Dynamic Distribution of Infectious Pancreatic Necrosis Virus (IPNV) Strains of Genogroups 1, 5, and 7 after Intraperitoneal Administration in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Isolation and Culture
2.2. Extraction of Viral Genome RNA
2.3. Virus Detection
2.4. Full-Length Amplification, Cloning, Nucleotide Sequencing, and Sequence Analysis
2.5. Artificial Infection
2.6. Ethics Statement
2.7. Quantitative PCR (qPCR) Analysis of IPNV
2.8. Titration
2.9. Statistical Analyses
3. Results
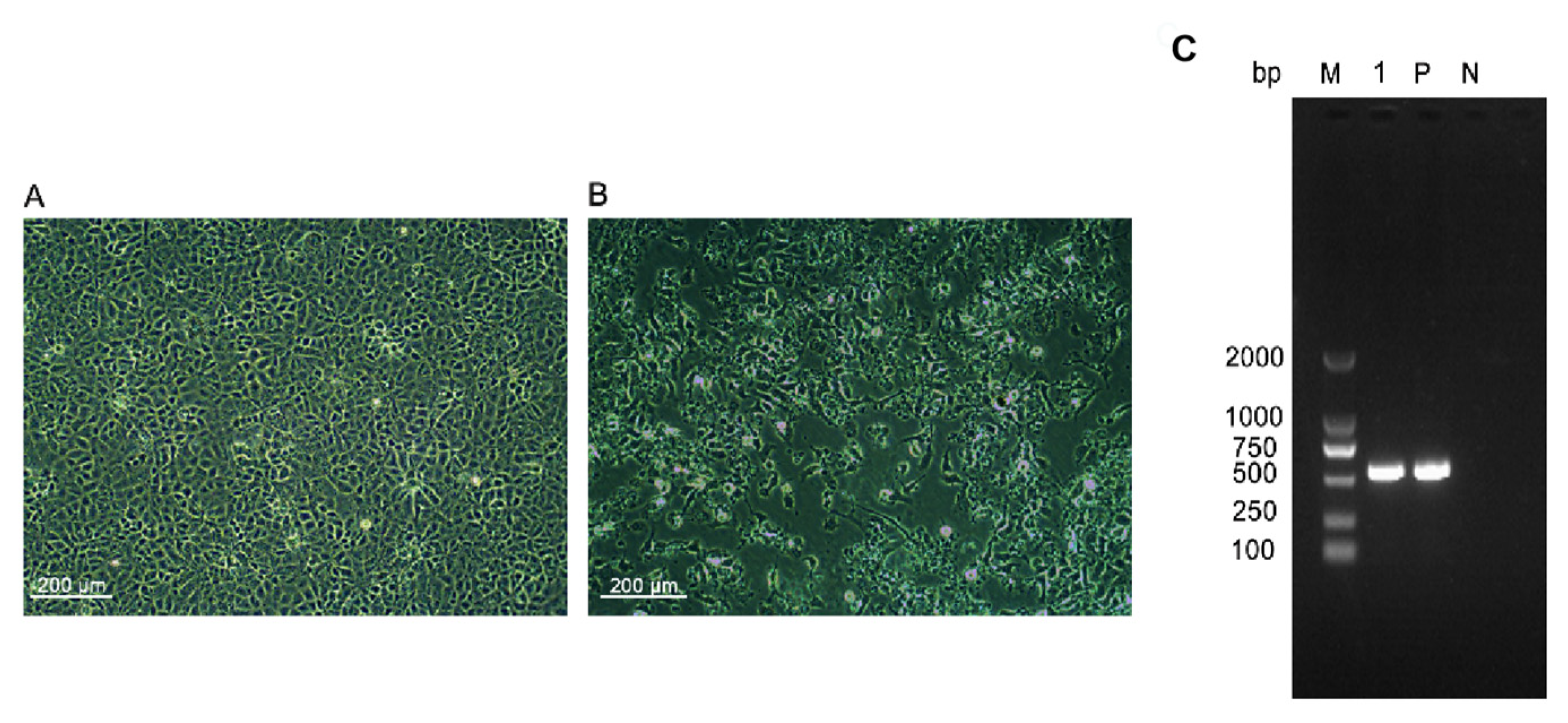
3.1. IPNV Detection in Rainbow Trout and IPNV-P202019 Isolation
3.2. Full-Length Genomic Sequencing and Phylogenetic Analyses
3.3. Tissues Distribution of Three Genogroups of IPNV in Rainbow Trout
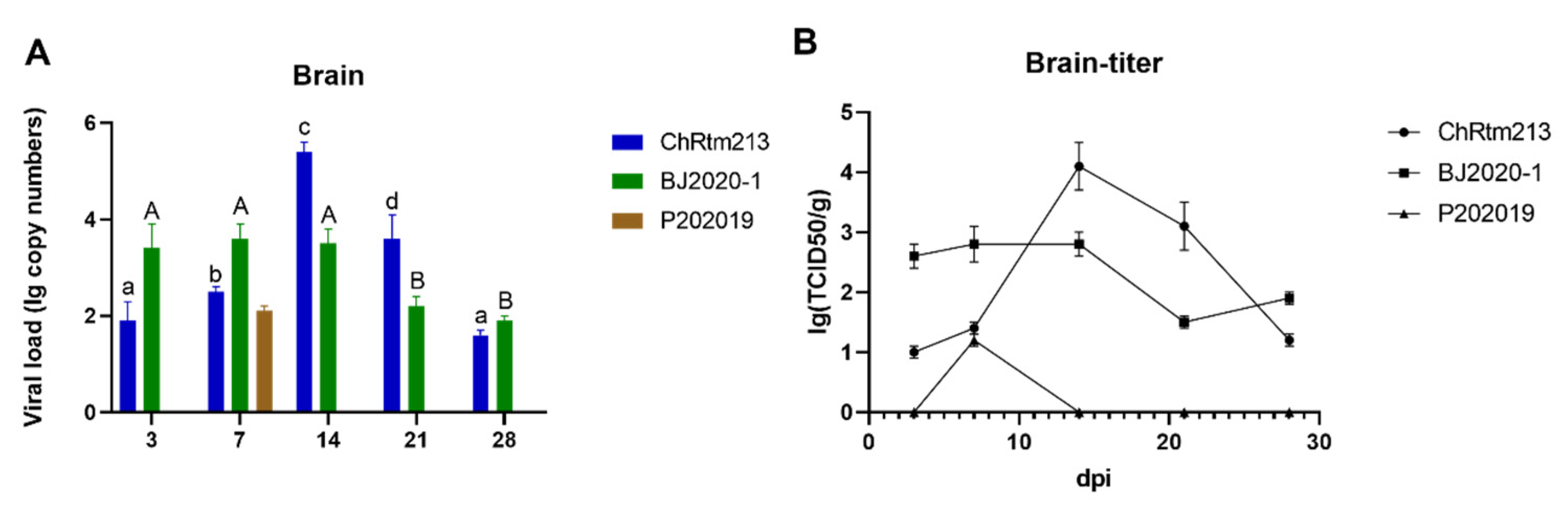
3.3.1. Brain
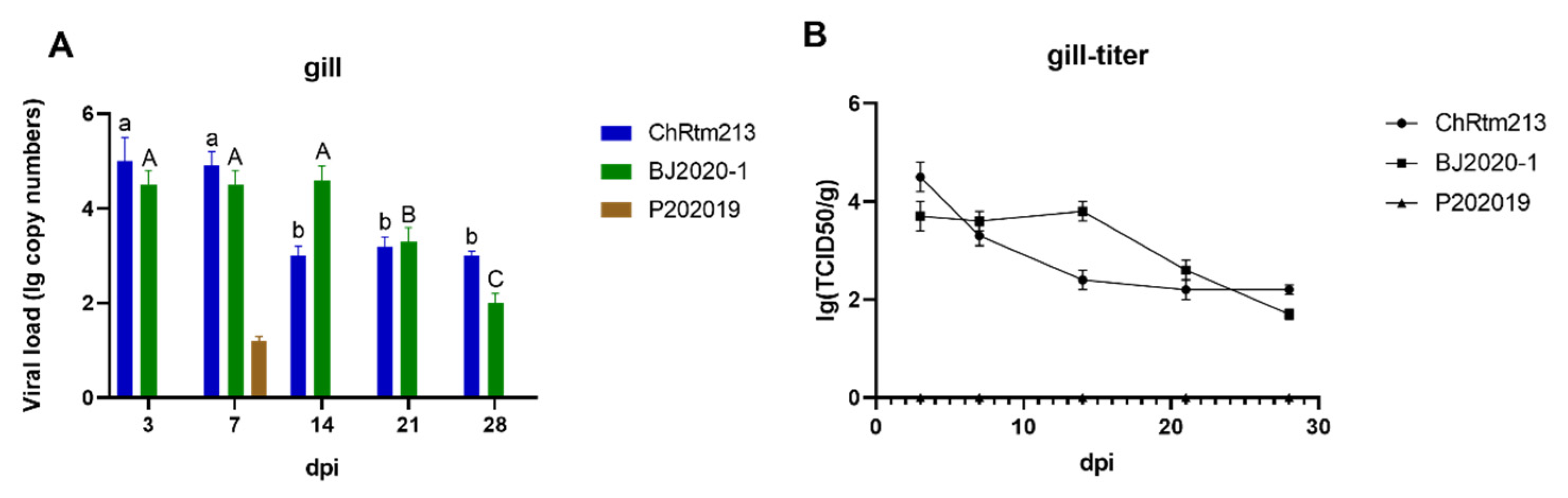
3.3.2. Gill
3.3.3. Heart
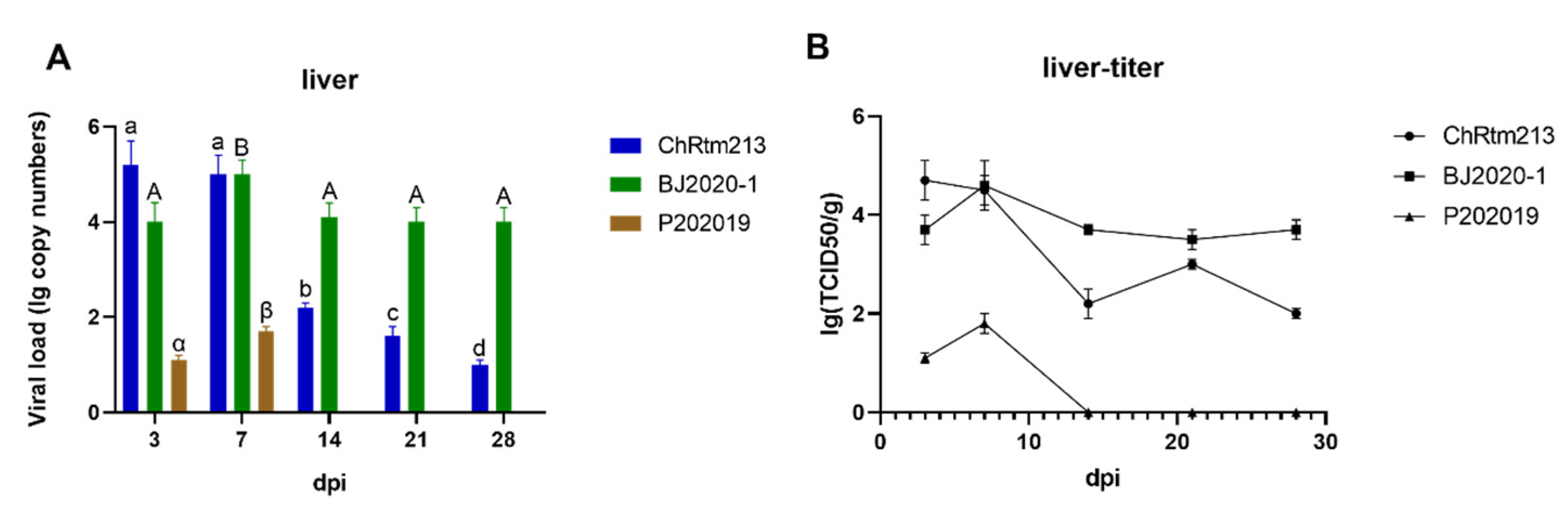
3.3.4. Liver
3.3.5. Spleen
3.3.6. Head Kidney
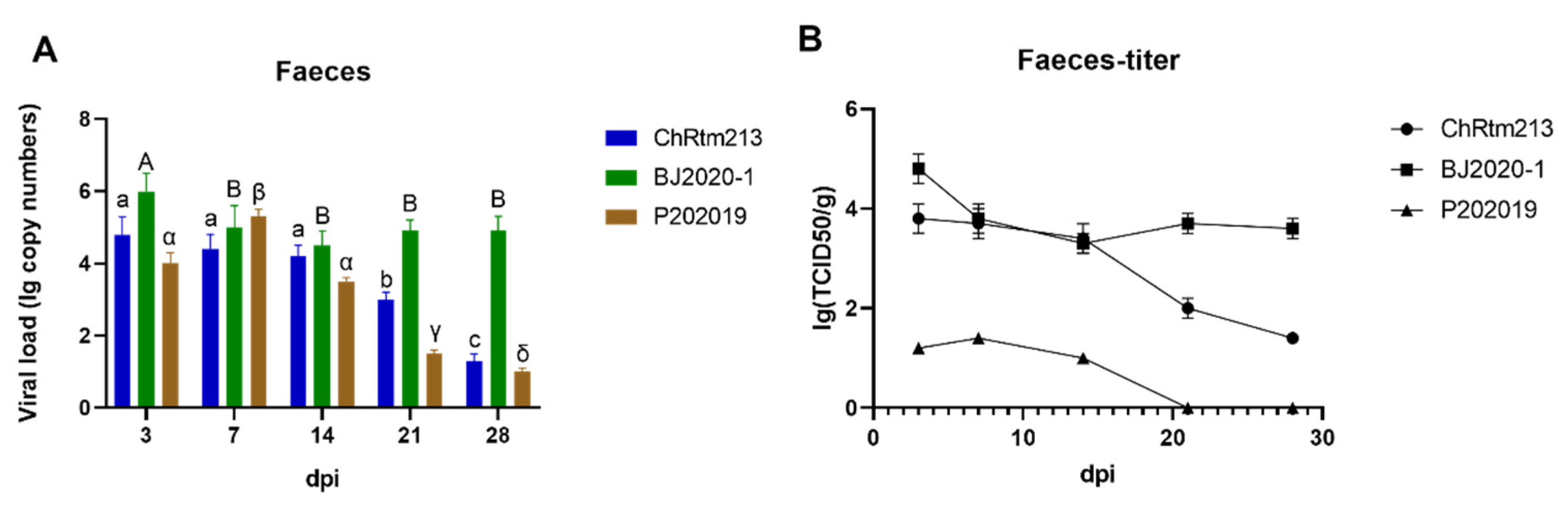
3.3.7. Faeces
3.3.8. Mucus
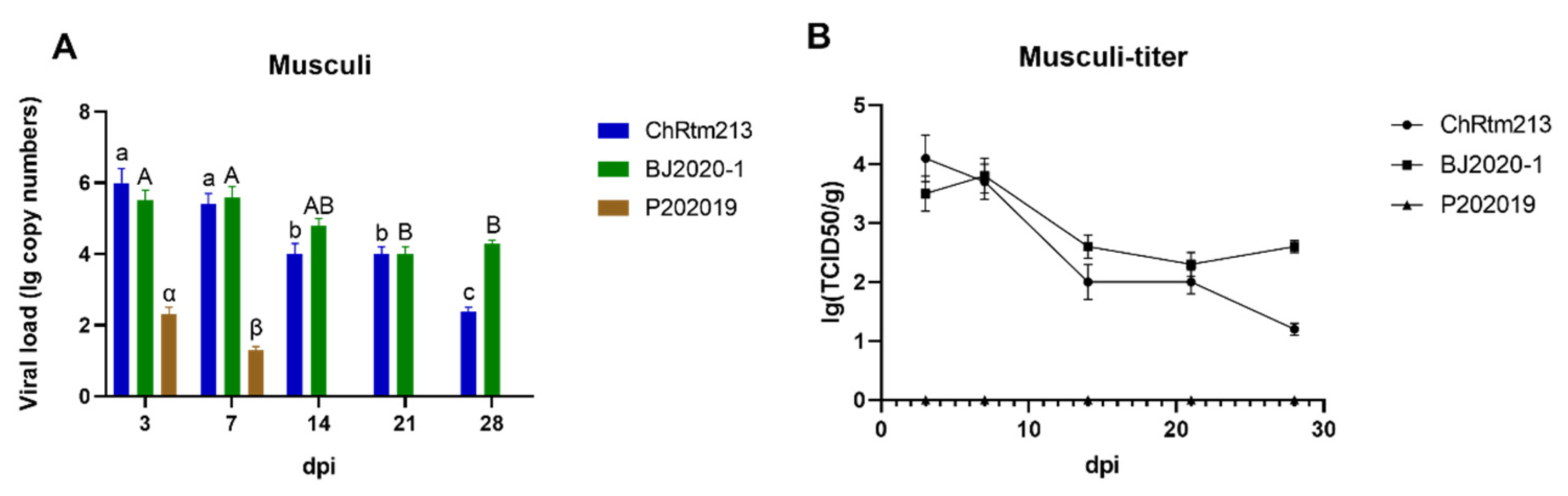
3.3.9. Musculi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, Y.; Zhao, J.; Ren, G.; Lu, T.; Xu, L. Infectious hematopoietic necrosis virus promoted infectious pancreatic necrosis virus multiplication during co-infection in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 561, 738649. [Google Scholar] [CrossRef]
- Dobos, P. The molecular biology of infectious pancreatic necrosis virus (IPNV). Annu. Rev. Fish Dis. 1995, 5, 25–54. [Google Scholar] [CrossRef]
- Benkaroun, J.; Muir, K.; Allshire, R.; Tamer, C.; Weidmann, M. Isolation of a New Infectious Pancreatic Necrosis Virus (IPNV) Variant from a Fish Farm in Scotland. Viruses 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Galloux, M.; Chevalier, C.; Henry, C.; Huet, J.-C.; Da Costa, B.; Delmas, B. Peptides resulting from the pVP2 C-terminal processing are present in infectious pancreatic necrosis virus particles. J. Gen. Virol. 2004, 85, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.; Castric, J.; Lamour, F.; Cabon, J.; Quentel, C. Study of the viral interference between infectious pancreatic necrosis virus (IPNV) and infectious haematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2008, 24, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D.; Eissler, Y.; Reyes-Lopez, F.E.; Kuznar, J.; Yáñez, J.M. Infectious pancreatic necrosis virus in salmonids: Molecular epidemiology and host response to infection. Rev. Aquac. 2021, 14, 751–769. [Google Scholar] [CrossRef]
- Duncan, R.; Mason, C.L.; Nagy, E.; Leong, J.-A.; Dobos, P. Sequence analysis of infectious pancreatic necrosis virus genome segment B and its encoded VP1 protein: A putative RNA-dependent RNA polymerase lacking the Gly-Asp-Asp motif. Virology 1991, 181, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; Enríquez, R. Factors associated with cellular infection by the infectious pancreatic necrosis virus (IPNV). Arch. Med. Vet. 2007, 39, 7–18. (In Spanish) [Google Scholar] [CrossRef][Green Version]
- Blake, S.; Ma, J.-Y.; Caporale, D.A.; Jairath, S.; Nicholson, B.L. Phylogenetic relationships of aquatic birnaviruses based on deduced amino acid sequences of genome segment A cDNA. Dis. Aquat. Org. 2001, 45, 89–102. [Google Scholar] [CrossRef]
- Nishizawa, T.; Kinoshita, S.; Yoshimizu, M. An approach for genogrouping of Japanese isolates of aquabirnaviruses in a new genogroup, VII, based on the VP2/NS junction region. J. Gen. Virol. 2005, 86, 1973–1978. [Google Scholar] [CrossRef]
- Duan, K.; Zhao, J.; Ren, G.; Shao, Y.; Lu, T.; Xu, L.; Tang, X.; Zhao, W.; Xu, L. Molecular Evolution of Infectious Pancreatic Necrosis Virus in China. Viruses 2021, 13, 488. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, B.; Li, W.; Li, Z. Isolation and identification of infectious pancreatic necrosis virus (IPNV) from imported rainbow trout (Salmo gairdneri) in P.R. China. Acta Hydrobiol. Sin. 1989, 13, 353–359. (In Chinese) [Google Scholar]
- Hu, X.; Wei, L.; Zhao, H.; Wu, B. Isolation and Identification of Infectious Pancreatic Necrosis Virus in Rainbow Trout. China Anim. Health Insp. 2012, 29, 27–30. (In Chinese) [Google Scholar]
- Sun, Y.J.; Chen, W.Y.; Xia, D.C.; Liu, G.C.; Zhuang, R.L. Isolation and Identification of Infectious Pancreatic Necrosis Virus of Rainbow Trout. Chin. J. Fish. 1993, 6, 50–56. (In Chinese) [Google Scholar]
- Glenney, G.W.; Barbash, P.A.; Coll, J.A.; Quartz, W.M. Isolation and Molecular Characterization of a Novel Infectious Pancreatic Necrosis Virus Strain in Returning Atlantic Salmon Salmo salar from the Connecticut River, USA. J. Aquat. Anim. Health 2012, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Herrera, E.; Salinas, J.; Torres, J.; Montero, A.B.; Barrón, B. Detection and Genotyping of an Infectious Pancreatic Necrosis Virus from Asymptomatic Rainbow Trout (Oncorhynchus mykiss) Facilities in Mexico. Intervirology 2008, 51, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Mejía, M.; Martínez, S.; Ortega, C.; Ulloa-Arvizu, R. Genotyping of Infectious Pancreatic Necrosis Virus Isolates from Mexico State. J. Aquat. Anim. Health 2011, 23, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Mejía, M.; Simón-Martínez, J.; Ulloa-Arvizu, R.; Salgado-Miranda, C.; Soriano-Vargas, E. Molecular characterization of the VP1 gene of a Mexican isolate of infectious pancreatic necrosis virus. Can. J. Vet. Res. 2010, 74, 218–222. [Google Scholar]
- Mutoloki, S.; Evensen, Ø. Sequence similarities of the capsid gene of Chilean and European isolates of infectious pancreatic necrosis virus point towards a common origin. J. Gen. Virol. 2011, 92, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D.; Eissler, Y.; Torres, P.; Jorquera, E.; Espinoza, J.C.; Kuznar, J. Detection and phylogenetic analysis of infectious pancreatic necrosis virus in Chile. Dis. Aquat. Org. 2015, 116, 173–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahne, W.; Thomsen, I. Infectious Pancreatic Necrosis: Detection of Virus and Antibodies in Rainbow Trout IPNV-Carrier (Salmo gairdneri). J. Vet. Med. Ser. B 1986, 33, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Cutrin, J.M.; Barja, J.L.; Nicholson, B.L.; Bandin, I.; Blake, S.; Dopazo, C.P. Restriction fragment length polymorphisms and sequence analysis: An approach for genotyping infectious pancreatic necrosis virus reference strains and other aquabirnaviruses isolated from northwestern Spain. Appl. Environ. Microb. 2004, 70, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Wehner, S.; Bekaert, M.; Di Paola, N.; Dilcher, M.; Muir, K.F.; Taggart, J.; Matejusova, I.; Weidmann, M. Molecular epidemiological study on Infectious Pancreatic Necrosis Virus isolates from aquafarms in Scotland over three decades. J. Gen. Virol. 2018, 99, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- McCowan, C.; Motha, J.; Crane, M.S.J.; Moody, N.J.G.; Crameri, S.; Hyatt, A.D.; Bradley, T. Isolation of a novel aquatic birnavirus from rainbow trout Oncorhynchus mykiss in Australia. Dis. Aquat. Org. 2015, 114, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhao, J.-Z.; Liu, M.; Lu, T.-Y.; Liu, H.-B.; Yin, J.; Xu, L.-M. Complete genomic sequence of an infectious pancreatic necrosis virus isolated from rainbow trout (Oncorhynchus mykiss) in China. Virus Genes 2017, 53, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, L.; Chen, G.; Duan, K.; Zhao, J.; Ren, G.; Shao, Y.; Zhang, Y.; Lu, T. Comparison of Sensitive Cell lines of Infectious Pancreatic Necrosis Virus and Viral Proliferation Characteristics in CHSE-214 Cell Line. Chin. J. Fish. 2022, 35, 1–8. (In Chinese) [Google Scholar]
- Xu, L.; Zhao, J.; Ren, G.; Dong, Y.; Lin, J.; Cao, Y.; Yin, J.; Liu, H.; Lu, T.; Zhang, Q. Co-infection of infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) caused high mortality in farmed rainbow trout (Oncorhynchus mykiss) in China. Aquaculture 2019, 512, 734386. [Google Scholar] [CrossRef]
- Alonso, M.; Saint-Jean, S.R.; Pérez-Prieto, S.I. Virulence of Infectious hematopoietic necrosis virus and Infectious pancreatic necrosis virus coinfection in rainbow trout (Oncorhynchus mykiss) and nucleotide sequence analysis of the IHNV glycoprotein gene. Arch. Virol. 2003, 148, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Jarp, J.; Gjevre, A.G.; Olsen, A.B.; Bruheim, T. Risk factors for furunculosis, infectious pancreatic necrosis and mortality in post-smolt of Atlantic salmon, Salmo solar L. J. Fish Dis. 1995, 18, 67–78. [Google Scholar] [CrossRef]
- Evensen, Ø.; Santi, N. Infectious Pancreatic Necrosis Virus. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 83–89. [Google Scholar]
- Munro, E.S.; Midtlyng, P.J. Infectious pancreatic necrosis and associated aquatic birnaviruses. In Fish Diseases and Disorders, 2nd ed.; Woo, P.T.K., Bruno, D.W., Eds.; CABI: Oxfordshire, UK, 2011; Volume 3. [Google Scholar]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. Birnaviruses of Aquatic Organisms. In Aquaculture Virology, 1st ed.; Kibenge, F.S.B., Godoy, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 237–250. [Google Scholar]
- Dopazo, C.P. The Infectious Pancreatic Necrosis Virus (IPNV) and its Virulence Determinants: What is Known and What Should be Known. Pathogens 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Tang, X.; Zhao, J.; Ren, G.; Shao, Y.; Lu, T.; He, B.; Xu, L. An inactivated vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2022, 127, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, J.; Liu, M.; Ren, G.; Jian, F.; Yin, J.; Feng, J.; Liu, H.; Lu, T. Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout. Sci. Rep. 2017, 7, 5700. [Google Scholar] [CrossRef] [PubMed]
- Tapia, D.; Kuznar, J.; Farlora, R.; Yáñez, J.M. Differential Transcriptomic Response of Rainbow Trout to Infection with Two Strains of IPNV. Viruses 2021, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Ahmadivand, S.; Behdani, M.; Hassanzadeh, R.; Taheri-Mirghaed, A. Transcription of adaptive-immune genes upon challenge with infectious pancreatic necrosis virus (IPNV) in DNA vaccinated rainbow trout (Oncorhynchus mykiss). Int. J. Aquat. Biol. 2016, 4, 353–359. [Google Scholar] [CrossRef]





| Strain | Genotype | Similarity with P202019 A Segment (%) | GenBank Accession No. | G + C (%) |
|---|---|---|---|---|
| IPNV-P202019 | 7 | 100 | OP272507 | 54.24 |
| YTAV Y-6 | 7 | 95.43 | AY283781 | 54.42 |
| MABV H1 | 7 | 95.40 | AY283783 | 54.52 |
| POBV | 7 | 94.56 | EU161285 | 54.00 |
| IPNV ChRtm213 | 1 | 84.44 | KX234591 | 55.02 |
| IPNV AM-98 | 1 | 84.15 | AY283780 | 55.30 |
| IPNV PA1 | 1 | 83.05 | MH010544 | 54.90 |
| IPNV VR299 | 1 | 83.94 | AF343572 | 54.92 |
| IPNV Antalya | 5 | 79.54 | MH614927 | 55.65 |
| MEIPNV1310 | 5 | 79.00 | KY315690 | 55.69 |
| S-IPNV/FS12-01 | 5 | 78.48 | DQ536090 | 55.61 |
| IPNV Connecticut-1 | 4 | 76.28 | JF440810 | 54.36 |
| Strain | Genotype | Similarity with P202019 B Segment (%) | GenBank Accession No. | G + C (%) |
|---|---|---|---|---|
| IPNV-P202019 | 7 | 100 | OP272508 | 52.34 |
| YTAV Y-6 | 7 | 95.32 | AY129662 | 52.65 |
| YTAV H1 | 7 | 95.25 | AY129665 | 52.76 |
| YTAV NC1 | 7 | 94.99 | AY129666 | 52.32 |
| IPNV ChRtm213 | 1 | 85.99 | KX234590 | 53.60 |
| IPNV 17F2 | 1 | 85.54 | AY780927 | 54.22 |
| IPNV PA1 | 1 | 84.35 | MH010545 | 54.10 |
| IPNV 20G1d | 1 | 80.65 | AY780931 | 53.41 |
| IPNV_SWE16_GG6 | 6 | 76.96 | ON409688 | 51.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Ren, G.; Zhao, J.; Lu, T.; Liu, Q.; Xu, L. Dynamic Distribution of Infectious Pancreatic Necrosis Virus (IPNV) Strains of Genogroups 1, 5, and 7 after Intraperitoneal Administration in Rainbow Trout (Oncorhynchus mykiss). Viruses 2022, 14, 2634. https://doi.org/10.3390/v14122634
Shao Y, Ren G, Zhao J, Lu T, Liu Q, Xu L. Dynamic Distribution of Infectious Pancreatic Necrosis Virus (IPNV) Strains of Genogroups 1, 5, and 7 after Intraperitoneal Administration in Rainbow Trout (Oncorhynchus mykiss). Viruses. 2022; 14(12):2634. https://doi.org/10.3390/v14122634
Chicago/Turabian StyleShao, Yizhi, Guangming Ren, Jingzhuang Zhao, Tongyan Lu, Qi Liu, and Liming Xu. 2022. "Dynamic Distribution of Infectious Pancreatic Necrosis Virus (IPNV) Strains of Genogroups 1, 5, and 7 after Intraperitoneal Administration in Rainbow Trout (Oncorhynchus mykiss)" Viruses 14, no. 12: 2634. https://doi.org/10.3390/v14122634
APA StyleShao, Y., Ren, G., Zhao, J., Lu, T., Liu, Q., & Xu, L. (2022). Dynamic Distribution of Infectious Pancreatic Necrosis Virus (IPNV) Strains of Genogroups 1, 5, and 7 after Intraperitoneal Administration in Rainbow Trout (Oncorhynchus mykiss). Viruses, 14(12), 2634. https://doi.org/10.3390/v14122634






