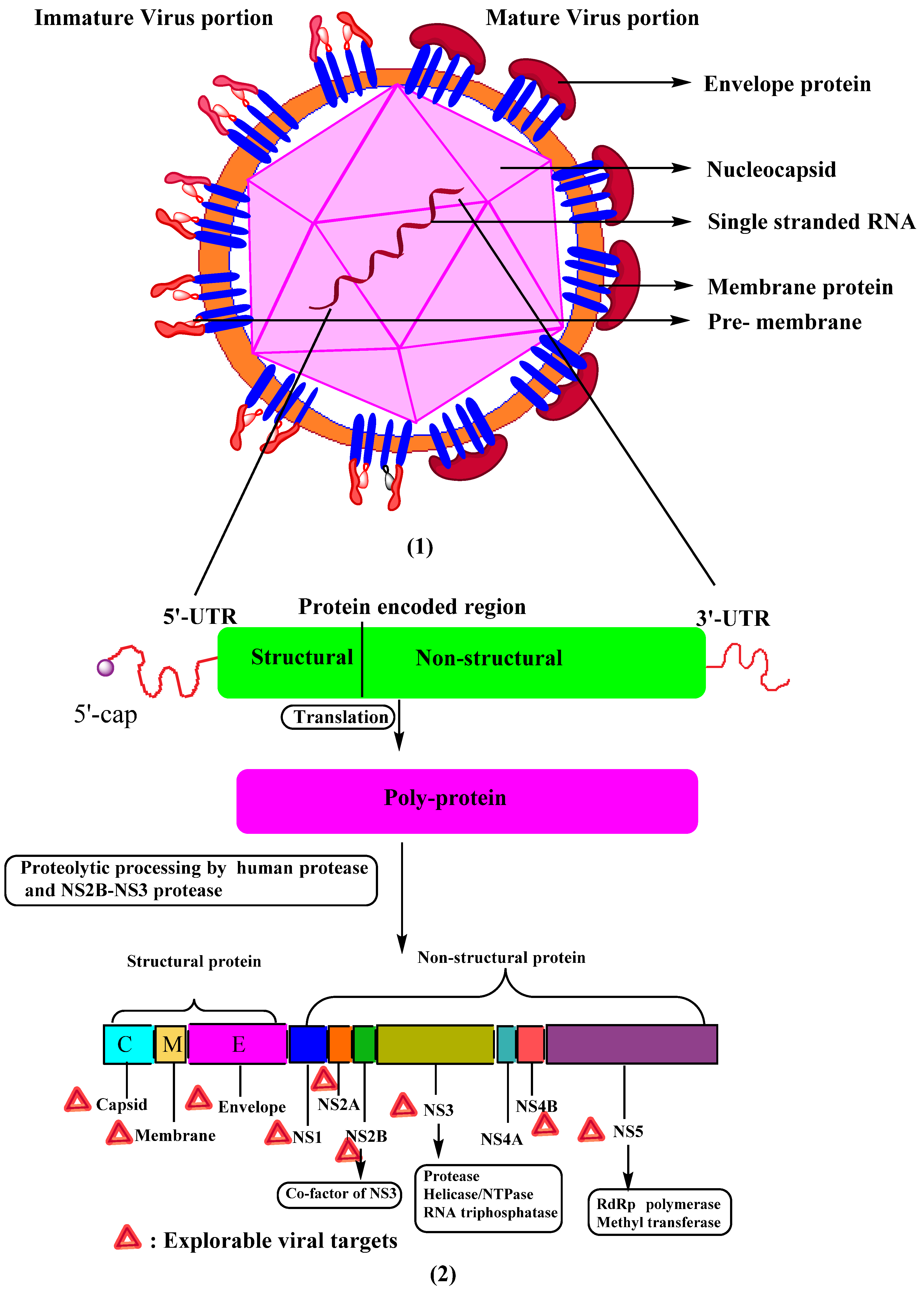
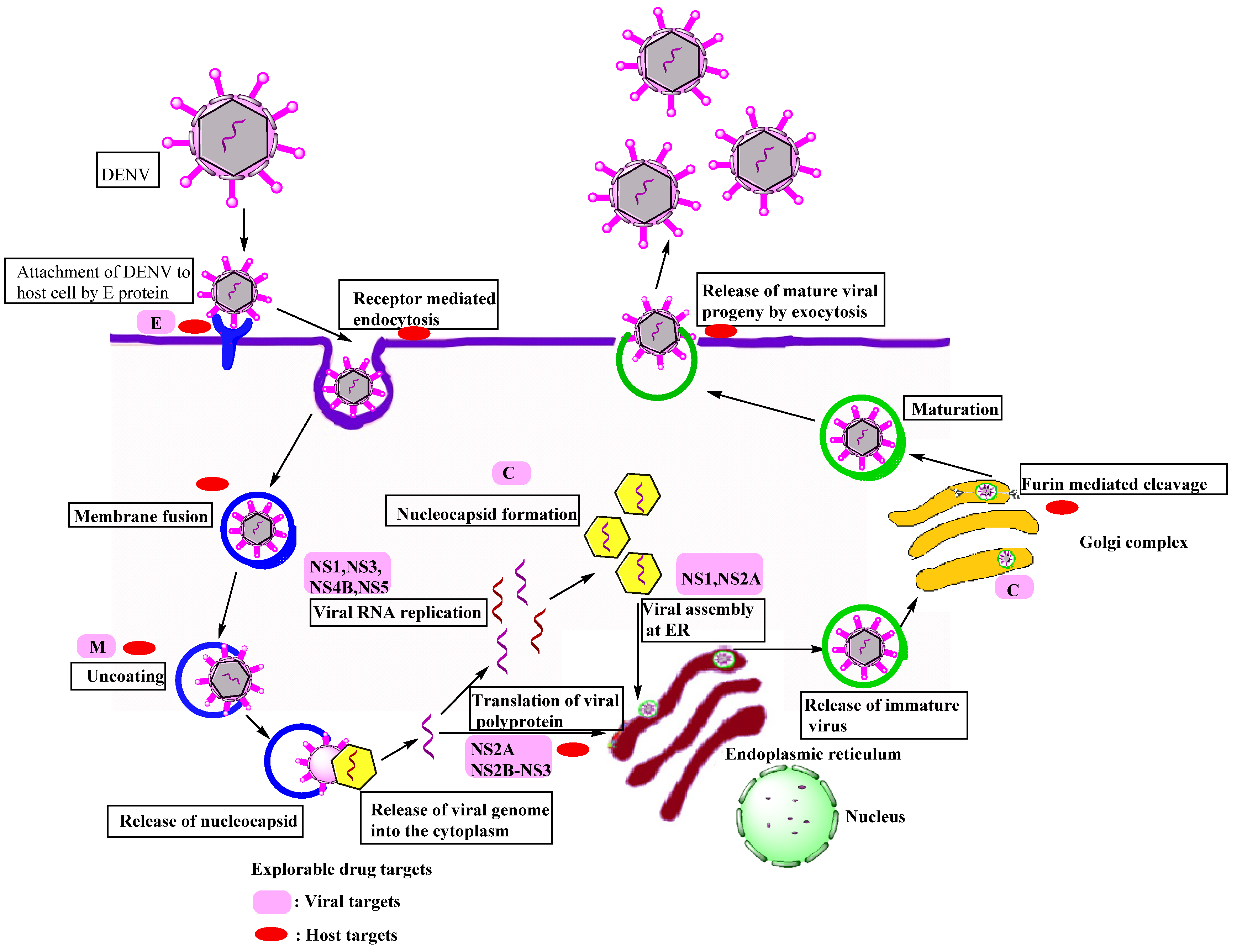
2. Structure and Biology of Dengue Virus
3. Pathogenesis
4. Explorable Drug Targets
4.1. Viral Targets
4.1.1. Structural Proteins
Envelope Protein (E)
- Fusion inhibitors: Peptides and small molecules bound to the β-OG pocket. The peptidic inhibitors have poor absorption from the gastrointestinal tract; thus, intravenous administration is required.
- Glycosidase inhibitors: α-glycosidase inhibitors mainly target the glycosylation process. They are only employed in limited conditions due to their severe toxicity and lack of selectivity. Some examples are deoxynojirimycin (DNJ) and castanospermine (CSP).
- Carbohydrate-binding agents: These agents block the connection between the DENV envelope N-glycans and the host cell. Some examples include concanavalin A and wheat germ agglutinin.
PrM/Membrane Protein (M)
Capsid Protein (C)
Non-Structural Protein
4.2. Host Targets
5. Drugs under Clinical Trials
6. Vector Control
7. Treatment
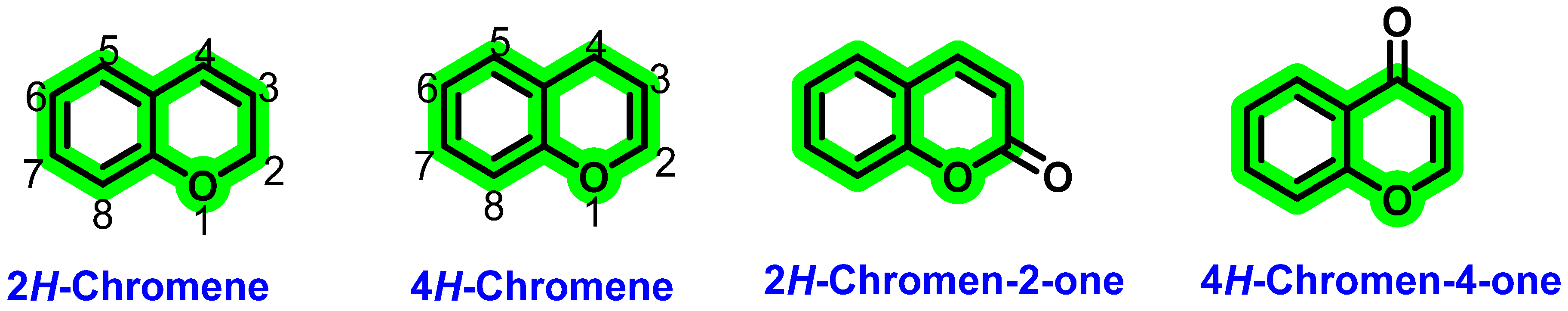
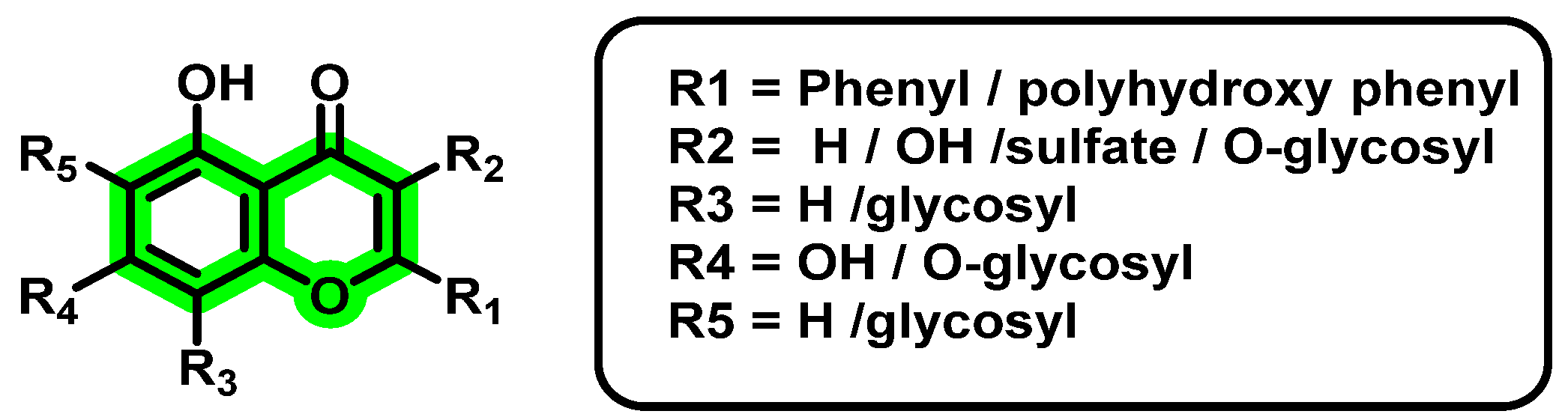
8. Chromene Derivatives against Dengue Virus
8.1. Chromene Analogues Acting on NS-1
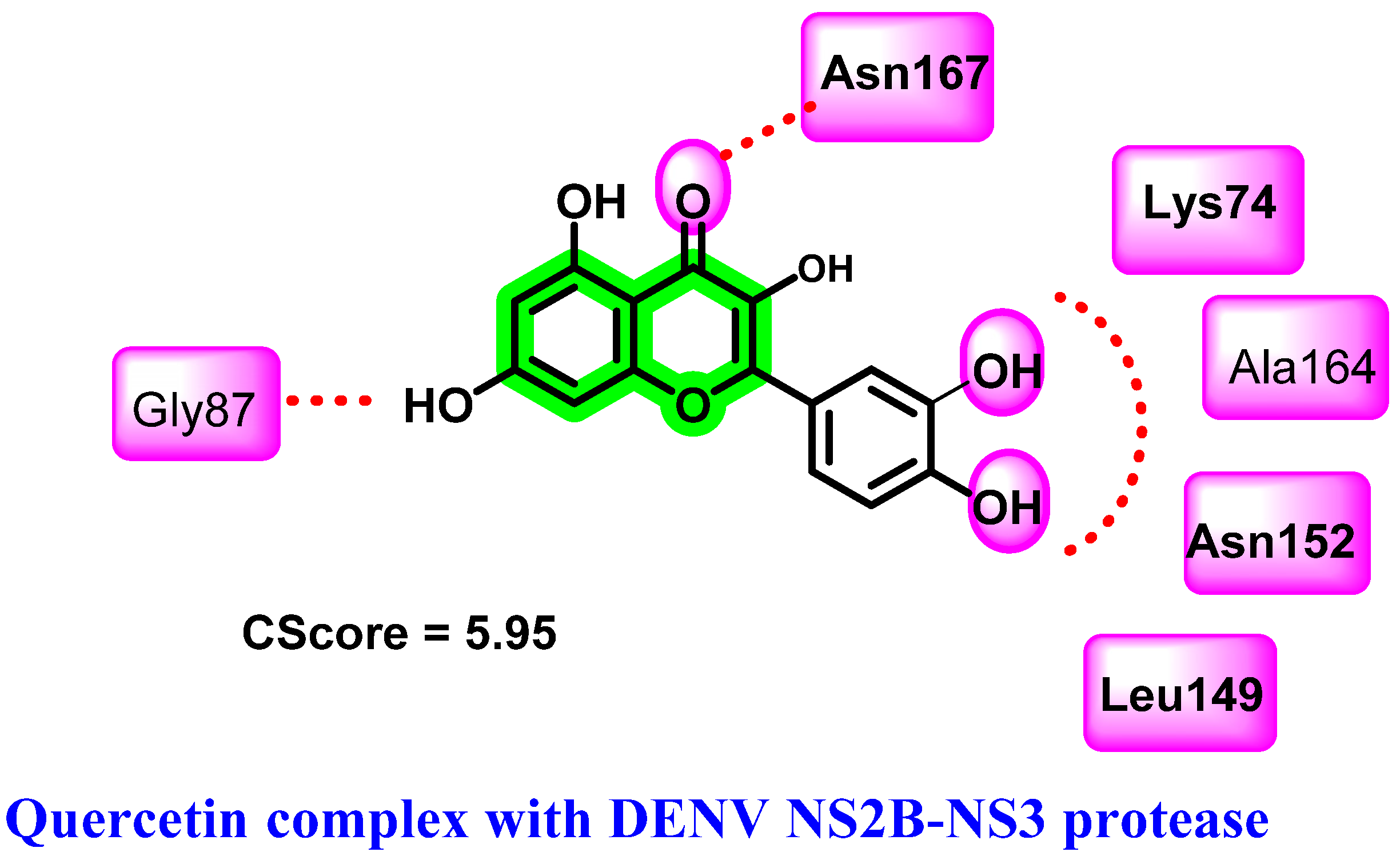
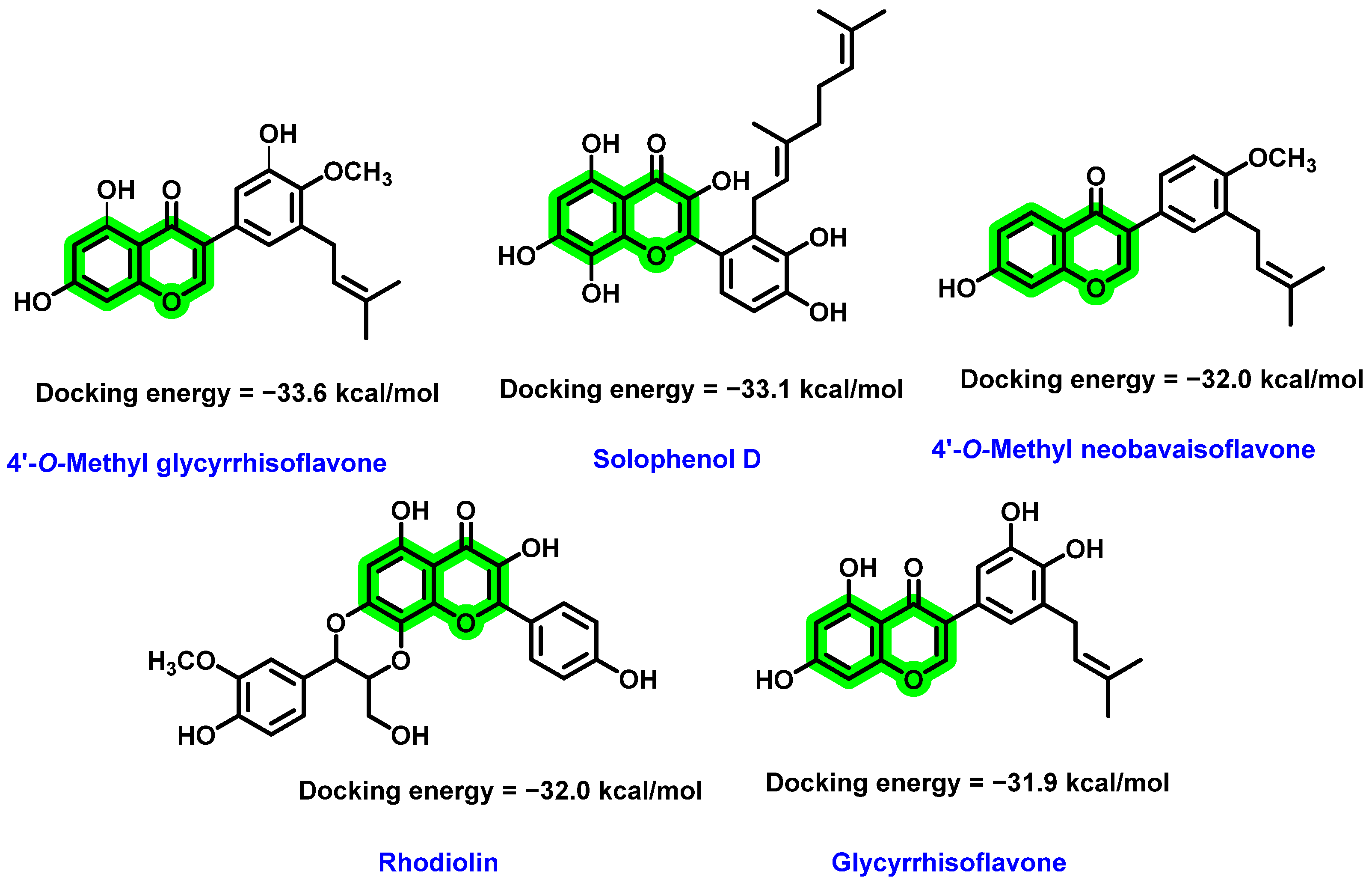
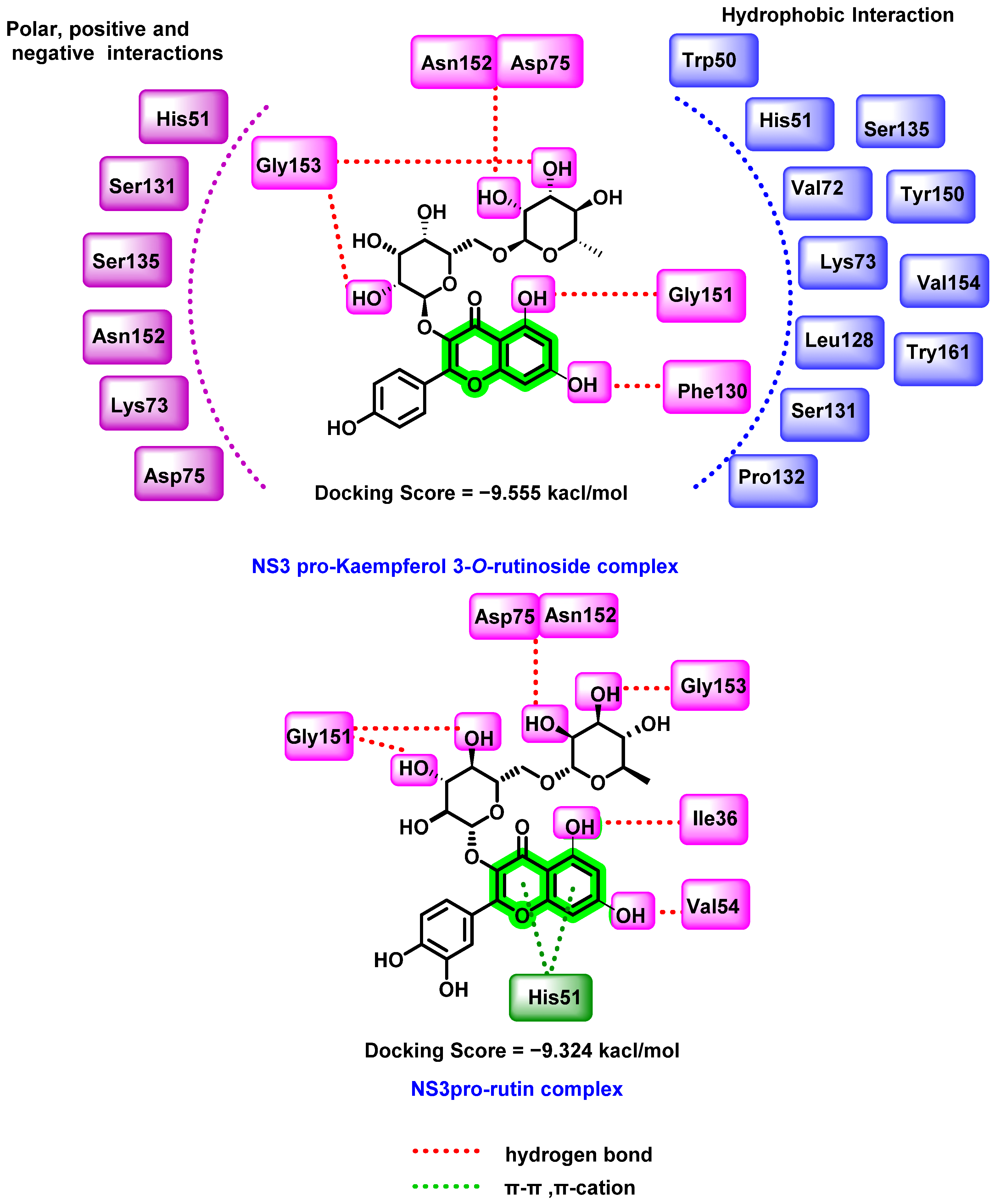
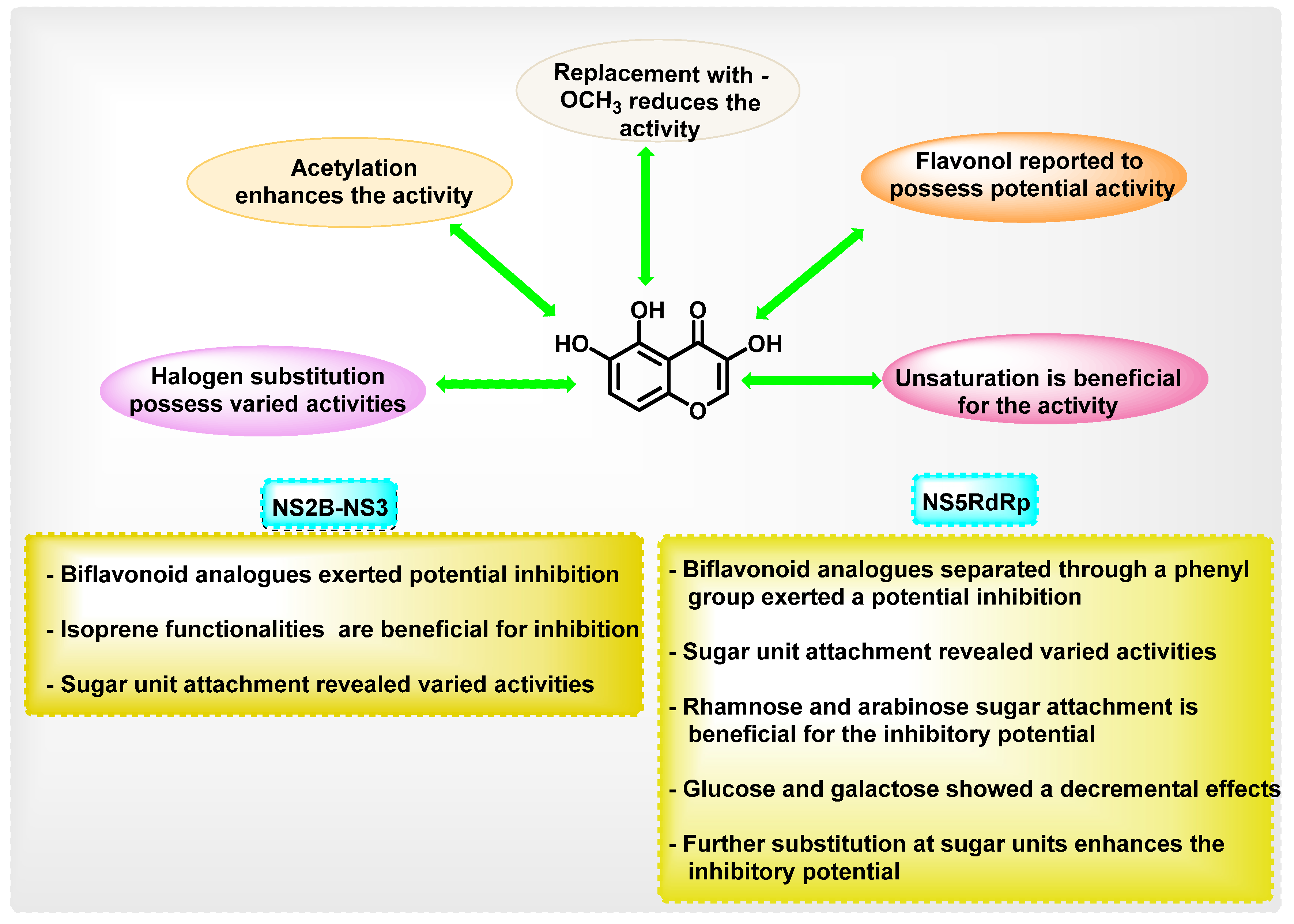
8.2. Chromene Analogues Acting on NS2B-NS3 Protease
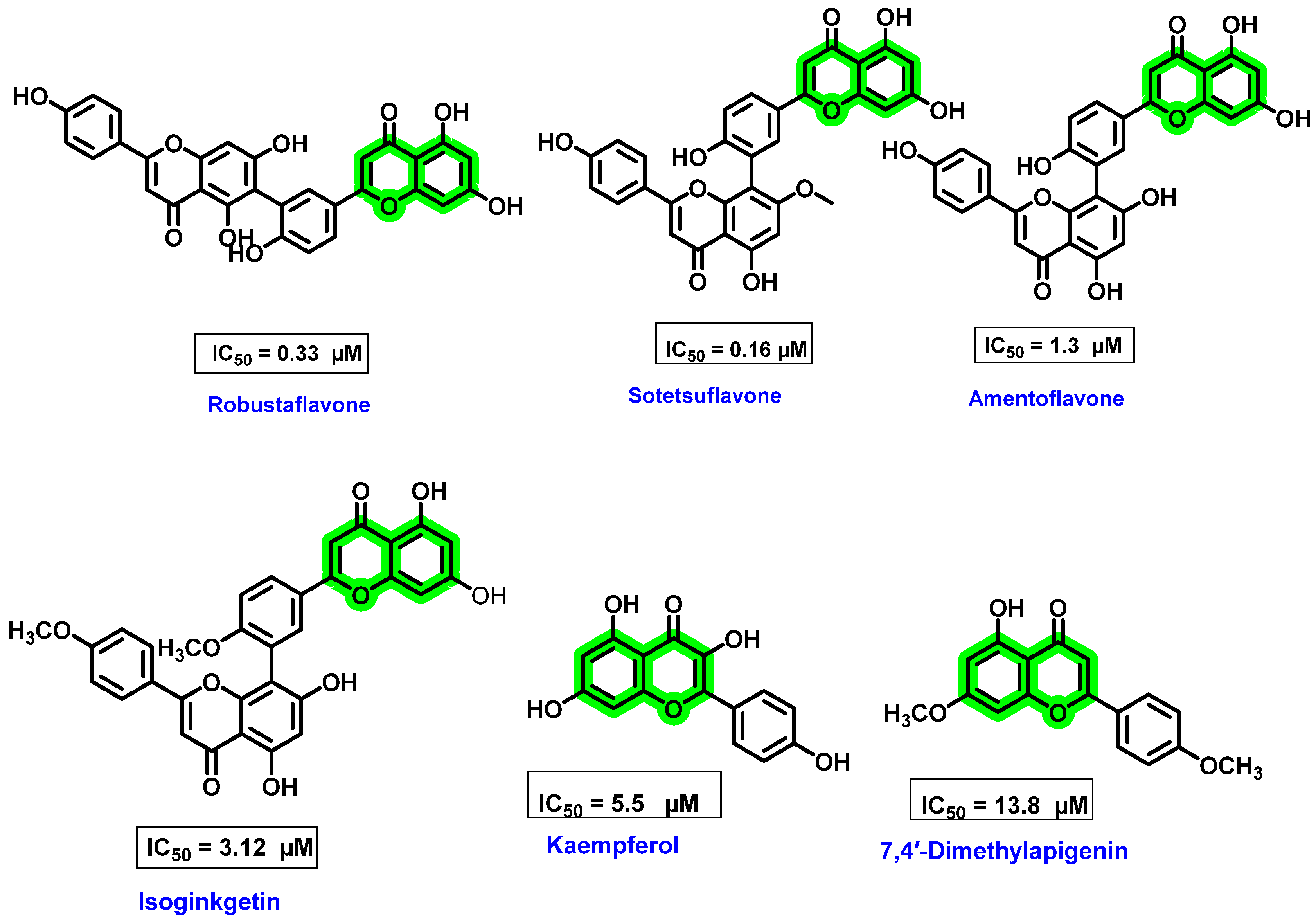
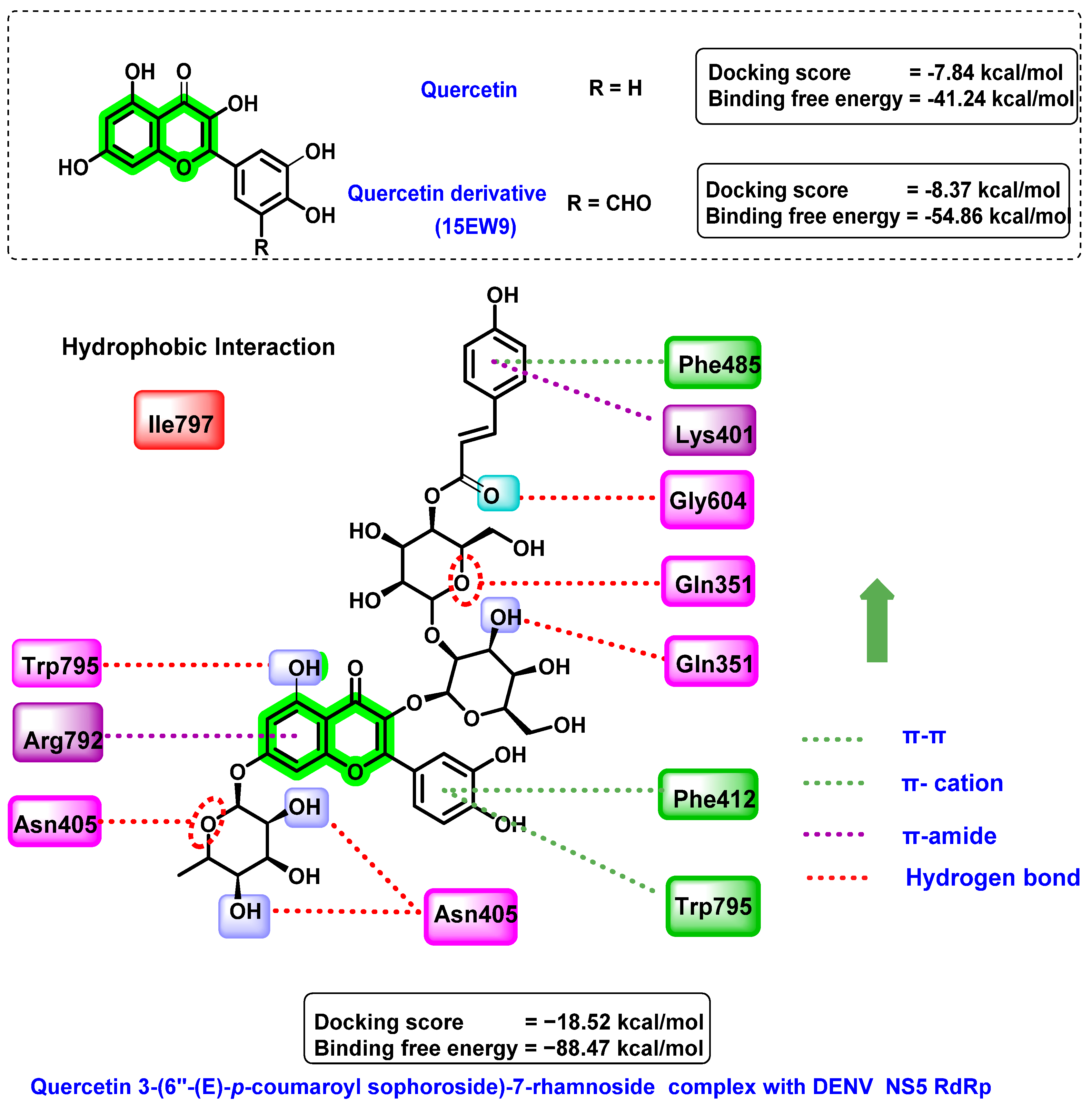
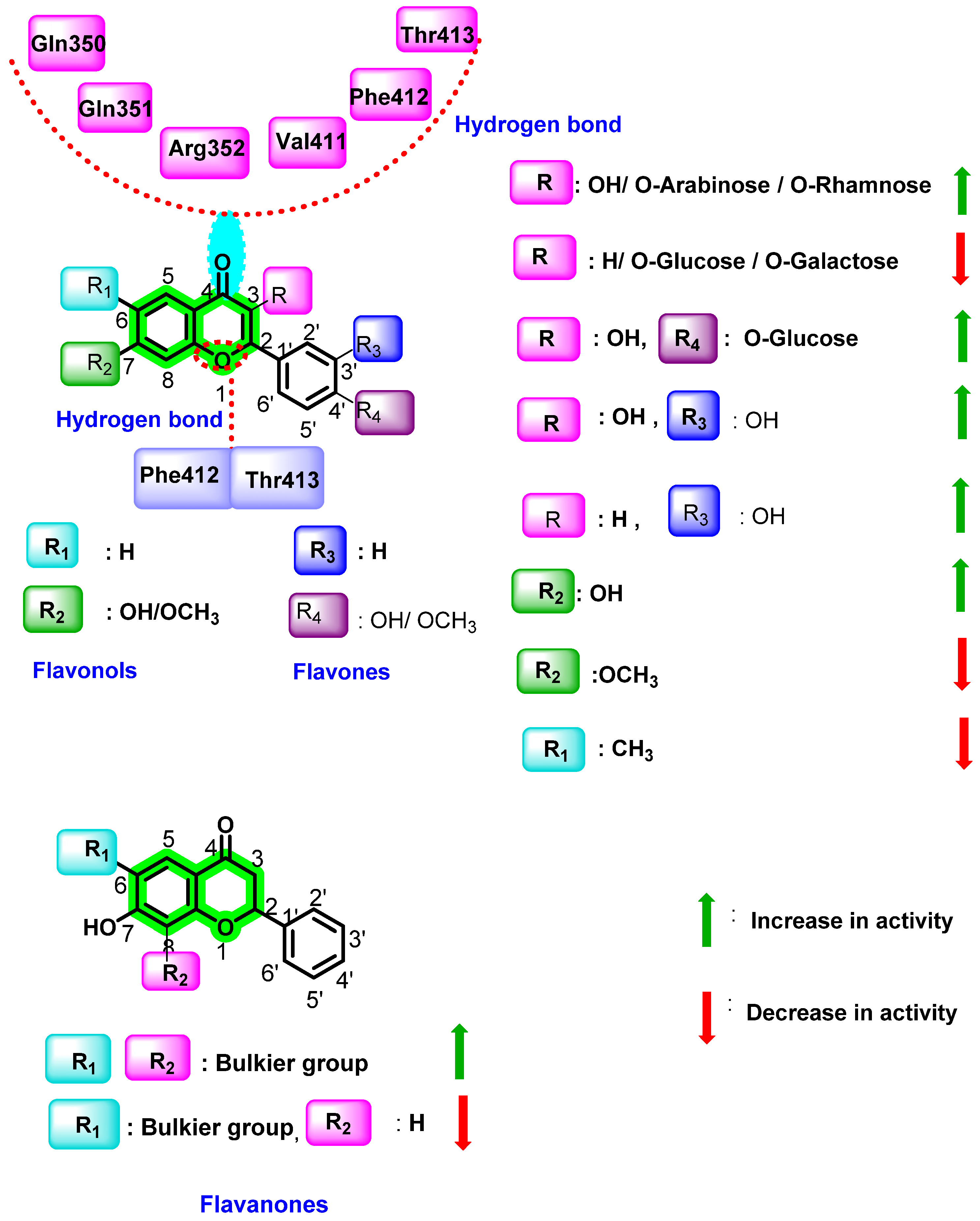
8.3. Chromene Analogues Acting on NS5 Protein
8.4. Chromene Analogues Acting on Multiple Targets
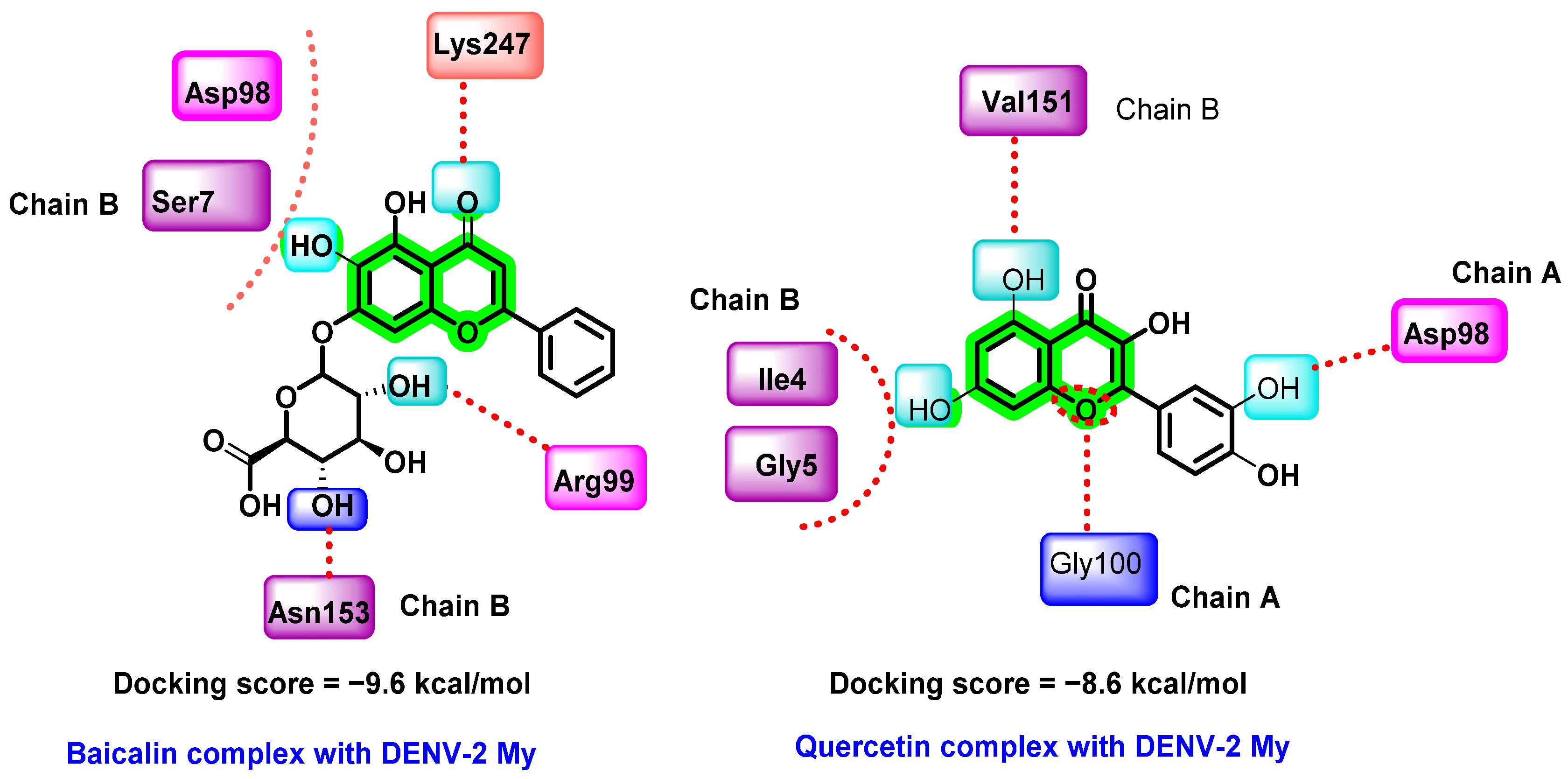
8.4.1. Chromene Analogues Acting on DENV-2 My/DENV-E Protein
8.4.2. Chromene Analogues Acting on DENV Polymerase and DENV Protease
8.4.3. Chromene Analogues Acting on DENV E Protein and NS5
8.5. Chromene Analogues Acting on Furin Enzyme
8.6. Miscellaneous Reports
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Dengue and Severe Dengue 2021. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed on 19 March 2022).
- Silva, N.M.; Santos, N.C.; Martins, I.C. Dengue and zika viruses: Epidemiological history, potential therapies, and promising vaccines. Trop. Med. Infect. Dis. 2020, 5, 150. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020. Available online: https://apps.who.int/iris/handle/10665/75303(2012) (accessed on 23 November 2022).
- Timiri, A.K.; Sinha, B.N.; Jayaprakash, V. Progress and prospects on DENV protease inhibitors. Eur. J. Med. Chem. 2016, 117, 125–143. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, M. Dengue epidemiology. J. Glob. Health 2019, 3, 37–45. [Google Scholar] [CrossRef]
- Rajapakse, S.; Rodrigo, C.; Rajapakse, A. Treatment of dengue fever. Infect. Drug. Resist. 2012, 5, 103–112. [Google Scholar] [CrossRef]
- Ahammad, F.; Rogayah, T.; Abd, T.; Mohamed, M. Contemporary strategies and current trends in designing antiviral drugs against dengue fever via targeting host-based approaches. Microorganisms 2019, 7, 296. [Google Scholar] [CrossRef]
- Pujar, G.V.; Sethu, A.K.; Bhagyalalitha, M.; Singh, M. Dengue structural proteins as antiviral drug targets: Current status in the drug discovery &development. Eur. J. Med. Chem. 2021, 221, 113527. [Google Scholar]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of dengue virus: Implications for flavivirus organization, maturation, and fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef]
- Uno, N.; Ross, T.M. Dengue virus and the host innate immune response. Emerg. Microbes Infect. 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- TuiskunenBäck, A.; Lundkvist, Å. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar]
- Smit, J.M.; Moesker, B.; Rodenhuis-zybert, I.; Wilschut, J. Flavivirus cell entry and membrane fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Byk, L.A.; Gamarnik, A.V. Properties and functions of the dengue virus capsid protein. Annu. Rev. Virol. 2016, 3, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P. Dengue drug discovery: Progress, challenges and outlook. Antivir. Res. 2019, 163, 156–178. [Google Scholar] [CrossRef]
- Nasar, S.; Rashid, N.; Iftikhar, S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 2020, 92, 941–955. [Google Scholar] [CrossRef]
- Gupta, P.; Mahajan, A. Approaches for the development of potential dengue inhibitors. Synth. Commun. 2020, 50, 2250–2265. [Google Scholar] [CrossRef]
- Behnam, M.A.M.; Nitsche, C.; Boldescu, V.; Klein, C.D. The medicinal chemistry of dengue virus. J. Med. Chem. 2016, 59, 5622–5649. [Google Scholar] [CrossRef]
- Alen, M.M.F.; Schols, D. Dengue virus entry as target for antiviral therapy. J. Trop. Med. 2012, 2012, 628475. [Google Scholar] [CrossRef]
- Naresh, P.; Selvaraj, A.; Shyam Sundar, P.; Murugesan, S.; Sathianarayanan, S.; Namboori PK, K.; Jubie, S. Targeting a conserved pocket (n-octyl-β-D-glucoside) on the dengue virus envelope protein by small bioactive molecule inhibitors. J. Biomol. Struct. Dyn. 2020, 40, 4866–4878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Patel, S.J.; Vangrevelinghe, E.; Hao, Y.X.; Rao, R.; Jaber, D.; Schul, W.; Gu, F.; Heudi, O.; Ngai, L.M.; et al. A small-molecule dengue virus entry inhibitor. Antimicrob. Agents Chemother. 2009, 53, 1823–1831. [Google Scholar] [CrossRef]
- Qi, R.F.; Zhang, L.; Chi, C.W. Biological characteristics of dengue virus and potential targets for drug design. Acta Biochim. Biophys. Sin 2008, 40, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Tripathi, I.P.; Tripathi, R.C. Genomics, proteomics and evolution of dengue virus. Brief. Funct. Genom. 2017, 16, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Keelapang, P.; Sriburi, R.; Supasa, S.; Panyadee, N.; Songjaeng, A.; Jairungsri, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Sittisombut, N. Alterations of pr-M cleavage and virus export in pr-M junction chimeric dengue viruses. J. Virol. 2004, 78, 2367–2381. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, J.; Zhang, X.; Zhou, Y.; Routh, A.L.; Kang, C.; Popov, V.L.; Chen, X.; Wang, Q.Y.; Dong, H.; et al. Dengue NS2A Protein Orchestrates Virus Assembly. Cell Host Microbe 2019, 26, 606–622.e8. [Google Scholar] [CrossRef] [PubMed]
- Gopala Reddy, S.B.; Chin, W.X.; Shivananju, N.S. Dengue virus NS2 and NS4: Minor proteins, mammoth roles. Biochem. Pharmacol. 2018, 154, 54–63. [Google Scholar] [CrossRef]
- Shrivastava, G.; García-Cordero, J.; León-Juárez, M.; Oza, G.; Tapia-Ramírez, J.; Villegas-Sepulveda, N.; Cedillo-Barrón, L. NS2A comprises a putative viroporin of Dengue virus 2. Virulence 2017, 8, 1450–1456. [Google Scholar] [CrossRef]
- Dighe, S.N.; Ekwudu, O.; Dua, K.; Chellappan, D.K.; Katavic, P.L.; Collet, T.A. Recent update on anti-dengue drug discovery. Eur. J. Med. Chem. 2019, 176, 431–455. [Google Scholar] [CrossRef]
- Wu, H.; Bock, S.; Snitko, M.; Berger, T.; Weidner, T.; Holloway, S.; Kanitz, M.; Diederich, W.E.; Steuber, H.; Walter, C.; et al. Novel dengue virus NS2B/NS3 protease inhibitors. Antimicrob. Agents Chemother. 2015, 59, 1100–1109. [Google Scholar] [CrossRef]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric inhibition of the NS2B-NS3 protease from dengue virus. ACS Chem. Biol. 2013, 8, 2744–2752. [Google Scholar] [CrossRef]
- Phoo, W.W.; El Sahili, A.; Zhang, Z.Z.; Chen, M.W.; Liew, C.W.; Lescar, J.; Vasudevan, S.G.; Luo, D. Crystal structures of full length DENV4 NS2B-NS3 reveal the dynamic interaction between NS2B and NS3. Antivir. Res. 2020, 182, 104900. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zou, J.; Wang, Q.Y.; Shi, P.Y. Targeting dengue virus NS4B protein for drug discovery. Antivir. Res. 2015, 118, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Sparacio, S.; Bartenschlager, R. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J. Biol. Chem. 2006, 281, 8854–8863. [Google Scholar] [CrossRef]
- Noble, C.G.; Chen, Y.L.; Dong, H.; Gu, F.; Lim, S.P.; Schul, W.; Wang, Q.Y.; Shi, P.Y. Strategies for development of dengue virus inhibitors. Antivir. Res. 2010, 85, 450–462. [Google Scholar] [CrossRef]
- Chan, C.Y.; Ooi, E.E. Dengue: An update on treatment options. Future Microbiol. 2015, 10, 2017–2031. [Google Scholar] [CrossRef]
- Gu, F.; Shi, P.-Y. The challenges of dengue drug discovery and development. Clin. Investig. 2014, 4, 683–685. [Google Scholar] [CrossRef]
- Hayashi, K.; Kamiya, M.; Hayashi, T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus and HIV. Planta Med. 2006, 3, 237–241. [Google Scholar]
- Gao, B.; Zhang, J.; Xie, L. Structure analysis of effective chemical compounds against dengue viruses isolated from Isatis tinctoria. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 3217473. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Kamisah, Y. Potential medicinal plants for the treatment of dengue fever and severer acute respiratory syndrome-coronavirus. Biomolecules 2021, 11, 1–25. [Google Scholar]
- Katiyar, M.K.; Dhakad, G.K.; Shivani, A.S.; Bhagat, S.; Katyal, T.; Kumar, R. Synthetic strategies and pharmacological activities of chromene and its derivatives: An overview. J. Mol. Struct. 2022, 1263, 133012. [Google Scholar] [CrossRef]
- Raj, V.; Lee, J. 2H/4H-Chromenes—A versatile biologically attractive Scaffold. Front. Chem. 2020, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef] [PubMed]
- Alaklabi, A.; Arif, I.A.; Ahamed, A.; Kumar, R.S.; Idhayadhulla, A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J. Biol. Sci. 2018, 25, 1387–1392. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomed. Rep. 2019, 10, 33–38. [Google Scholar] [CrossRef]
- al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Sridhar SN, C.; George, G.; Verma, A.; Paul, A.T. Natural products-based pancreatic lipase inhibitors for obesity treatment. In Natural Bio-Active Compounds; Springer: Singapore, 2019; Volume 1, pp. 149–191. [Google Scholar]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: A systematic review of preclinical evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mathew, B.; Aleya, L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci. Total Environ. 2020, 700, 134836. [Google Scholar] [CrossRef]
- SCiumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Zandi, K.; Lani, R.; Wong, P.F.; Teoh, B.T.; Sam, S.S.; Johari, J.; Mustafa, M.R.; Abu Bakar, S. Flavone enhances dengue virus type-2 (NGC strain) infectivity and replication in Vero cells. Molecules 2021, 17, 2437. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Baby, J.; Devan, A.R.; Kumar, A.R.; Gorantla, J.N.; Nair, B.; Aishwarya, T.S.; Nath, L.R. Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: A review. J. Food Biochem. 2021, 45, e13761. [Google Scholar] [CrossRef] [PubMed]
- Keramagi, A.R.; Skariyachan, S. Prediction of binding potential of natural leads against the prioritized drug targets of chikungunya and dengue viruses by computational screening. 3 Biotech 2018, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Shetty, S.; Bhide, R.; Narayanan, S. Evaluation of Platelet Augmentation Activity of Carica papaya Leaf Aqueous Extract in Rats. J. Pharmacogn. Phytochem. 2013, 1, 57–60. [Google Scholar]
- Sarwar, M.W.; Riaz, A.; Dilshad, S.M.R.; Al-Qahtani, A.; Nawaz-Ul-Rehman, M.S.; Mubin, M. Structure activity relationship (SAR) and quantitative structure activity relationship (QSAR) studies showed plant flavonoids as potential inhibitors of dengue NS2B-NS3 protease. BMC Struct. Biol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Thapa, M.; Kim, Y.; Desper, J.; Chang, K.-O.; Hua, D.H. Synthesis and antiviral activity of substituted quercetins. Bioorg. Med. Chem. Lett. 2012, 22, 353–356. [Google Scholar] [CrossRef]
- Gravina, H.D.; Tafuri, N.F.; Silva Júnior, A.; Fietto, J.L.R.; Oliveira, T.T.; Diaz, M.A.N.; Almeida, M.R. In vitro assessment of the antiviral potential of trans-cinnamic acid, quercetin and morin against equid herpesvirus 1. Res. Vet. Sci. 2011, 91, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Senthilvel, P.; Lavanya, P.; Kumar, K.M.; Swetha, R.; Anitha, P.; Bag, S.; Sarveswari, S. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents Dengue 2 viral assembly. Bioinformation 2013, 9, 889–895. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; Da Silva, M.F.D.G.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.N.; Setzer, W.N. An in-silico investigation of phytochemicals as antiviral agents against dengue fever. Comb. Chem. High Throughput Screen. 2016, 19, 516–536. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Bharadwaj, S.; Afroz, S.; Khan, N.; Ansari, M.A.; Yadava, U.; Tripathi, R.C.; Tripathi, I.P.; Mishra, S.K.; Kang, S.G. Anti-dengue infectivity evaluation of bioflavonoid from Azadirachta indica by dengue virus serine protease inhibition. J. Biomol. Struct. Dyn. 2019, 39, 1417–1430. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Pereira, W.L.; Da Silveira Oliveira, A.F.C.; Da Silva, A.M.; De Oliveira, A.S.; Da Silva, M.L.; Da Silva, C.C.; De Paula, S.O. Natural products as source of potential dengue antivirals. Molecules 2014, 19, 8151–8176. [Google Scholar] [CrossRef]
- Leardkamolkarn, V.; Sirigulpanit, W.; Phurimsak, C.; Kumkate, S.; Himakoun, L.; Sripanidkulchai, B. The inhibitory actions of Houttuynia cordata aqueous extract on dengue virus and dengue-infected cells. J. Food Biochem. 2012, 36, 86–92. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef]
- De Morais, S.M. Antiviral activities of extracts and phenolic components of two Spondias species against dengue virus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 405–413. [Google Scholar]
- Ismail, N.A.; Jusoh, S.A. Molecular docking and molecular dynamics simulation studies to predict flavonoid binding on the surface of DENV-2 E protein. Interdiscip. Sci. Comput. Life Sci. 2017, 9, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; Sousa, D.P.D. Antiviral role of phenolic compounds against dengue virus: A review. Biomolecules 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Canard, B.; Figadère, B.; Guillemot, J.; Nour, M. Biflavonoids of Dacrydium balansae with Potent Inhibitory Activity on Dengue 2 NS5 Polymerase. Planta Med. 2012, 78, 672–677. [Google Scholar]
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B.; et al. Structure-activity relationship study of biflavonoids on the dengue virus polymerase DENV-NS5 RdRp. Planta Med. 2013, 79, 1313–1318. [Google Scholar] [CrossRef]
- Coulerie, P.; Maciuk, A.; Eydoux, C.; Hnawia, E.; Lebouvier, N.; Figadère, B.; Guillemot, J.; Nour, M. New inhibitors of the DENV-NS5 RdRp from Carpolepis laurifolia as potential antiviral drugs for dengue treatment. Rec. Nat. Prod. 2014, 8, 286–289. [Google Scholar]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Biomol. Struct. Dyn. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Zandi, K.; Lim, T.; Rahim, N.; Shu, M.; Teoh, B.; Sam, S.; Danlami, M.; Tan, K.; Abubakar, S. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement. Altern. Med. 2013, 13, 91. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the japanese encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, S.; Gromiha, M.M. Quercetin derivatives as non-nucleoside inhibitors for dengue polymerase: Molecular docking, molecular dynamics simulation, and binding free energy calculation. J. Biomol. Struct. Dyn. 2017, 35, 2895–2909. [Google Scholar]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, S.; Gromiha, M.M. Structural basis of flavonoids as dengue polymerase inhibitors: Insights from QSAR and docking studies. J. Biomol. Struct. Dyn. 2019, 37, 104–115. [Google Scholar] [CrossRef]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Swarbrick, C.M.D.; Chan, K.W.K.; Luo, D.; Zhang, W.; Lai, X.; Li, G.; Vasudevan, S.G. Luteolin escape mutants of dengue virus map to prM and NS2B and reveal viral plasticity during maturation. Antivir. Res. 2018, 154, 87–96. [Google Scholar] [CrossRef]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017, 143, 176–185. [Google Scholar] [CrossRef]
- Rakers, C.; Schwerdtfeger, S.M.; Mortier, J.; Duwe, S.; Wolff, T.; Wolber, G.; Melzig, M.F. Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 4312–4317. [Google Scholar] [CrossRef]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
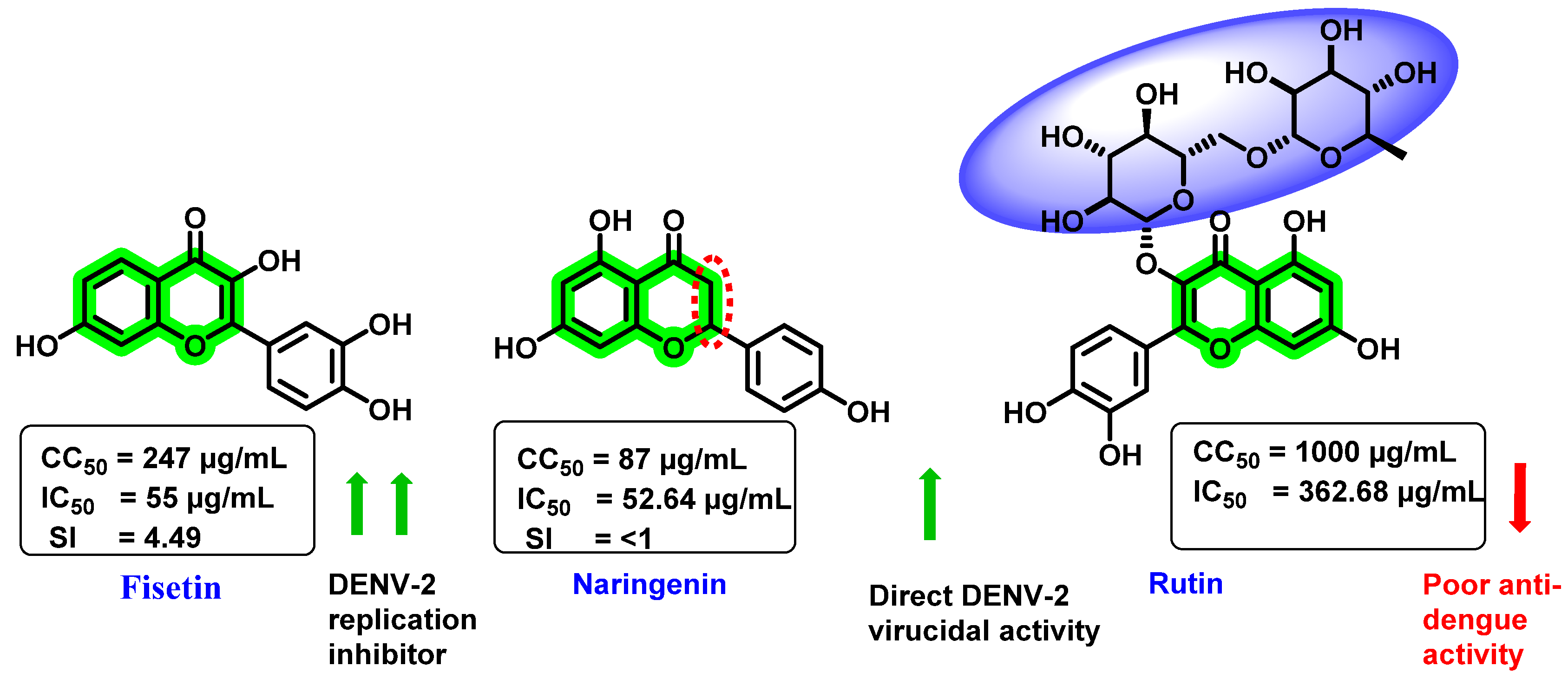
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abubakar, S. In vitro antiviral activity of fisetin, rutin and naringenin against dengue virus type-2. J. Med. Plant Res. 2011, 5, 5534–5539. [Google Scholar]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antivir. Res. 2011, 89, 26–34. [Google Scholar] [CrossRef]
- Simões, L.R.; Maciel, G.M.; Brandão, G.C.; Kroon, E.G.; Castilho, R.O.; Oliveira, A.B. Antiviral activity of Distictella elongata (Vahl) Urb. (Bignoniaceae), a potentially useful source of anti-dengue drugs from the state of Minas Gerais, Brazil. Lett. Appl. Microbiol. 2011, 53, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Suroengrit, A.; Yuttithamnon, W.; Srivarangkul, P.; Pankaew, S.; Kingkaew, K.; Chavasiri, W.; Boonyasuppayakorn, S. Halogenated chrysins inhibit dengue and zika virus infectivity. Sci. Rep. 2017, 7, 13696. [Google Scholar] [CrossRef] [PubMed]



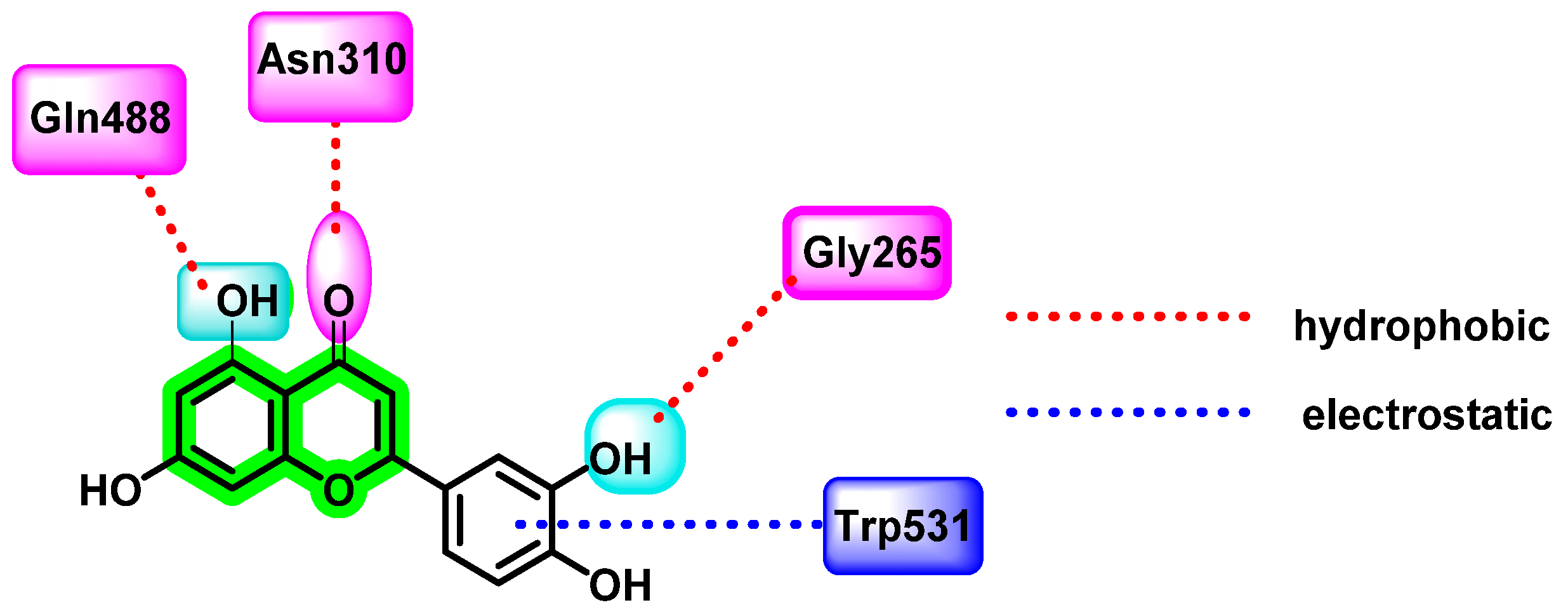
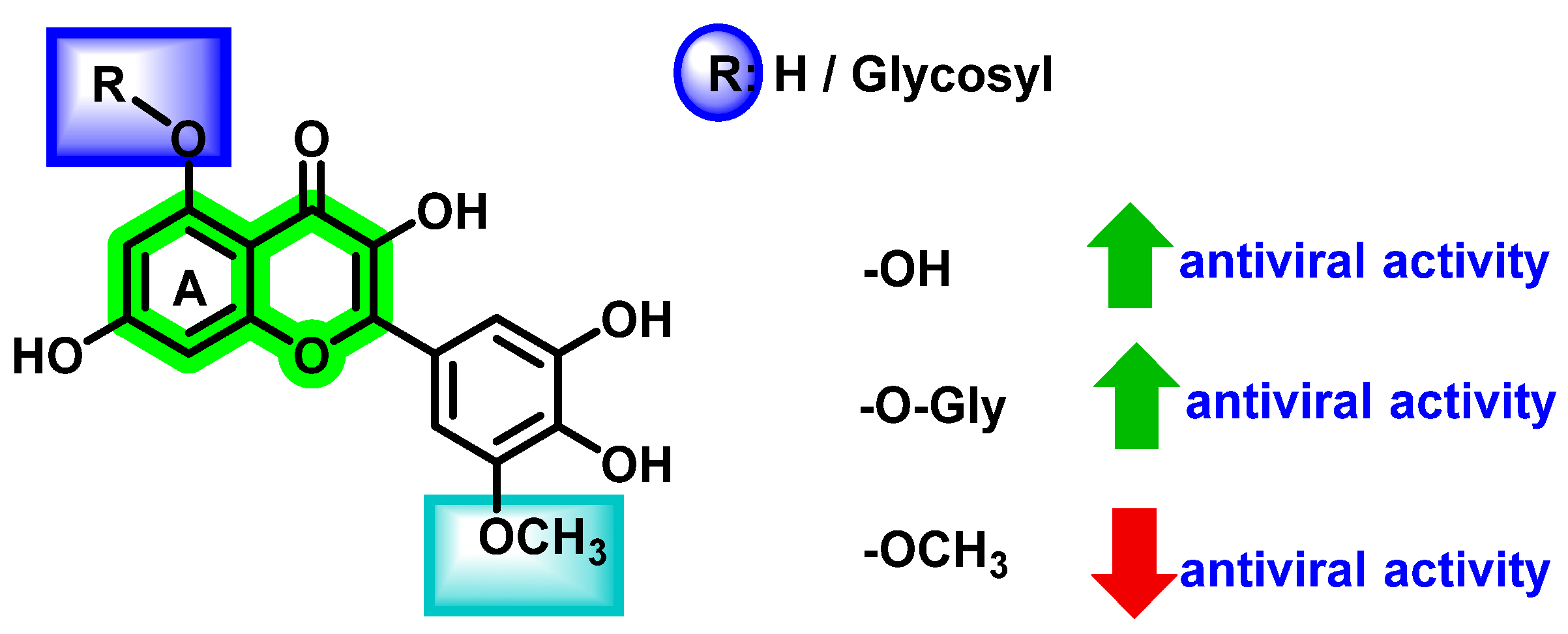
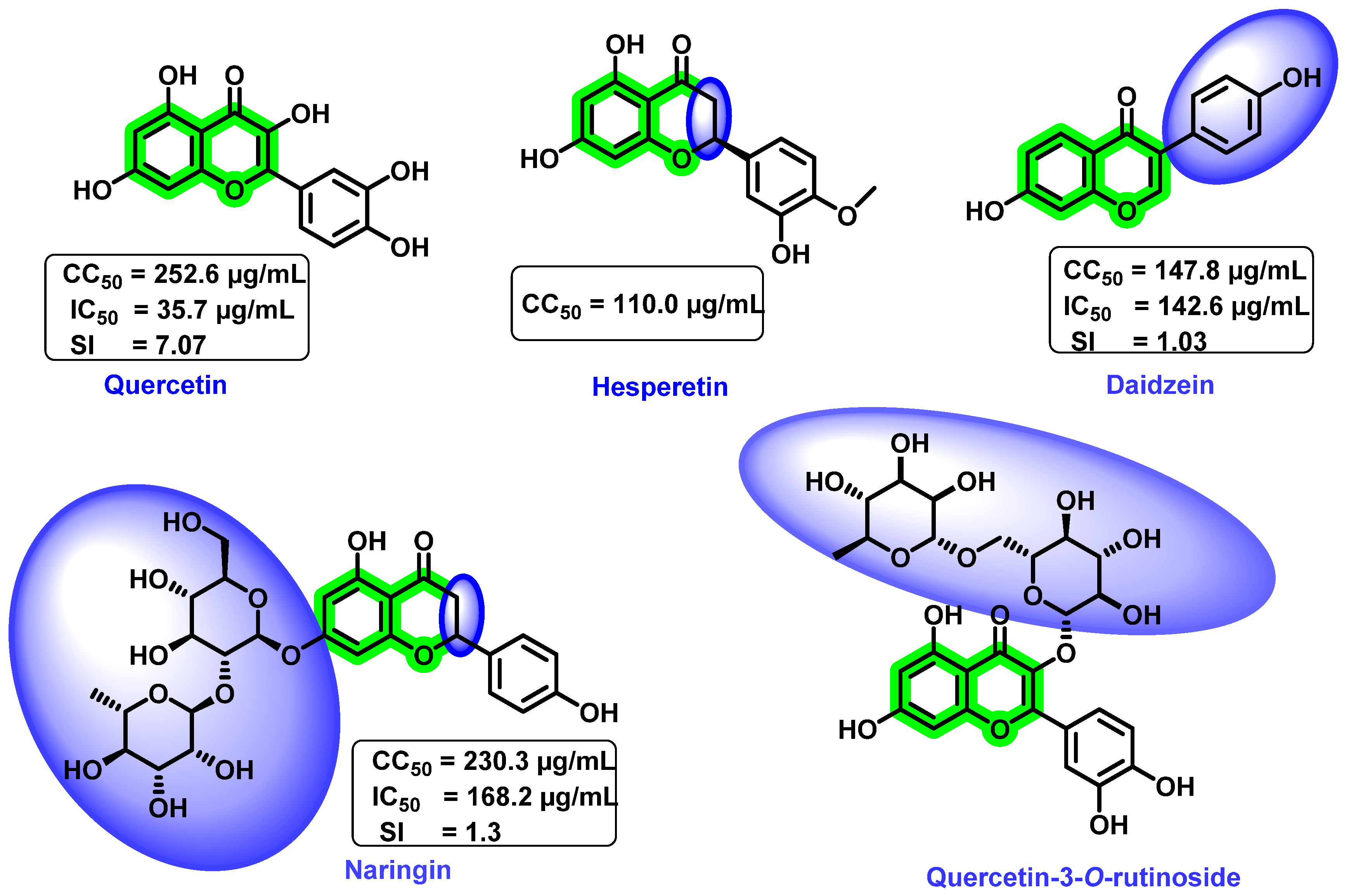
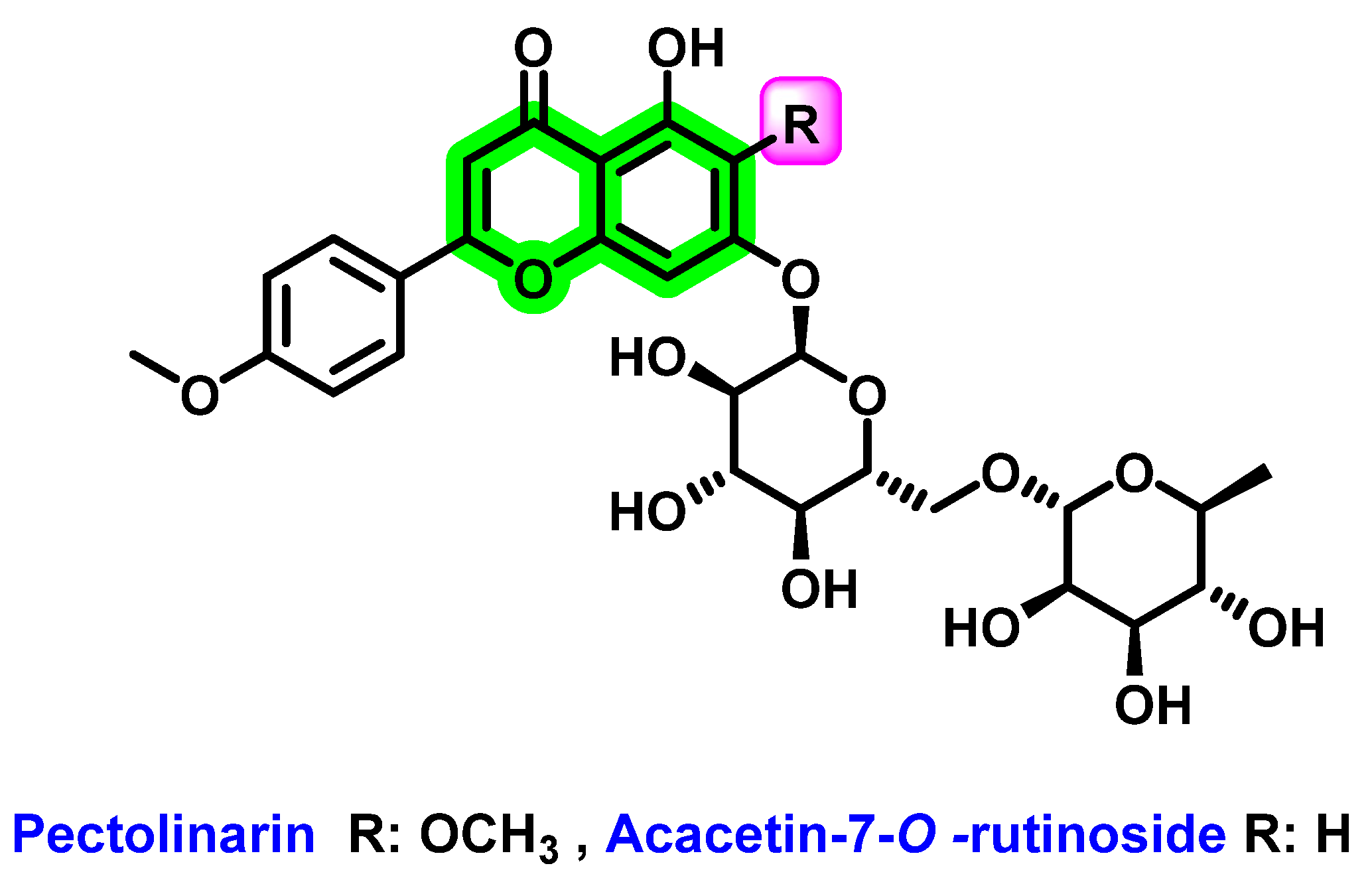

| Non-Structural Protein | Structural Features | Molecular Mass (KDa) | Functions |
|---|---|---|---|
| NS1 | Multifunctional protein with the exposed hydrophobic region. | 43–48 |
|
| NS2A |
| 42 |
|
| NS2B |
| 15 |
|
| NS3 |
| 70 |
|
| NS4A |
| 16 |
|
| NS4B |
| 27 |
|
| NS5 |
| 103 |
|
| Drug | Sponsor | Trial Identifier | Phase | Location |
|---|---|---|---|---|
| AT-752 | Atea Pharmaceuticals, Inc. | NCT05366439 | I | New York, United States |
| AT-752 in dengue infected patients | Atea Pharmaceuticals, Inc. | NCT05466240 | II | Brazil |
| AT-752 in healthy subjects | Atea Pharmaceuticals, Inc. | NCT04722627 | I | Victoria, Australia |
| JNJ-64281802 (For Dengue prevention) | Janssen Research & Development, LLC | NCT05201794 | II | Brazil |
| JNJ-64281802 (With confirmed dengue fever patients) | Janssen Research & Development, LLC | NCT04906980 | II | Singapore |
| JNJ-64281802 against DENV-3 | NIAID | NCT05048875 | II | Maryland and Vermont, United States |
| Zanamivir–Prevent Vascular Permeability in Dengue (ZAP-DENGUE) | George Washington University | NCT04597437 | Early Phase I | - |
| Ivermectin | Mahidol University | NCT02045069 | II/III | - |
| Ivermectin in paediatric dengue patients | Mahidol University | NCT03432442 | II | Bangkok, Thailand |
| Chloroquine | University of Sao Paulo | NCT00849602 | I/II | Ribeirão Preto, SP, Brazil |
| Celgosivir | Singapore General Hospita | NCT01619969 | I/II | Singapore |
| Melatonin | Ilocos Training and Regional Medical Centre | NCT05034809 | II | - |
| Balapiravir | Hoffmann-La Roche | NCT01096576 | I | Ho Chi Minh City, Vietnam |
| NT-proBNP and Troponin in Dengue children | Le PhoucTruyen | NCT04837430 | - | Ho Chi Minh City, Vietnam |
| Montelukast | Phramongkutklao College of Medicine and Hospital | NCT04673422 | II/III | Bangkok and Songkhla, Thailand |
| Ketotifen | National University Hospital, Singapore | NCT02673840 | IV | Singapore |
| AV-1 | AbViro LLC | NCT04273217 | I | Texas, United States |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmapalan, B.T.; Biswas, R.; Sankaran, S.; Venkidasamy, B.; Thiruvengadam, M.; George, G.; Rebezov, M.; Zengin, G.; Gallo, M.; Montesano, D.; et al. Inhibitory Potential of Chromene Derivatives on Structural and Non-Structural Proteins of Dengue Virus. Viruses 2022, 14, 2656. https://doi.org/10.3390/v14122656
Dharmapalan BT, Biswas R, Sankaran S, Venkidasamy B, Thiruvengadam M, George G, Rebezov M, Zengin G, Gallo M, Montesano D, et al. Inhibitory Potential of Chromene Derivatives on Structural and Non-Structural Proteins of Dengue Virus. Viruses. 2022; 14(12):2656. https://doi.org/10.3390/v14122656
Chicago/Turabian StyleDharmapalan, Babitha Thekkiniyedath, Raja Biswas, Sathianarayanan Sankaran, Baskar Venkidasamy, Muthu Thiruvengadam, Ginson George, Maksim Rebezov, Gokhan Zengin, Monica Gallo, Domenico Montesano, and et al. 2022. "Inhibitory Potential of Chromene Derivatives on Structural and Non-Structural Proteins of Dengue Virus" Viruses 14, no. 12: 2656. https://doi.org/10.3390/v14122656
APA StyleDharmapalan, B. T., Biswas, R., Sankaran, S., Venkidasamy, B., Thiruvengadam, M., George, G., Rebezov, M., Zengin, G., Gallo, M., Montesano, D., Naviglio, D., & Shariati, M. A. (2022). Inhibitory Potential of Chromene Derivatives on Structural and Non-Structural Proteins of Dengue Virus. Viruses, 14(12), 2656. https://doi.org/10.3390/v14122656













