Viral Coinfections
Abstract
1. Introduction
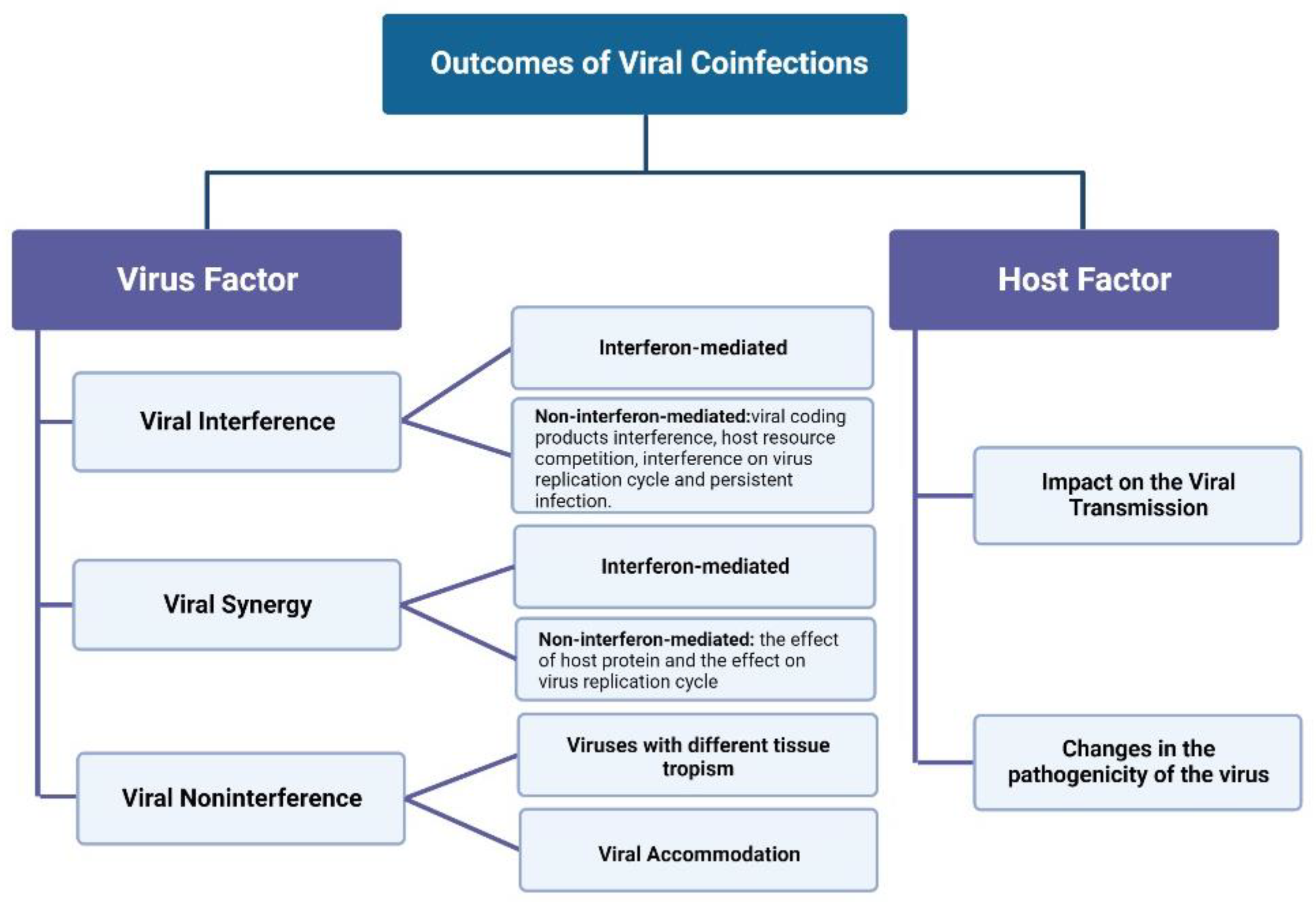
2. Virus–Virus Interaction in Coinfections
2.1. Viral Interference
2.2. Viral Synergy
2.3. Viral Noninterference
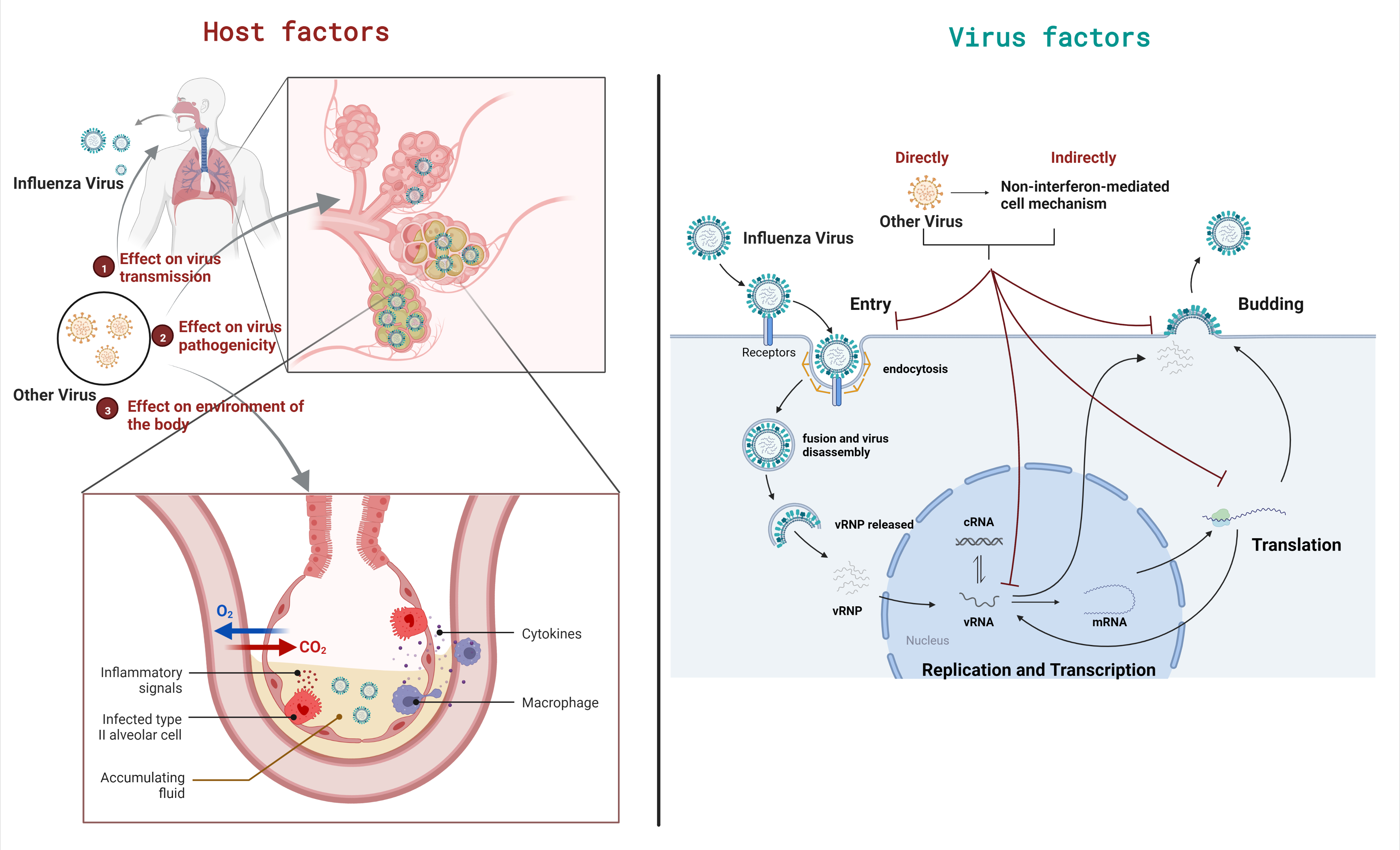
3. Outcome of Viral Coinfections on Host
3.1. Effects on Virus Transmission
3.2. Effects on Viral Pathogenicity
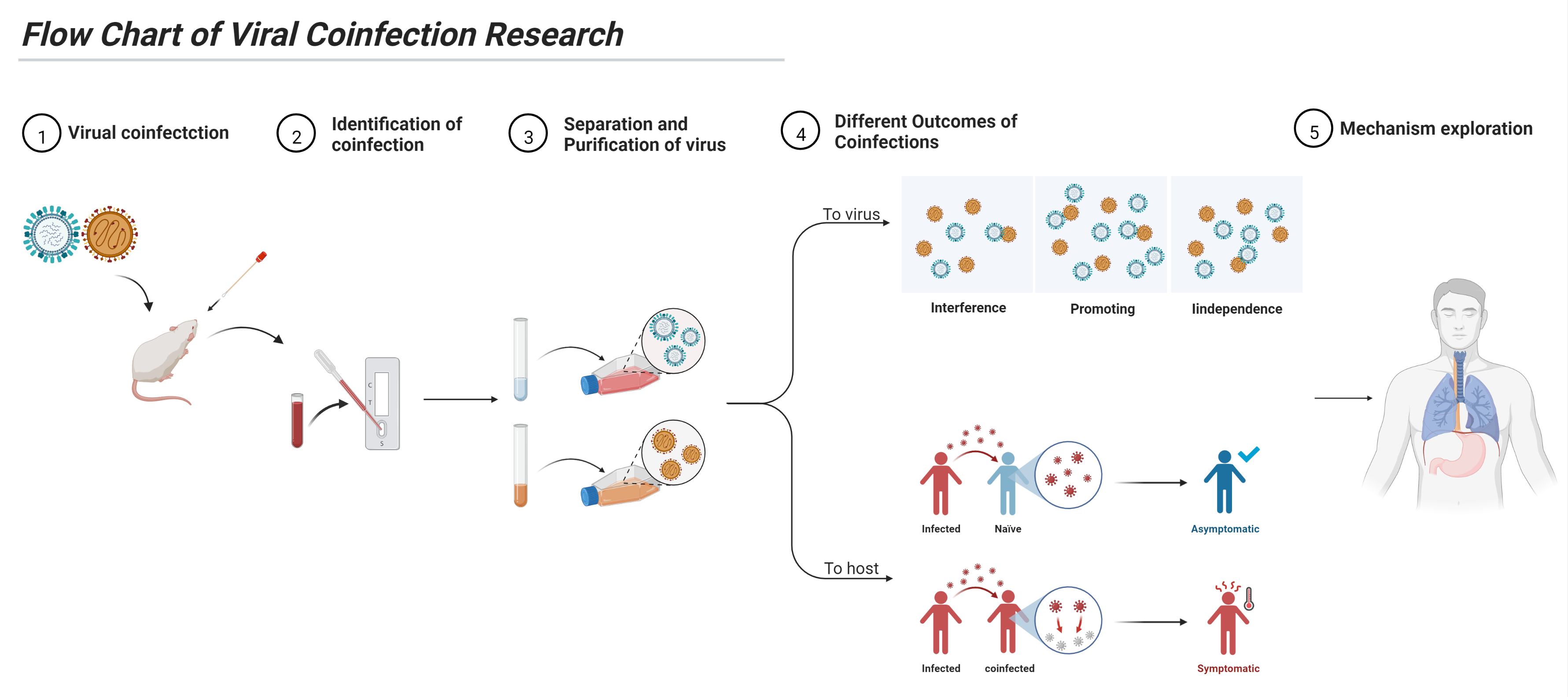
4. Study of Viral Coinfection
4.1. Identification
- (i)
- (ii)
- Application of digital droplet PCR (ddPCR) makes it possible to identify two highly similar viruses [153]. This method improves the accuracy and sensitivity of coinfection detection.
- (iii)
- The transmission electron microscopy detection method of a gold nanoparticle gene probe also has applications in coinfection detection [154]. This method makes detection more convenient, which is conducive to clinical detection.
- (iv)
- Fayyadh et al. used multicolor imaging with self-assembled quantum dot probes to image and successfully detect H1N1, H3N2, and H9N2 influenza viruses in coinfected cells [155]. This method provides a basis for in vitro detection of coinfection, which is more direct and easier to operate than traditional detection.
- (v)
4.2. Viral Separation and Purification
- (i)
- The purification of viruses by CPE is a mainstream method for virus isolation in coinfection, but it requires the selection of suitable cell lines, where one virus can produce obvious CPE while the other virus does not produce obvious CPE. The disadvantage of this method is whether or not some traditional virus isolation cell lines are sensitive to another virus, and coinfection may affect the formation of CPE. At present, it is feasible to separate snakehead retrovirus (SnRV) from grouper nervous necrosis virus (GNNV) by SGF-1 [165]; FMDV from PPRV [130] or single serotype FMDV from multiple serotypes ofFMDV [142] by BHK21; IAV from respiratory viruses by suspended MDCK cells (MDCK-S) and adherent MDCK cells (MDCK-A) [166]; porcine epidemic diarrhea (PEDV) from porcine kobuvirus 1 (PKV) by Vero cells [157]; Hepatitis E virus (HEV) from porcine sapelovirus (PSV) by N1380 cells [167]; and porcine circovirus 2 (PCV2) from porcine parvovirus (PPV) by PK-15 [168].
- (ii)
- An endpoint dilution assay is used to isolate two viruses with a highly similar host range/orientation but different replication rates. However, the separation success rate is usually low. It needs subsequent molecular-level detection and multi-generation blind passages for verification. Beperet et al. successfully isolated two different subtypes of alphabaculoviruses from coinfection samples by an endpoint dilution assay [162]. Dormitorio et al. successfully detected avian influenza virus (AIV) from suspicious allantoine fluid samples using this method [169].
- (iii)
- The Ab neutralization method is suitable for different serotype viruses or two viruses with a distant genetic relationship. This method has a high success rate, but it needs to be verified by subsequent multi-generation blind passages. For the coinfection of multiple serotypes of the same virus, the serotype is generally determined first, and then the 2-dimensional microneutralization test (2D-MNT) corresponding to the serotype is carried out. Mahajan used 2D-MNT to isolate and purify multiple serotype viruses from coinfection samples of FMDV [142].
- (iv)
- The organic solvent treatment method has certain limitations. Whether an organic solvent can kill one virus without affecting another virus needs to be verified. The choice of organic solvent is crucial. At present, it is feasible to remove PPRV with an organic solvent in coinfection of FMDV and PPRV [130]. The use of 5% H2O2 can completely inactivate the infectious laryngotracheitis virus, while the infectivity of NDV, infectious bronchitis virus, and AIV is reduced without being fully inactivated [172].
- (v)
- Hemadsorption is suitable for virus isolation from non-hemagglutinating viruses. The integrity of this method for virus isolation is uncertain and the virus needs to be transferred to susceptible cell lines for amplification. At present, it is feasible to remove PPRV in coinfections of FMDV and PPRV [130]. Hemadsorption is useful for viruses such as IAV, parainfluenza virus, and mumps virus, which express their hemagglutinin proteins on the plasma membrane of infected cells [161].
- (vi)
- Acid/alkali treatment is suitable for the separation of one PH-sensitive virus and another non-PH-sensitive virus. However, due to the difference in PH sensitivity of the isolated virus and the misdetection of molecular detection methods, this method has some notable limitations. Acidic environments (PH < 6.6) can effectively inhibit AIV replication [173]. The optimum survival range of the plague virus is from pH 6 to pH 11, while that of NDV is from pH 2 to pH 11 [174]. Thus, we can isolate viruses from coinfection samples by acid/alkali treatment.
- (vii)
- Reverse genetic system rescues viruses. Some viruses have a mature reverse genetics system. We can isolate the complete genome fragments of the virus from the positive samples and then obtain complete or defective viruses. The disadvantage of this method is that constructing the system necessitates a considerable workload, and it is not suitable for the separation of two related viruses.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salas-Benito, J.S.; De Nova-Ocampo, M. Viral Interference and Persistence in Mosquito-Borne Flaviviruses. J. Immunol. Res. 2015, 2015, 873404. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-J.; Lee, S.-D. Hepatitis B virus/hepatitis C virus coinfection: Epidemiology, clinical features, viral interactions and treatment. J. Gastroenterol. Hepatol. 2008, 23, 512–520. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Nakajima, N.; Sato, Y.; Takahashi, K.; Accola, M.; Chiba, S.; Fan, S.; Neumann, G.; Rehrauer, W.; Suzuki, T.; et al. SARS-CoV-2 Interference of Influenza Virus Replication in Syrian Hamsters. J. Infect. Dis. 2022, 225, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.D.; Sordillo, E.M.; Gitman, M.R.; Paniz Mondolfi, A.E. Coinfection in SARS-CoV-2 infected patients: Where are influenza virus and rhinovirus/enterovirus? J. Med. Virol. 2020, 92, 1699–1700. [Google Scholar] [CrossRef]
- Rojek, J.M.; Campbell, K.P.; Oldstone, M.B.; Kunz, S. Old World arenavirus infection interferes with the expression of functional alpha-dystroglycan in the host cell. Mol. Biol. Cell 2007, 18, 4493–4507. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Li, W.; Sui, J.; Marasco, W.; Choe, H.; Farzan, M. Influenza A virus neuraminidase limits viral superinfection. J. Virol. 2008, 82, 4834–4843. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Perez-Simó, M.; Muñoz, M.; Bohorquez, J.A.; Rosell, R.; Summerfield, A.; Domingo, M.; Ruggli, N.; Ganges, L. Efficacy of a live attenuated vaccine in classical swine fever virus postnatally persistently infected pigs. Vet. Res. 2015, 46, 78. [Google Scholar] [CrossRef]
- Muñoz-González, S.; Pérez-Simó, M.; Colom-Cadena, A.; Cabezón, O.; Bohórquez, J.A.; Rosell, R.; Pérez, L.J.; Marco, I.; Lavín, S.; Domingo, M.; et al. Classical Swine Fever Virus vs. Classical Swine Fever Virus: The Superinfection Exclusion Phenomenon in Experimentally Infected Wild Boar. PLoS ONE 2016, 11, e0149469. [Google Scholar] [CrossRef]
- Goto, H.; Ihira, H.; Morishita, K.; Tsuchiya, M.; Ohta, K.; Yumine, N.; Tsurudome, M.; Nishio, M. Enhanced growth of influenza A virus by coinfection with human parainfluenza virus type 2. Med. Microbiol. Immunol. 2016, 205, 209–218. [Google Scholar] [CrossRef]
- Kent, R.J.; Crabtree, M.B.; Miller, B.R. Transmission of West Nile Virus by Culex quinquefasciatus Say Infected with Culex Flavivirus Izabal. PLoS Negl. Trop. Dis. 2010, 4, e671. [Google Scholar] [CrossRef]
- Bellecave, P.; Gouttenoire, J.; Gajer, M.; Brass, V.; Koutsoudakis, G.; Blum, H.E.; Bartenschlager, R.; Nassal, M.; Moradpour, D. Hepatitis B and C virus coinfection: A novel model system reveals the absence of direct viral interference. Hepatology 2009, 50, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kanthong, N.; Khemnu, N.; Sriurairatana, S.; Pattanakitsakul, S.-N.; Malasit, P.; Flegel, T.W. Mosquito cells accommodate balanced, persistent co-infections with a densovirus and Dengue virus. Dev. Comp. Immunol. 2008, 32, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, C.; Alonso, M.C.; Dopazo, C.P.; Bandín, I. In vivo study of viral haemorrhagic septicaemia virus and infectious pancreatic necrosis virus coexistence in Senegalese sole (Solea senegalensis). J. Fish Dis. 2017, 40, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, P.; Chakraborty, S. Biology of viral satellites and their role in pathogenesis. Curr. Opin. Virol. 2018, 33, 96–105. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. The Subviral Agents. In Virus Taxonomy; Elsevier: San Diego, CA, USA, 2012; pp. 1211–1219. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Genus-Umbravirus. In Virus Taxonomy; Elsevier: San Diego, CA, USA, 2012; pp. 1191–1195. [Google Scholar] [CrossRef]
- La Scola, B.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E.; et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008, 455, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Haller, O. Jean Lindenmann: From viral interference to interferon and beyond (1924–2015). J. Interferon Cytokine Res. 2015, 35, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, F. Viral interference and interferon. Ric. Clin. Lab. 1975, 5, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Essaidi-Laziosi, M.; Geiser, J.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C. Interferon-Dependent and Respiratory Virus-Specific Interference in Dual Infections of Airway Epithelia. Sci. Rep. 2020, 10, 10246. [Google Scholar] [CrossRef] [PubMed]
- Cribier, B.; Schmitt, C.; Rey, D.; Lang, J.M.; Kirn, A.; Stoll-Keller, F. Role of endogenous interferon in hepatitis C virus (HCV) infection and in coinfection by HIV and HCV. Res. Virol. 1996, 147, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.J.; Ijezie, E.C.; Balemba, O.B.; Miura, T.A. Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model. J. Virol. 2018, 92, e00881-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hinson, E.R.; Cresswell, P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2007, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Olfat, F.; Phoon, M.C.; Hsu, J.P.; Howe, J.L.C.; Seet, J.E.; Chin, K.C.; Chow, V.T.K. In vivo and in vitro studies on the antiviral activities of viperin against influenza H1N1 virus infection. J. Gen. Virol. 2012, 93, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Yaneva, R.; Cresswell, P. Viperin: A multifunctional, interferon-inducible protein that regulates virus replication. Cell Host Microbe 2011, 10, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 2013, 371, 67–104. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J. Antiviral Properties of ISG15. Viruses 2010, 2, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, J.B.; Moran, J.V. The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition. PLoS Genet. 2015, 11, e1005121. [Google Scholar] [CrossRef] [PubMed]
- Carthagena, L.; Parise, M.C.; Ringeard, M.; Chelbi-Alix, M.K.; Hazan, U.; Nisole, S. Implication of TRIM alpha and TRIMCyp in interferon-induced anti-retroviral restriction activities. Retrovirology 2008, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, J.; Zhang, C.; Huang, S.; Nunnari, G.; Wang, F.X.; Tong, X.; Gao, L.; Nikisher, K.; Zhang, H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J. Virol. 2006, 80, 7645–7657. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, L.D.; Zalinger, Z.B.; Li, Y.; Wright, P.W.; Elliott, R.; Rose, K.M.; Silverman, R.H.; Weiss, S.R. Activation of RNase L by Murine Coronavirus in Myeloid Cells Is Dependent on Basal Oas Gene Expression and Independent of Virus-Induced Interferon. J. Virol. 2016, 90, 3160–3172. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nagata, K.; Kato, A.; Ishihama, A. Interferon-inducible mouse Mx1 protein that confers resistance to influenza virus is GTPase. J. Biol. Chem. 1991, 266, 21404–21408. [Google Scholar] [CrossRef] [PubMed]
- Streitenfeld, H.; Boyd, A.; Fazakerley, J.K.; Bridgen, A.; Elliott, R.M.; Weber, F. Activation of PKR by Bunyamwera virus is independent of the viral interferon antagonist NSs. J. Virol. 2003, 77, 5507–5511. [Google Scholar] [CrossRef]
- Lalle, E.; Sacchi, A.; Abbate, I.; Vitale, A.; Martini, F.; D’Offizi, G.; Antonucci, G.; Castilletti, C.; Poccia, F.; Capobianchi, M.R. Activation of interferon response genes and of plasmacytoid dendritic cells in HIV-1 positive subjects with GB virus C co-infection. Int. J. Immunopathol. Pharmacol. 2008, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Liu, J.; Li, M.; Xie, J.; Lv, Q.; Deng, W.; Zhou, N.; Zhou, Y.; Song, J.; et al. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. 2020, 30, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Leuven, J.T.V.; Gonzalez, A.J.; Ijezie, E.C.; Wixom, A.Q.; Clary, J.L.; Naranjo, M.N.; Ridenhour, B.J.; Miller, C.R.; Miura, T.A. Rhinovirus Reduces the Severity of Subsequent Respiratory Viral Infections by Interferon-Dependent and -Independent Mechanisms. mSphere 2021, 6, e00479-21. [Google Scholar] [CrossRef]
- Polacino, P.; Kaplan, G.; Palma, E.L. Homologous interference by a foot-and-mouth disease virus strain attenuated for cattle. Arch. Virol. 1985, 86, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Brown, D.T. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J. Virol. 1985, 54, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Koo, R.; Stollar, V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology 1977, 82, 69–83. [Google Scholar] [CrossRef]
- Karpf, A.R.; Lenches, E.; Strauss, E.G.; Strauss, J.H.; Brown, D.T. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 1997, 71, 7119–7123. [Google Scholar] [CrossRef]
- Marcus, P.I.; Carver, D.H. Intrinsic interference: A new type of viral interference. J. Virol. 1967, 1, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zebovitz, E.; Brown, A. Interference among group A arboviruses. J. Virol. 1968, 2, 1283–1289. [Google Scholar] [CrossRef]
- Brinton, M.A. Host factors involved in West Nile virus replication. Ann. N. Y. Acad. Sci. 2001, 951, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Riis, B.; Rattan, S.I.; Clark, B.F.; Merrick, W.C. Eukaryotic protein elongation factors. Trends Biochem. Sci. 1990, 15, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, T.; Abbott, C.M.; Harrich, D. The unexpected roles of eukaryotic translation elongation factors in RNA virus replication and pathogenesis. Microbiol. Mol. Biol. Rev. MMBR 2013, 77, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kelen, K.; Beyaert, R.; Inzé, D.; De Veylder, L. Translational control of eukaryotic gene expression. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 143–168. [Google Scholar] [CrossRef] [PubMed]
- De Nova-Ocampo, M.; Villegas-Sepúlveda, N.; del Angel, R.M. Translation elongation factor-1alpha, La, and PTB interact with the 3’ untranslated region of dengue 4 virus RNA. Virology 2002, 295, 337–347. [Google Scholar] [CrossRef]
- Davis, W.G.; Blackwell, J.L.; Shi, P.Y.; Brinton, M.A. Interaction between the cellular protein eEF1A and the 3’-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J. Virol. 2007, 81, 10172–10187. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.L.; Brinton, M.A. Translation elongation factor-1 alpha interacts with the 3’ stem-loop region of West Nile virus genomic RNA. J. Virol. 1997, 71, 6433–6444. [Google Scholar] [CrossRef]
- Yocupicio-Monroy, M.; Padmanabhan, R.; Medina, F.; del Angel, R.M. Mosquito La protein binds to the 3’ untranslated region of the positive and negative polarity dengue virus RNAs and relocates to the cytoplasm of infected cells. Virology 2007, 357, 29–40. [Google Scholar] [CrossRef] [PubMed]
- García-Montalvo, B.M.; Medina, F.; del Angel, R.M. La protein binds to NS5 and NS3 and to the 5’ and 3’ ends of Dengue 4 virus RNA. Virus Res. 2004, 102, 141–150. [Google Scholar] [CrossRef]
- Gomila, R.C.; Martin, G.W.; Gehrke, L. NF90 binds the dengue virus RNA 3’ terminus and is a positive regulator of dengue virus replication. PLoS ONE 2011, 6, e16687. [Google Scholar] [CrossRef]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.R.; Suomalainen, M.; Varadarajan, S.; Garoff, H.; Helenius, A. Multiple mechanisms for the inhibition of entry and uncoating of superinfecting Semliki Forest virus. Virology 1997, 231, 59–71. [Google Scholar] [CrossRef]
- New, C.; Lee, Z.-Y.; Tan, K.S.; Wong, A.H.; Wang, D.Y.; Tran, T. Tetraspanins: Host Factors in Viral Infections. Int. J. Mol. Sci. 2021, 22, 11609. [Google Scholar] [CrossRef]
- Kim, G.N.; Kang, C.Y. Utilization of homotypic and heterotypic proteins of vesicular stomatitis virus by defective interfering particle genomes for RNA replication and virion assembly: Implications for the mechanism of homologous viral interference. J. Virol. 2005, 79, 9588–9596. [Google Scholar] [CrossRef]
- Chotkowski, H.L.; Ciota, A.T.; Jia, Y.; Puig-Basagoiti, F.; Kramer, L.D.; Shi, P.Y.; Glaser, R.L. West Nile virus infection of Drosophila melanogaster induces a protective RNAi response. Virology 2008, 377, 197–206. [Google Scholar] [CrossRef]
- Sánchez-Vargas, I.; Scott, J.C.; Poole-Smith, B.K.; Franz, A.W.; Barbosa-Solomieu, V.; Wilusz, J.; Olson, K.E.; Blair, C.D. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathog. 2009, 5, e1000299. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Jupatanakul, N.; Ramirez, J.L.; Kang, S.; Romero-Vivas, C.M.; Mohammed, H.; Dimopoulos, G. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl. Trop. Dis. 2013, 7, e2295. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hong, H.; Yue, J.; Wu, Y.; Li, X.; Jiang, L.; Li, L.; Li, Q.; Gao, G.; Yang, X. Inhibitory effect of small interfering RNA on dengue virus replication in mosquito cells. Virol. J. 2010, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, G.P. Flavivirus RNAi suppression: Decoding non-coding RNA. Curr. Opin. Virol. 2014, 7, 55–60. [Google Scholar] [CrossRef]
- Lemm, J.A.; Rice, C.M. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J. Virol. 1993, 67, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Lemm, J.A.; Rümenapf, T.; Strauss, E.G.; Strauss, J.H.; Rice, C.M. Polypeptide requirements for assembly of functional Sindbis virus replication complexes: A model for the temporal regulation of minus- and plus-strand RNA synthesis. EMBO J. 1994, 13, 2925–2934. [Google Scholar] [CrossRef]
- Shirako, Y.; Strauss, J.H. Regulation of Sindbis virus RNA replication: Uncleaved P123 and nsP4 function in minus-strand RNA synthesis, whereas cleaved products from P123 are required for efficient plus-strand RNA synthesis. J. Virol. 1994, 68, 1874–1885. [Google Scholar] [CrossRef]
- Nunes, F.M.; Aleixo, A.C.; Barchuk, A.R.; Bomtorin, A.D.; Grozinger, C.M.; Simões, Z.L. Non-Target Effects of Green Fluorescent Protein (GFP)-Derived Double-Stranded RNA (dsRNA-GFP) Used in Honey Bee RNA Interference (RNAi) Assays. Insects 2013, 4, 90–103. [Google Scholar] [CrossRef]
- Flenniken, M.L.; Andino, R. Non-Specific dsRNA-Mediated Antiviral Response in the Honey Bee. PLoS ONE 2013, 8, e77263. [Google Scholar] [CrossRef] [PubMed]
- Steck, F.T.; Rubin, H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology 1966, 29, 642–653. [Google Scholar] [CrossRef]
- Le Guern, M.; Levy, J.A. Human immunodeficiency virus (HIV) type 1 can superinfect HIV-2-infected cells: Pseudotype virions produced with expanded cellular host range. Proc. Natl. Acad. Sci. USA 1992, 89, 363–367. [Google Scholar] [CrossRef]
- Michel, N.; Allespach, I.; Venzke, S.; Fackler, O.T.; Keppler, O.T. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. CB 2005, 15, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Hrecka, K.; Swigut, T.; Schindler, M.; Kirchhoff, F.; Skowronski, J. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 2005, 79, 10650–10659. [Google Scholar] [CrossRef] [PubMed]
- Geleziunas, R.; Bour, S.; Wainberg, M.A. Cell surface down-modulation of CD4 after infection by HIV-1. FASEB J. 1994, 8, 593–600. [Google Scholar] [CrossRef]
- Willey, R.L.; Maldarelli, F.; Martin, M.A.; Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992, 66, 7193–7200. [Google Scholar] [CrossRef]
- Breiner, K.M.; Schaller, H.; Knolle, P.A. Endothelial cell-mediated uptake of a hepatitis B virus: A new concept of liver targeting of hepatotropic microorganisms. Hepatology 2001, 34, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Nethe, M.; Berkhout, B.; van der Kuyl, A.C. Retroviral superinfection resistance. Retrovirology 2005, 2, 52. [Google Scholar] [CrossRef]
- Schneider-Schaulies, J.; Schnorr, J.J.; Brinckmann, U.; Dunster, L.M.; Baczko, K.; Liebert, U.G.; Schneider-Schaulies, S.; ter Meulen, V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc. Natl. Acad. Sci. USA 1995, 92, 3943–3947. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Joyce, M.A.; Addison, W.R.; Fischer, K.P.; Tyrrell, D.L. Superinfection exclusion in duck hepatitis B virus infection is mediated by the large surface antigen. J. Virol. 2004, 78, 7925–7937. [Google Scholar] [CrossRef] [PubMed]
- Bratt, M.A.; Rubin, H. Specific interference among strains of Newcastle disease virus. II. Comparison of interference by active and inactive virus. Virology 1968, 35, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.E.; Sanfridson, A.; Ottinger, J.S.; Doyle, C.; Cullen, B.R. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 1993, 177, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Rikkonen, M.; Peränen, J.; Kääriäinen, L. Nuclear and nucleolar targeting signals of Semliki Forest virus nonstructural protein nsP2. Virology 1992, 189, 462–473. [Google Scholar] [CrossRef]
- Ranki, M.; Ulmanen, I.; Kääriäinen, L. Semliki Forest virus-specific nonstructural protein is associated with ribosomes. FEBS Lett. 1979, 108, 299–302. [Google Scholar] [CrossRef]
- Simon, K.O.; Cardamone, J.J., Jr.; Whitaker-Dowling, P.A.; Youngner, J.S.; Widnell, C.C. Cellular mechanisms in the superinfection exclusion of vesicular stomatitis virus. Virology 1990, 177, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Dowling, P.; Youngner, J.S.; Widnell, C.C.; Wilcox, D.K. Superinfection exclusion by vesicular stomatitis virus. Virology 1983, 131, 137–143. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, B.; Lim, P.Y.; Yuan, Z.; Bernard, K.A.; Shi, P.Y. Exclusion of West Nile virus superinfection through RNA replication. J. Virol. 2009, 83, 11765–11776. [Google Scholar] [CrossRef] [PubMed]
- Geib, T.; Sauder, C.; Venturelli, S.; Hässler, C.; Staeheli, P.; Schwemmle, M. Selective virus resistance conferred by expression of Borna disease virus nucleocapsid components. J. Virol. 2003, 77, 4283–4290. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, V.; Hoffmann, S.; Herian, U.; Penin, F.; Bartenschlager, R. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J. Virol. 2003, 77, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Appel, N.; Koutsoudakis, G.; Kallis, S.; Lohmann, V.; Pietschmann, T.; Bartenschlager, R. Analysis of hepatitis C virus superinfection exclusion by using novel fluorochrome gene-tagged viral genomes. J. Virol. 2007, 81, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Claus, C.; Tzeng, W.P.; Liebert, U.G.; Frey, T.K. Rubella virus-induced superinfection exclusion studied in cells with persisting replicons. J. Gen. Virol. 2007, 88, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Dowling, P.; Youngner, J.S. Viral interference-dominance of mutant viruses over wild-type virus in mixed infections. Microbiol. Rev. 1987, 51, 179–191. [Google Scholar] [CrossRef]
- Beperet, I.; Irons, S.L.; Simón, O.; King, L.A.; Williams, T.; Possee, R.D.; López-Ferber, M.; Caballero, P. Superinfection exclusion in alphabaculovirus infections is concomitant with actin reorganization. J. Virol. 2014, 88, 3548–3556. [Google Scholar] [CrossRef]
- Frielle, D.W.; Kim, P.B.; Keene, J.D. Inhibitory effects of vesicular stomatitis virus on cellular and influenza viral RNA metabolism and protein synthesis. Virology 1989, 172, 274–284. [Google Scholar] [CrossRef]
- Norkin, L.C. Persistent infections of green monkey kidney cells initiated with temperature-sensitive mutants of simian virus 40. Virology 1980, 107, 375–388. [Google Scholar] [CrossRef]
- Norkin, L.C. Rhesus monkeys kidney cells persistently infected with Simian Virus 40: Production of defective interfering virus and acquisition of the transformed phenotype. Infect. Immun. 1976, 14, 783–792. [Google Scholar] [CrossRef]
- Ahmed, R.; Chakraborty, P.R.; Fields, B.N. Genetic variation during lytic reovirus infection: High-passage stocks of wild-type reovirus contain temperature-sensitive mutants. J. Virol. 1980, 34, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Wilkie, M.L. Persistent infection of Aedes albopictus C6/36 cells by Bunyamwera virus. Virology 1986, 150, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.J.; Stollar, V. Differential sensitivity of infectious and defective-interfering particles of Sindbis virus to ultraviolet irradiation. Virology 1980, 103, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Peleg, J.; Stollar, V. Homologous interference in Aedes aegypti cell cultures infected with Sindbis virus. Arch. Fur Die Gesamte Virusforsch. 1974, 45, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Shenk, T.E.; Koshelnyk, K.A.; Stollar, V. Temperature-sensitive virus from Aedes albopictus cells chronically infected with Sindbis virus. J. Virol. 1974, 13, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.; Birrer, M.; Udem, S.; Bloom, B.R. Complementation analysis of measles virus mutants isolated from persistently infected lymphoblastoid cell lines. J. Virol. 1980, 33, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Frielle, D.W.; Huang, D.D.; Youngner, J.S. Persistent infection with influenza A virus: Evolution of virus mutants. Virology 1984, 138, 103–117. [Google Scholar] [CrossRef]
- Sivaram, A.; Barde, P.V.; Gokhale, M.D.; Singh, D.K.; Mourya, D.T. Evidence of co-infection of chikungunya and densonucleosis viruses in C6/36 cell lines and laboratory infected Aedes aegypti (L.) mosquitoes. Parasites Vectors 2010, 3, 95. [Google Scholar] [CrossRef]
- Wei, W.; Shao, D.; Huang, X.; Li, J.; Chen, H.; Zhang, Q.; Zhang, J. The pathogenicity of mosquito densovirus (C6/36DNV) and its interaction with dengue virus type II in Aedes albopictus. Am. J. Trop. Med. Hyg. 2006, 75, 1118–1126. [Google Scholar] [CrossRef]
- Burivong, P.; Pattanakitsakul, S.-N.; Thongrungkiat, S.; Malasit, P.; Flegel, T.W. Markedly reduced severity of Dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV). Virology 2004, 329, 261–269. [Google Scholar] [CrossRef]
- Dasgupta, R.; Selling, B.; Rueckert, R. Flock house virus: A simple model for studying persistent infection in cultured Drosophila cells. Arch. Virology. Suppl. 1994, 9, 121–132. [Google Scholar] [CrossRef]
- Rechavi, O.; Minevich, G.; Hobert, O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell 2011, 147, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Free, H.M.; Zietlow, S.L.; Paskewitz, S.M.; Aksoy, S.; Shi, L.; Fuchs, J.; Hu, C.; Christensen, B.M. Replication of flock house virus in three genera of medically important insects. J. Med. Entomol. 2007, 44, 102–110. [Google Scholar] [CrossRef]
- Goic, B.; Vodovar, N.; Mondotte, J.A.; Monot, C.; Frangeul, L.; Blanc, H.; Gausson, V.; Vera-Otarola, J.; Cristofari, G.; Saleh, M.C. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat. Immunol. 2013, 14, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Dowling, P.; Youngner, J.S. Vaccinia rescue of VSV from interferon-induced resistance: Reversal of translation block and inhibition of protein kinase activity. Virology 1983, 131, 128–136. [Google Scholar] [CrossRef]
- Cheng, X.M.; Uchida, T.; Xia, Y.C.; Umarova, R.; Liu, C.J.; Chen, P.J.; Gaggar, A.; Suri, V.; Mucke, M.M.; Vermehren, J.; et al. Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection. J. Clin. Investig. 2020, 130, 3205–3220. [Google Scholar] [CrossRef]
- Hao, X.Q.; Li, Y.C.; Chen, H.; Chen, B.; Liu, R.H.; Wu, Y.D.; Xiao, X.Y.; Zhou, P.; Li, S.J. Canine Circovirus Suppresses the Type I Interferon Response and Protein Expression but Promotes CPV-2 Replication. Int. J. Mol. Sci. 2022, 23, 6382. [Google Scholar] [CrossRef]
- He, B.; Paterson, R.G.; Stock, N.; Durbin, J.E.; Durbin, R.K.; Goodbourn, S.; Randall, R.E.; Lamb, R.A. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: The multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 2002, 303, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Wansley, E.K.; Grayson, J.M.; Parks, G.D. Apoptosis induction and interferon signaling but not IFN-β promoter induction by an SV5 P/V mutant are rescued by coinfection with wild-type SV5. Virology 2003, 316, 41–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, X.; Zhou, D.; Zhou, J.; Xue, J.; Cheng, Z. Marek’s Disease Virus and Reticuloendotheliosis Virus Coinfection Enhances Viral Replication and Alters Cellular Protein Profiles. Front. Vet. Sci. 2022, 9, 854007. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, P.P.; Cheng, Z.Q.; Yang, J.; Bi, J.M.; Wang, G.H. Avian leukosis virus subgroup J and reticuloendotheliosis virus coinfection induced TRIM62 regulation of the actin cytoskeleton. J. Vet. Sci. 2020, 21, e49. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.I.; Siqueira-Neto, J.L.; McKerrow, J.H. Location, Location, Location: Five Facts about Tissue Tropism and Pathogenesis. PLoS Pathog. 2016, 12, e1005519. [Google Scholar] [CrossRef] [PubMed]
- Ure, A.E.; Forslund, O. Characterization of Human Papillomavirus Type 154 and Tissue Tropism of Gammapapillomaviruses. PLoS ONE 2014, 9, e89342. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. Update on viral accommodation, a model for host-viral interaction in shrimp and other arthropods. Dev. Comp. Immunol. 2007, 31, 217–231. [Google Scholar] [CrossRef]
- Flegel, T.W. Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 2012, 110, 166–173. [Google Scholar] [CrossRef]
- Johansson, M.W.; Söderhäll, K. The prophenoloxidase activating system and associated proteins in invertebrates. Prog. Mol. Subcell. Biol. 1996, 15, 46–66. [Google Scholar] [CrossRef]
- Namikoshi, A.; Wu, J.L.; Yamashita, T.; Nishizawa, T.; Nishioka, T.; Arimoto, M.; Muroga, K. Vaccination trials with Penaeus japonicus to induce resistance to white spot syndrome virus. Aquaculture 2004, 229, 25–35. [Google Scholar] [CrossRef]
- Venegas, C.A.; Nonaka, L.; Mushiake, K.; Nishizawa, T.; Murog, K. Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV). Dis. Aquat. Org. 2000, 42, 83–89. [Google Scholar] [CrossRef]
- Khanobdee, K.; Soowannayan, C.; Flegel, T.W.; Ubol, S.; Withyachumnarnkul, B. Evidence for apoptosis correlated with mortality in the giant black tiger shrimp Penaeus monodon infected with yellow head virus. Dis. Aquat. Org. 2002, 48, 79–90. [Google Scholar] [CrossRef][Green Version]
- Sahtout, A.H.; Hassan, M.D.; Shariff, M. DNA fragmentation, an indicator of apoptosis, in cultured black tiger shrimp Penaeus monodon infected with white spot syndrome virus (WSSV). Dis. Aquat. Org. 2001, 44, 155–159. [Google Scholar] [CrossRef]
- Wongprasert, K.; Khanobdee, K.; Glunukarn, S.S.; Meeratana, P.; Withyachumnarnkul, B. Time-course and levels of apoptosis in various tissues of black tiger shrimp Penaeus monodon infected with white-spot syndrome virus. Dis. Aquat. Org. 2003, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Shell, E.J.; Fokam, E.B.; Mason, P.W.; Hanley, K.A.; Estes, D.M.; Weaver, S.C. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology 2007, 358, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, A.; Chan, Y.C. Dengue Type 2 Virus in Naturally Infected Aedes albopictus Mosquitoes in Singapore. Science 1965, 149, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Holmes, E.C.; Fokam, E.B.; Faye, O.; Diallo, M.; Sall, A.A.; Weaver, S.C. Evolutionary processes among sylvatic dengue type 2 viruses. J. Virol. 2007, 81, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Rotzén-Östlund, M.; Zweygberg-Wirgart, B.; Rubinova, S.; Brytting, M. Does viral interference affect spread of influenza? Eurosurveillance 2009, 14, 19354. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, T.; Robison, A.; Young, M.C.; Black, W.C.; Foy, B.D.; Ebel, G.D.; Ruckert, C. Sequential Infection of Aedes aegypti Mosquitoes with Chikungunya Virus and Zika Virus Enhances Early Zika Virus Transmission. Insects 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Kaaden, O.R. Foot-and-mouth disease in camelids: A review. Vet. J. 2004, 168, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Barua, S.; Riyesh, T.; Chaubey, K.K.; Rawat, K.D.; Khandelwal, N.; Mishra, A.K.; Sharma, N.; Chandel, S.S.; Sharma, S.; et al. Complexities in Isolation and Purification of Multiple Viruses from Mixed Viral Infections: Viral Interference, Persistence and Exclusion. PLoS ONE 2016, 11, e0156110. [Google Scholar] [CrossRef]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol. Infect. 2015, 143, 37–47. [Google Scholar] [CrossRef]
- Goka, E.; Vallely, P.; Mutton, K.; Klapper, P. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir Viruses 2013, 7, 1079–1087. [Google Scholar] [CrossRef]
- Tang, M.B.; Yu, C.P.; Chen, S.C.; Chen, C.H. Co-Infection of Adenovirus, Norovirus and Torque Teno Virus in Stools of Patients with Acute Gastroenteritis. Southeast Asian J. Trop. Med. Public Health 2014, 45, 1326–1336. [Google Scholar] [PubMed]
- Amin, J.; Law, M.G.; Bartlett, M.; Kaldor, J.M.; Dore, G.J. Causes of death after diagnosis of hepatitis B or hepatitis C infection: A large community-based linkage study. Lancet 2006, 368, 938–945. [Google Scholar] [CrossRef]
- Liang, L.; He, C.; Lei, M.; Li, S.; Hao, Y.; Zhu, H.; Duan, Q. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA Cell Biol. 2005, 24, 485–490. [Google Scholar] [CrossRef]
- Calixto, R.; Oliveira, G.; Lima, M.; Andrade, A.C.; Trindade, G.D.; De Oliveira, D.B.; Kroon, E.G. A Model to Detect Autochthonous Group 1 and 2 Brazilian Vaccinia virus Coinfections: Development of a qPCR Tool for Diagnosis and Pathogenesis Studies. Viruses 2018, 10, 15. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, C.; Li, Y.; Yan, D.; Zhang, X.; Jiang, D.; Yang, S.; Li, L. Impact of Coinfection With SARS-CoV-2 and Influenza on Disease Severity: A Systematic Review and Meta-Analysis. Front. Public Heal. 2021, 9, 773130. [Google Scholar] [CrossRef]
- Xiang, T.D.; Zheng, X. Interaction between hepatitis B virus and SARS-CoV-2 infections. World J. Gastroenterol. 2021, 27, 782–793. [Google Scholar] [CrossRef]
- Martinez-Roig, A.; Salvado, M.; Caballero-Rabasco, M.A.; Sanchez-Buenavida, A.; Lopez-Segura, N.; Bonet-Alcaina, M. Viral Coinfection in Childhood Respiratory Tract Infections. Arch. Bronconeumol. 2015, 51, 5–9. [Google Scholar] [CrossRef]
- Zeremski, M.; Martinez, A.D.; Talal, A.H. Editorial Commentary: Management of Hepatitis C Virus in HIV-Infected Patients in the Era of Direct-Acting Antivirals. Clin. Infect. Dis. 2014, 58, 880–882. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Acosta-Reyes, J.; Navarro-Lechuga, E.; Corchuelo, S.; Rico, A.; Parra, E.; Tolosa, N.; Pardo, L.; González, M.; Martìn-Rodriguez-Hernández, J.; et al. Dengue, chikungunya and zika virus coinfection: Results of the national surveillance during the zika epidemic in Colombia. Epidemiol. Infect. 2019, 147, e77. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, G.K.; Subramaniam, S.; Biswal, J.K.; Pattnaik, B. Selective isolation of foot-and-mouth disease virus from coinfected samples containing more than one serotype. Braz. J. Microbiol. 2021, 52, 2447–2454. [Google Scholar] [CrossRef]
- Scotta, M.C.; Chakr, V.C.; de Moura, A.; Becker, R.G.; de Souza, A.P.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Semple, M.G.; Cowell, A.; Dove, W.; Greensill, J.; McNamara, P.S.; Halfhide, C.; Shears, P.; Smyth, R.L.; Hart, C.A. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J. Infect. Dis. 2005, 191, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. New Engl. J. Med. 2017, 376, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Salassa, B.; Daziano, E.; Bonino, F.; Lavarini, C.; Smedile, A.; Chiaberge, E.; Rosina, F.; Brunetto, M.R.; Pessione, E.; Spezia, C. Serological diagnosis of hepatitis B and delta virus (HBV/HDV) coinfection. J. Hepatol. 1991, 12, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Masyeni, S.; Santoso, M.S.; Widyaningsih, P.D.; Asmara, D.G.W.; Nainu, F.; Harapan, H.; Sasmono, R.T. Serological cross-reaction and coinfection of dengue and COVID-19 in Asia: Experience from Indonesia. Int. J. Infect. Dis. 2021, 102, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Afonso, C.L.; Miller, P.J.; Shepherd, E.; DeJesus, E.; Smith, D.; Pantin-Jackwood, M.J. Effect of Infection with a Mesogenic Strain of Newcastle Disease Virus on Infection with Highly Pathogenic Avian Influenza Virus in Chickens. Avian. Dis. 2016, 60, 269–278. [Google Scholar] [CrossRef]
- Bara, J.J.; Muturi, E.J. Effect of mixed infections of Sindbis and La Crosse viruses on replication of each virus in vitro. Acta Trop. 2014, 130, 71–75. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.R.; Pei, Y.; Xue, J.; Ruan, S.F.; Zhang, G.Z. Development and Application of an MRT-qPCR Assay for Detecting Coinfection of Six Vertically Transmitted or Immunosuppressive Avian Viruses. Front. Microbiol. 2020, 11, 1581. [Google Scholar] [CrossRef]
- Xu, Z.X.; Peng, Y.; Yang, M.H.; Li, X.H.; Wang, J.; Zou, R.R.; Liang, J.H.; Fang, S.S.; Liu, Y.X.; Yang, Y. Simultaneous detection of Zika, chikungunya, dengue, yellow fever, West Nile, and Japanese encephalitis viruses by a two-tube multiplex real-time RT-PCR assay. J. Med. Virol. 2022, 94, 2528–2536. [Google Scholar] [CrossRef]
- Sanchez-Ponce, Y.; Varela-Fascinetto, G.; Romo-Vazquez, J.C.; Lopez-Martinez, B.; Sanchez-Huerta, J.L.; Parra-Ortega, I.; Fuentes-Panana, E.M.; Morales-Sanchez, A. Simultaneous Detection of Beta and Gamma Human Herpesviruses by Multiplex qPCR Reveals Simple Infection and Coinfection Episodes Increasing Risk for Graft Rejection in Solid Organ Transplantation. Viruses 2018, 10, 730. [Google Scholar] [CrossRef]
- Leibovitch, E.C.; Brunetto, G.S.; Caruso, B.; Fenton, K.; Ohayon, J.; Reich, D.S.; Jacobson, S. Coinfection of Human Herpesviruses 6A (HHV-6A) and HHV-6B as Demonstrated by Novel Digital Droplet PCR Assay. PLoS ONE 2014, 9, e92328. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Luo, X.; Ning, Q. Detection of HBV and HCV coinfection by TEM with Au nanoparticle gene probes. J. Huazhong Univ. Sci. Technol. 2007, 27, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Fayyadh, T.K.; Ma, F.Y.; Qin, C.; Zhang, X.W.; Li, W.; Zhang, X.E.; Zhang, Z.P.; Cui, Z.Q. Simultaneous detection of multiple viruses in their co-infected cells using multicolour imaging with self-assembled quantum dot probes. Microchim. Acta 2017, 184, 2815–2824. [Google Scholar] [CrossRef]
- Srisomwat, C.; Yakoh, A.; Avihingsanon, A.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. An alternative label-free DNA sensor based on the alternating-current electroluminescent device for simultaneous detection of human immunodeficiency virus and hepatitis C co-infection. Biosens. Bioelectron. 2022, 196, 113719. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, M.-E.; Trujillo-Ortega, M.-E.; Alcaraz-Estrada, S.-L.; Lozano-Aguirre-Beltran, L.; Sandoval-Jaime, C.; Taboada-Ramirez, B.I.; Sarmiento-Silva, R.-E. Molecular Detection and Characterization of Porcine Epidemic Diarrhea Virus and Porcine Aichivirus C Coinfection in Mexico. Viruses 2021, 13, 738. [Google Scholar] [CrossRef]
- Bialasiewicz, S.; McVernon, J.; Nolan, T.; Lambert, S.B.; Zhao, G.; Wang, D.; Nissen, M.D.; Sloots, T.P. Detection of a divergent Parainfluenza 4 virus in an adult patient with influenza like illness using next-generation sequencing. BMC Infect. Dis. 2014, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bester, R.; Burger, J.T.; Maree, H.J. Next-generation sequencing for virus detection: Covering all the bases. Virol. J. 2016, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- van Engelenburg, F.A.; Terpstra, F.G.; Schuitemaker, H.; Moorer, W.R. The virucidal spectrum of a high concentration alcohol mixture. J. Hosp. Infect. 2002, 51, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Leland Diane, S.; Ginocchio Christine, C. Role of Cell Culture for Virus Detection in the Age of Technology. Clin. Microbiol. Rev. 2007, 20, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Beperet, I.; Simón, O.; López-Ferber, M.; Lent, J.v.; Williams, T.; Caballero, P.; Johnson, K.N. Mixtures of Insect-Pathogenic Viruses in a Single Virion: Towards the Development of Custom-Designed Insecticides. Appl. Environ. Microbiol. 2021, 87, e02180-20. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-F.; Su, C.-L.; Shu, P.-Y.; Yang, C.-F.; Liao, T.-L.; Cheng, C.-H.; Hu, H.-C.; Huang, J.-H. Concurrent Isolation of Chikungunya Virus and Dengue Virus from a Patient with Coinfection Resulting from a Trip to Singapore. J. Clin. Microbiol. 2010, 48, 4586–4589. [Google Scholar] [CrossRef]
- Davidson, I.; Nagar, S.; Haddas, R.; Ben-Shabat, M.; Golender, N.; Lapin, E.; Altory, A.; Simanov, L.; Ribshtein, I.; Panshin, A.; et al. Avian Influenza Virus H9N2 Survival at Different Temperatures and pHs. Avian Dis. 2010, 54, 725–728. [Google Scholar] [CrossRef]
- Lee, K.W.; Chi, S.C.; Cheng, T.M. Interference of the life cycle of fish nodavirus with fish retrovirus. J. Gen. Virol. 2002, 83, 2469–2474. [Google Scholar] [CrossRef]
- Harada, Y.; Takahashi, H.; Trusheim, H.; Roth, B.; Mizuta, K.; Hirata-Saito, A.; Ogane, T.; Odagiri, T.; Tashiro, M.; Yamamoto, N. Comparison of suspension MDCK cells, adherent MDCK cells, and LLC-MK2 cells for selective isolation of influenza viruses to be used as vaccine seeds. Influenza Other Respir. Viruses 2020, 14, 204–209. [Google Scholar] [CrossRef]
- Zhang, W.; Kataoka, M.; Doan, H.Y.; Wu, F.-T.; Takeda, N.; Muramatsu, M.; Li, T.-C. Isolation and Characterization of Hepatitis E Virus Subtype 4b Using a PLC/PRF/5 Cell-Derived Cell Line Resistant to Porcine Sapelovirus Infection. Jpn. J. Infect. Dis. 2021, 74, 573–575. [Google Scholar] [CrossRef]
- Kim, J.; Chae, C. A comparison of virus isolation, polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine circovirus 2 and porcine parvovirus in experimentally and naturally coinfected pigs. J. Vet. Diagn. Investig. 2004, 16, 45–50. [Google Scholar] [CrossRef]
- Dormitorio, T.V.; Giambrone, J.J. Limiting dilution studies to detect avian influenza viruses from questionable allantoic fluid samples. J. Dairy Sci. 2010, 93, 544. [Google Scholar]
- El Zowalatys, M.E.; Chander, Y.; Redig, P.T.; El Latif, H.K.A.; El Sayed, M.A.; Goyal, S.M. Selective isolation of Avian influenza virus (AIV) from cloacal samples containing AIV and Newcastle disease virus. J. Vet. Diagn. Investig. 2011, 23, 330–332. [Google Scholar] [CrossRef]
- Mi, S.J.; Guo, S.B.; Xing, C.N.; Xiao, C.T.; He, B.; Wu, B.; Xia, X.Z.; Tu, C.C.; Gong, W.J. Isolation and Characterization of Porcine Astrovirus 5 from a Classical Swine Fever Virus-Infected Specimen. J. Virol. 2021, 95, e01513-20. [Google Scholar] [CrossRef]
- Neighbor, N.K.; Newberry, L.A.; Bayyari, G.R.; Skeeles, J.K.; Beasley, J.N.; McNew, R.W. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult. Sci. 1994, 73, 1511–1516. [Google Scholar] [CrossRef]
- Brown, J.D.; Goekjian, G.; Poulson, R.; Valeika, S.; Stallknecht, D.E. Avian influenza virus in water: Infectivity is dependent on pH, salinity and temperature. Vet. Microbiol. 2009, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Moses, H.E.; Brandly, C.A.; Jones, E.E. The pH Stability of Viruses of Newcastle Disease and Fowl Plague. Science 1947, 105, 477–479. [Google Scholar] [CrossRef]
- Hematian, A.; Sadeghifard, N.; Mohebi, R.; Taherikalani, M.; Nasrolahi, A.; Amraei, M.; Ghafourian, S. Traditional and Modern Cell Culture in Virus Diagnosis. Osong Public Health Res. Perspect. 2016, 7, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Waner, J.L. Mixed viral infections: Detection and management. Clin. Microbiol. Rev. 1994, 7, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Brumback, B.G.; Wade, C.D. Simultaneous culture for adenovirus, cytomegalovirus, and herpes simplex virus in same shell vial by using three-color fluorescence. J. Clin. Microbiol. 1994, 32, 2289–2290. [Google Scholar] [CrossRef]
- Weinberg, A.; Brewster, L.; Clark, J.; Simoes, E. Evaluation of R-Mix shell vials for the diagnosis of viral respiratory tract infections. J. Clin. Virol. 2004, 30, 100–105. [Google Scholar] [CrossRef]
- Lim, G.; Park, T.S.; Suh, J.T.; Lee, H.J. Comparison of R-mix virus culture and multiplex reverse transcriptase-PCR for the rapid detection of respiratory viruses. Korean J. Lab. Med. 2010, 30, 289–294. [Google Scholar] [CrossRef][Green Version]
- Barenfanger, J.; Drake, C.; Mueller, T.; Troutt, T.; O’Brien, J.; Guttman, K. R-Mix cells are faster, at least as sensitive and marginally more costly than conventional cell lines for the detection of respiratory viruses. J. Clin. Virol. 2001, 22, 101–110. [Google Scholar] [CrossRef]
- Dunn, J.J.; Woolstenhulme, R.D.; Langer, J.; Carroll, K.C. Sensitivity of respiratory virus culture when screening with R-mix fresh cells. J. Clin. Microbiol. 2004, 42, 79–82. [Google Scholar] [CrossRef]
- St George, K.; Patel, N.M.; Hartwig, R.A.; Scholl, D.R.; Jollick, J.A., Jr.; Kauffmann, L.M.; Evans, M.R.; Rinaldo, C.R., Jr. Rapid and sensitive detection of respiratory virus infections for directed antiviral treatment using R-Mix cultures. J. Clin. Virol. 2002, 24, 107–115. [Google Scholar] [CrossRef]
- Gillim-Ross, L.; Taylor, J.; Scholl, D.R.; Ridenour, J.; Masters, P.S.; Wentworth, D.E. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 2004, 42, 3196–3206. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Poudel, B. Tools to detect influenza virus. Yonsei Med. J. 2013, 54, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.Y.; Chan, P.K.; Peiris, M.; Tsang, D.N.; Que, T.L.; Shortridge, K.F.; Cheung, P.T.; To, W.K.; Ho, E.T.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Choi, W.S.; Noh, J.Y.; Baek, J.H.; Seo, Y.B.; Lee, J.; Song, J.Y.; Park, D.W.; Lee, J.S.; Cheong, H.J.; Kim, W.J. Suboptimal effectiveness of the 2011-2012 seasonal influenza vaccine in adult Korean populations. PLoS ONE 2015, 10, e0098716. [Google Scholar] [CrossRef]
- Schmutzhard, J.; Merete Riedel, H.; Zweygberg Wirgart, B.; Grillner, L. Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. J. Clin. Virol. 2004, 29, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Hite, S.; Duane, V.; Yan, H. CV-1 and MRC-5 mixed cells for simultaneous detection of herpes simplex viruses and varicella zoster virus in skin lesions. J. Clin. Virol. 2002, 24, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Woreta, T.A.; Chalasani, N. Fatty Liver Disease in Human Immunodeficiency Virus-Hepatitis B Virus Coinfection: A Cause for Concern COMMENT. Clin. Infect. Dis. 2021, 73, E3286–E3287. [Google Scholar] [CrossRef]
- Kim, Z.; Lee, J.H. Coinfection with severe acute respiratory syndrome coronavirus-2 and other respiratory viruses at a tertiary hospital in Korea. J Clin Lab Anal 2021, 35, e23868. [Google Scholar] [CrossRef]
- Zhong, P.P.; Zhang, H.L.; Chen, X.F.; Lv, F.F. Clinical characteristics of the lower respiratory tract infection caused by a single infection or coinfection of the human parainfluenza virus in children. J. Med. Virol. 2019, 91, 1625–1632. [Google Scholar] [CrossRef]
- Mavilia, M.G.; Wu, G.Y. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J. Clin. Transl. Hepatol. 2018, 6, 296–305. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Ali, G. Report on Influenza A and B Viruses: Their Coinfection in a Saudi Leukemia Patient. Biomed Res. Int. 2013, 2013, 290609. [Google Scholar] [CrossRef]
- Yapali, S.; Bayrakci, B.; Gunsar, F.; Ersoz, G.; Karasu, Z.; Akarca, U.S. Delta virus coinfection does not increase, but hcv coinfection increase the hbsag loss, in chronic hbv infection. J. Hepatol. 2011, 54, S159–S160. [Google Scholar] [CrossRef]
- Kim, E.H.; Nguyen, T.Q.; Casel, M.A.B.; Rollon, R.; Kim, S.M.; Kim, Y.I.; Yu, K.M.; Jang, S.G.; Yang, J.; Poo, H.; et al. Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4(+) T Cell Responses. J. Virol. 2022, 96, e01873-21. [Google Scholar] [CrossRef]
- Pires, C.A.A.; Quaresma, J.A.S.; Aarao, T.L.D.; de Souza, J.R.; Macedo, G.M.M.; Neto, F.; Xavier, M.B. Expression of interleukin-1 beta and interleukin-6 in leprosy reactions in patients with human immunodeficiency virus coinfection. Acta Trop. 2017, 172, 213–216. [Google Scholar] [CrossRef]
- Kanthong, N.; Khemnu, N.; Pattanakitsakul, S.-N.; Malasit, P.; Flegel, T.W. Persistent, triple-virus co-infections in mosquito cells. BMC Microbiol. 2010, 10, 14. [Google Scholar] [CrossRef]
- Cook, J.K.; Huggins, M.B.; Orbell, S.J.; Mawditt, K.; Cavanagh, D. Infectious bronchitis virus vaccine interferes with the replication of avian pneumovirus vaccine in domestic fowl. Avian Pathol. J. WVPA 2001, 30, 233–242. [Google Scholar] [CrossRef]
- Gelb, J., Jr.; Ladman, B.S.; Licata, M.J.; Shapiro, M.H.; Campion, L.R. Evaluating viral interference between infectious bronchitis virus and Newcastle disease virus vaccine strains using quantitative reverse transcription-polymerase chain reaction. Avian Dis. 2007, 51, 924–934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Miller, P.J.; Afonso, C.L.; Spackman, E.; Kapczynski, D.R.; Shepherd, E.; Smith, D.; Swayne, D.E. Experimental co-infections of domestic ducks with a virulent Newcastle disease virus and low or highly pathogenic avian influenza viruses. Vet. Microbiol. 2015, 177, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sloutskin, A.; Yee, M.B.; Kinchington, P.R.; Goldstein, R.S. Varicella-zoster virus and herpes simplex virus 1 can infect and replicate in the same neurons whether co- or superinfected. J. Virol. 2014, 88, 5079–5086. [Google Scholar] [CrossRef]
- White, P.A.; Li, Z.; Zhai, X.; Marinos, G.; Rawlinson, W.D. Mixed viral infection identified using heteroduplex mobility analysis (HMA). Virology 2000, 271, 382–389. [Google Scholar] [CrossRef]
- Diallo, I.S.; Taylor, J.; Gibson, J.; Hoad, J.; De Jong, A.; Hewitson, G.; Corney, B.G.; Rodwell, B.J. Diagnosis of a naturally occurring dual infection of layer chickens with fowlpox virus and gallid herpesvirus 1 (infectious laryngotracheitis virus). Avian Pathol. J. WVPA 2010, 39, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Otta, S.K.; Arulraj, R.; Ezhil Praveena, P.; Manivel, R.; Panigrahi, A.; Bhuvaneswari, T.; Ravichandran, P.; Jithendran, K.P.; Ponniah, A.G. Association of dual viral infection with mortality of Pacific white shrimp (Litopenaeus vannamei) in culture ponds in India. VirusDisease 2014, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Vaziry, A.; Silim, A.; Bleau, C.; Frenette, D.; Lamontagne, L. Dual infections with low virulent chicken infectious anaemia virus (lvCIAV) and intermediate infectious bursal disease virus (iIBDV) in young chicks increase lvCIAV in thymus and bursa while decreasing lymphocyte disorders induced by iIBDV. Avian Pathol. J. WVPA 2013, 42, 88–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ge, X.-Y.; Wang, N.; Zhang, W.; Hu, B.; Li, B.; Zhang, Y.-Z.; Zhou, J.-H.; Luo, C.-M.; Yang, X.-L.; Wu, L.-J.; et al. Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol. Sin. 2016, 31, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Zampiero, A.; Bianchini, S.; Mori, A.; Scala, A.; Tagliabue, C.; Sciarrabba, C.S.; Fossali, E.; Piralla, A.; Principi, N. Epidemiology and Clinical Characteristics of Respiratory Infections Due to Adenovirus in Children Living in Milan, Italy, during 2013 and 2014. PLoS ONE 2016, 11, e0152375. [Google Scholar] [CrossRef] [PubMed]
- González Álvarez, D.A.; López Cortés, L.F.; Cordero, E. Impact of HIV on the severity of influenza. Expert Rev. Respir. Med. 2016, 10, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Fan, C.; Liu, S.; Zhou, J.; Jin, Y.; Zheng, X.; Wang, Q.; Liu, J.; Yang, H.; Gu, J.; et al. Cellular proteomic analysis of porcine circovirus type 2 and classical swine fever virus coinfection in porcine kidney-15 cells using isobaric tags for relative and absolute quantitation-coupled LC-MS/MS. Electrophoresis 2017, 38, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Shinjoh, M.; Omoe, K.; Saito, N.; Matsuo, N.; Nerome, K. In vitro growth profiles of respiratory syncytial virus in the presence of influenza virus. Acta Virol. 2000, 44, 91–97. [Google Scholar] [PubMed]
- Hurt, A.C.; Nor’e, S.S.; McCaw, J.M.; Fryer, H.R.; Mosse, J.; McLean, A.R.; Barr, I.G. Assessing the viral fitness of oseltamivir-resistant influenza viruses in ferrets, using a competitive-mixtures model. J. Virol. 2010, 84, 9427–9438. [Google Scholar] [CrossRef] [PubMed]
- Dobrescu, I.; Levast, B.; Lai, K.; Delgado-Ortega, M.; Walker, S.; Banman, S.; Townsend, H.; Simon, G.; Zhou, Y.; Gerdts, V.; et al. In vitro and ex vivo analyses of co-infections with swine influenza and porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2014, 169, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Rozeboom, L.E.; Kassira, E.N. Dual infections of mosquitoes with strains of West Nile virus. J. Med. Entomol. 1969, 6, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Pesko, K.; Mores, C.N. Effect of sequential exposure on infection and dissemination rates for West Nile and St. Louis encephalitis viruses in Culex quinquefasciatus. Vector-Borne Zoonotic Dis. 2009, 9, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, D.; Castro, A.; Haines, H. Demonstration of interference between dengue virus types in cultured mosquito cells using monoclonal antibody probes. J. Gen. Virol. 1982, 59, 273–282. [Google Scholar] [CrossRef] [PubMed]
| Coinfecting Viruses | Outcome | Method(s) of Detection | Method(s) of Purification | Cause Mechanisms | Effect on Host | Reference (Published Year) |
|---|---|---|---|---|---|---|
| HIV and HBV | NA | liver biopsies | NA | NA | Occurrence of complications and increased incidence of nonalcoholic fatty liver disease (NALFD) | [189] (2021) |
| COVID-19 and CoV 229E/OC43, AdV, HRV, FluA | Independence | MRT-qPCR | NA | NA | No obvious trend change | [190] (2021) |
| HPIV and HRV, RSV, AdV, HCoV, HboV, FluB, HMPV, FluA | NA | multiplex PCR | NA | NA | Alleviation of clinical symptoms in coinfection hosts | [191] (2019) |
| HBV and HCV | Noninterference (in vitro) coinfection interfered HBV (in vivo) | PCR, serologic profiles | NA | MiRNA 122 mediated by HCV core protein inhibits HBV replication. | A faster progression and high incidence of hepatocellular carcinoma | [192] (2018) |
| DENV, CHIKV, and ZIKV | NA | MRT-qPCR | NA | NA | Mean viraemia was significantly lower in coinfections compared to monoinfections. ZIKV- DENV coinfection did not significantly differ from reported ZIKV monoinfections. Coinfection by ZIKV–CHIKV could affect foetal death | [141] (2019) |
| FluA and hPIV2 | coinfection enhanced FluA | Virus titration and Immunofluorescent staining | Cell fusion induced by hPIV2 infection promotes FluA replication. | NA | [9] (2016) | |
| FluA and FluB | Noninterference | RT-PCR | Using Embryonating Chicken Eggs | NA | Patients presented typical influenza-like disease symptoms including fever > 39°C, myalgia, pharyngitis, and cough. | [193] (2013) |
| HBV, HCV, and HDV | Interference (HCV to HBV) Noninterference (HDV to HBV) | hepatitis B surface antigen loss rates | NA | NA | NA | [194] (2011) |
| RV and FluA | coinfection interfered FluA | Virus titration | NA | RV inhibits FluA replication by activating innate immune defense. | Reduced mortality in mice | [22] (2018) |
| SARS-CoV-2 and FluA | NA | Virus titration | NA | Coinfections caused severe lymphopenia in peripheral blood, resulting in reduced total IgG, neutralizing antibody titers, and CD4+ T cell responses against each virus. | The coinfection of SARS-CoV-2 with IAV enhanced disease severity. | [195] (2022) |
| Leprosy virus and HIV | Noninterference | clinical form and type of leprosy reaction | NA | HIV coinfected patients and patients with leprosy alone expressed similar levels of IL-1β and IL-6. | No change in tissue immunological behavior in patients coinfected with HIV and leprosy. | [196] (2017) |
| MDV and REV | Synergy | Confocal imaging, Western blotting, and qRT-PCR | Using the pfu and TCID50 methods | Two virus synergistic replication in vitro is related to innate immune pathway, Akt pathway, and cell adhesion and migration pathway. | Coinfection with Marek’s disease virus (MDV) and reticuloendotheliosis virus (REV) causes synergistic pathogenic effects and serious losses to the poultry industry. | [112] (2022) |
| DNV and CHIKV | Noninterference | RT-qPCR | NA | NA | The viruses could stably co-exist both in the cell lines and adult mosquitoes. | [100] (2010) |
| DNV and DENV | Interference (DNV to DENV) | Immunostaining for flow cytometry | Cell inoculated virus | NA | NA | [102] (2004) |
| DENV, DNV and JEV | Noninterference | Flow cytometry and IFA | Cell inoculated virus | NA | Triple co-infections of viruses can be easily established without signs of disease in C6/36 mosquito cells by sequential viral challenge followed by serial split passage of whole cells. | [197] (2010) |
| IBV and APV | Interference (IBV to APV) | RT-PCR | NA | NA | NA | [198] (2001) |
| IBV and NDV | Interference (IBV to NDV) | qRT-PCR | NA | NA | NA | [199] (2007) |
| HPAIV and NDV | Interference (NDV to HPAIV) | Virus titration | NA | This viral interference is titer dependent. | HPAIV replication was affected and an increase in survival was found in all coinfected groups when compared to the HPAIV single-inoculated group. | [148] (2016) |
| SINV and LACV | BHK cell: Enhancement(both SINV and LACV) C6/36 cell: coinfection don’t affect LACV; enhanced SINV | qRT-PCR | CPE | NA | NA | [149] (2014) |
| Sindbis Virus and other alphaviruses | Interference | Plaque assays | NA | This interference depends on a central role for the alphavirus trans-acting protease that processes the nonstructural proteins. | Mosquito cells persistently infected with Sindbis virus are broadly able to exclude other alphaviruses | [40] (1997) |
| WNV and CxFV | Noninterference (in vitro) Coinfection enhanced WNV (in vivo) | Plaque assays, qRT-PCR, and IFA | NA | The WNV titer in CxFV Izabal (+) C6/36 cells did not reach the maximum titer observed in CxFV Izabal (−) cells due to death of cells caused by CxFV Izabal. | NA | [10] (2010) |
| AIV and NDV | Interference | RT-PCR and serology | NA | NA | Coinfection with LPAIV had no impact on clinical signs; ducks coinfected with HPAIV survived for shorter duration. | [200] (2015) |
| HSV and VZV | Interference (superinfection exclusion, SE) | Laser confocal | Fluorescent virus rescue | The downregulation of heparan sulfate proteoglycan 2 (HSPG2) that alphaherpesvirus receptor may partially account for the exclusion. | NA | [201] (2014) |
| HMPV and HRSV | NA | ELISA and RT-PCR | NA | NA | Increased hospitalization rates | [144] (2005) |
| HCV and TTV | NA | PCR-HMA | NA | A generic method based upon PCR and heteroduplex mobility analysis (HMA) can be used to rapidly determine coinfection with two strains of the homologous virus. | NA | [202] (2000) |
| GaHV-1 and FWPV | NA | PCR | Using Embryonating Chicken Eggs and CPE | NA | NA | [203] (2010) |
| WSSV and IHHNV | NA | PCR and histopathology | NA | NA | Except for typical clinical symptoms of WSSV infection, coinfected shrimps did not have any other external deformities. | [204] (2014) |
| lvCIAV and iIBDV | Synergy | PCR, RT-PCR and ELISA | NA | LvCIAV infection attenuated subsequent iIBDV infection-induced T cell recruitment and subsequent B cell depletion in the bursa. | Without occurrence of clinical signs | [205] (2013) |
| Multiple coronaviruses | Noninterference | RT-PCR | NA | Bats are natural hosts of coronavirus and potential zoonotic sources of viral pathogens. | NA | [206] (2016) |
| HAdV, HEV, RSV and HRV | Noninterference | xTAG RVP Fast v2 and qRT-PCR | NA | NA | Lower frequency of lower respiratory tract infections, lower wheezing rates and higher hospitalization rates | [207] (2016) |
| HIV and FluA | Synergy | NA | NA | NA | Higher risk of influenza infection | [208] (2016) |
| PCV2 and CSFV | NA | proteomic profiling | NA | Mitochondrial dysfunction, nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated oxidative stress response and apoptosis signaling pathways might be the specifical targets during PCV2-CSFV coinfection. | NA | [209] (2017) |
| PPRV and FMDV | Interference | qPT-PCR | Plaque assays, neutralization with antibodies and Viral RNA transfection | NA | NA | [130] (2016) |
| RSV and FluA | Interference | Virus titration and IFA | NA | FluA blocks the growth of RSV by competing with RSV for protein synthesis and selective budding. | NA | [210] (2000) |
| Two different FluA | Interference | Virus titration, RT-PCR and qRT-PCR | Plaque assays | H3N2 and H1N1 have different abilities to inhibit the replication and transmission of their respective drug-resistant virus mutants. | NA | [211] (2010) |
| PRRSV and SIV | Interference | IFA and qRT-PCR | Plaque assays and cell inoculated virus | PRRSV and SIV demonstrate additive effects on the expression of several types of virally induced transcripts. | NA | [212] (2014) |
| Two different VACV | Synergy (lung) Interference (spleen) | qPCR | NA | NA | NA | [136] (2018) |
| Two different WNV | Interference | Virus titration | NA | This interference depends on blocking the transmission of superinfecting virus. | NA | [213] (1969) |
| SLEV and WNV | Interference | qRT-PCR | NA | This interference depends on blocking the transmission of superinfecting virus. | NA | [214] (2009) |
| DENV1 and DENV3 | Interference | IFA | NA | This interference depends on blocking the transmission of superinfecting virus. | NA | [215] (1982) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Wang, C.; Zhang, Y. Viral Coinfections. Viruses 2022, 14, 2645. https://doi.org/10.3390/v14122645
Du Y, Wang C, Zhang Y. Viral Coinfections. Viruses. 2022; 14(12):2645. https://doi.org/10.3390/v14122645
Chicago/Turabian StyleDu, Yanting, Chen Wang, and Ying Zhang. 2022. "Viral Coinfections" Viruses 14, no. 12: 2645. https://doi.org/10.3390/v14122645
APA StyleDu, Y., Wang, C., & Zhang, Y. (2022). Viral Coinfections. Viruses, 14(12), 2645. https://doi.org/10.3390/v14122645






