Dynamics and Patterning of 5-Hydroxytryptamine 2 Subtype Receptors in JC Polyomavirus Entry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Antibodies, and Reagents
2.2. Generation of 5-HT2R-Dendra2 Fusion Constructs
2.3. Generation of HEK293A Stable Cell Lines for 5-HT2R-Dendra2
2.4. Indirect Immunofluorescence Detection and Quantification of JCPyV Infection
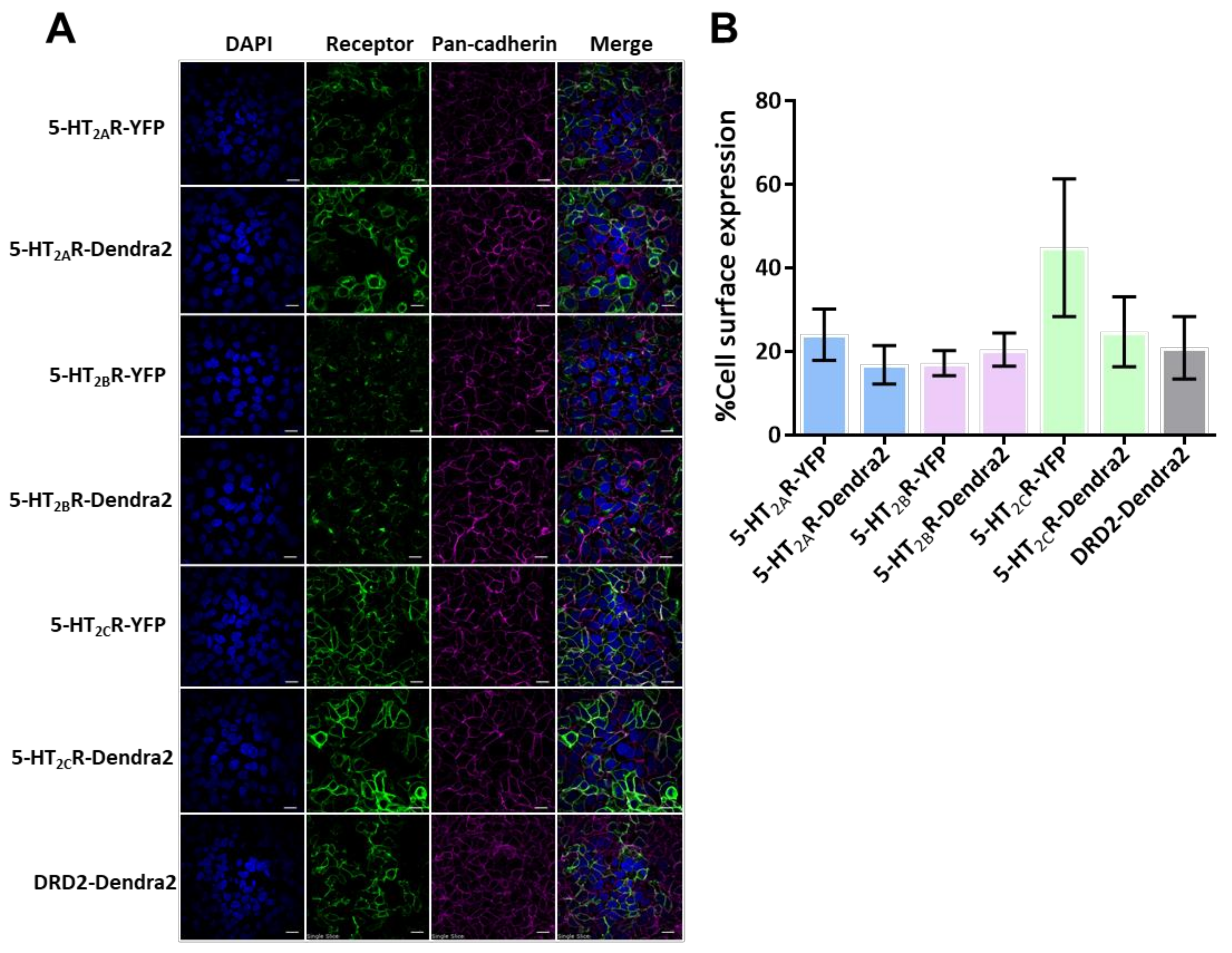
2.5. Cell-Surface Expression of 5-HT2R-Dendra2 Fusion Proteins in HEK293A Stable Cells
2.6. Analysis of JCPyV Attachment in Stable Cells
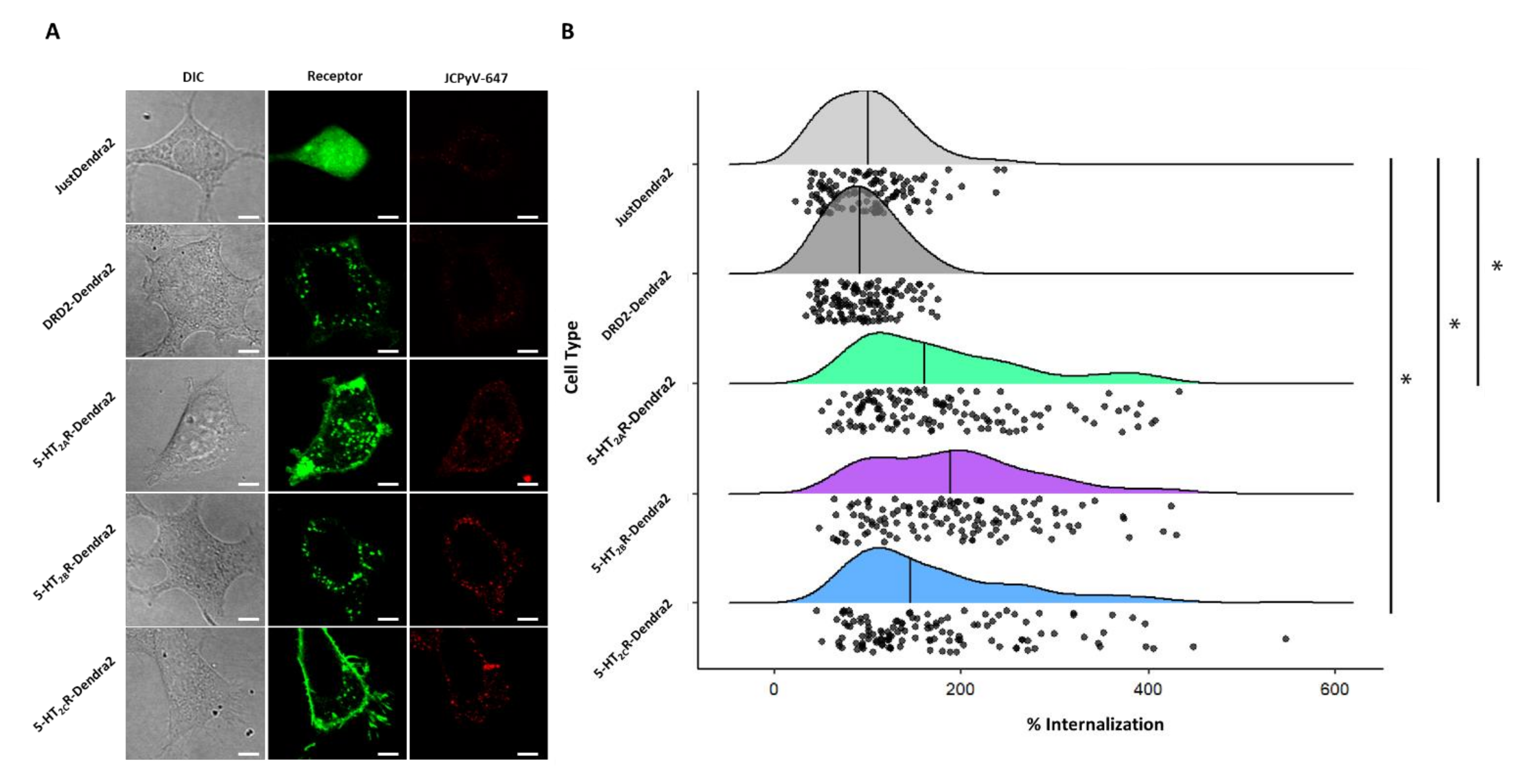
2.7. Quantification of JCPyV Entry in Stable Cells
2.8. Preparing Samples for Two-Color FPALM Experiment
2.9. Super-Resolution Fluorescence Photoactivation Localization Microscopy (FPALM)
2.10. Glucose Oxidase Imaging Buffer
2.11. Multicolor Widefield FPALM Imaging of JCPyV and Mock Infected Samples
3. Results
3.1. 5-HT2R-Dendra2 Forms Clusters on the Cell Surface of Stably Transfected Cells
3.2. Cell-Surface Characterization of 5-HT2Rs Using FPALM-TIRF Microscopy
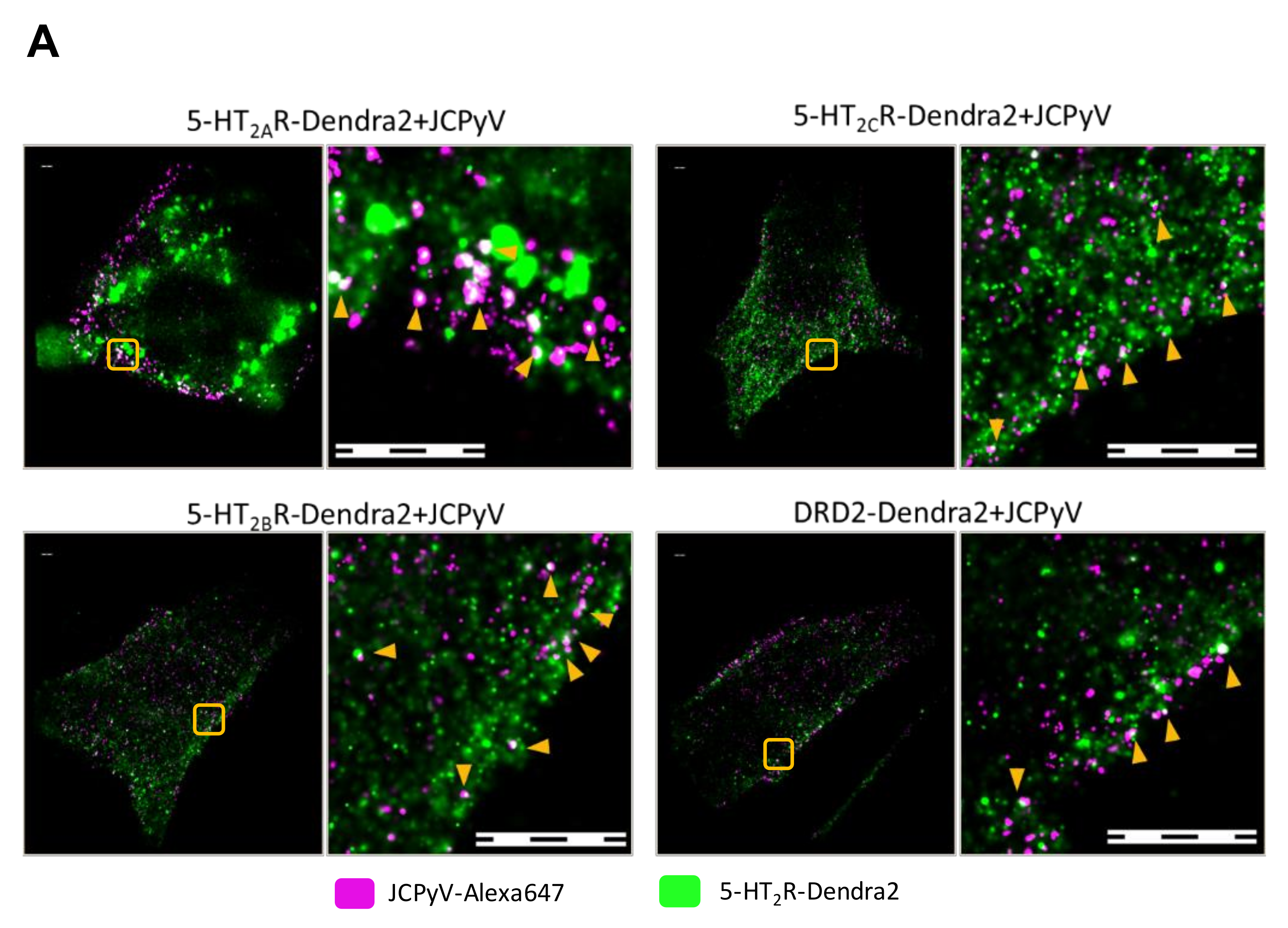
3.3. Manders’ Colocalization Analysis: 5-HT2Rs Colocalize with JCPyV during Virus Entry
3.4. JCPyV Attachment Is Not Enhanced in Cells Expressing 5-HT2 Receptors
3.5. JCPyV Entry Is Enhanced in 5-HT2R-Expressing Cells
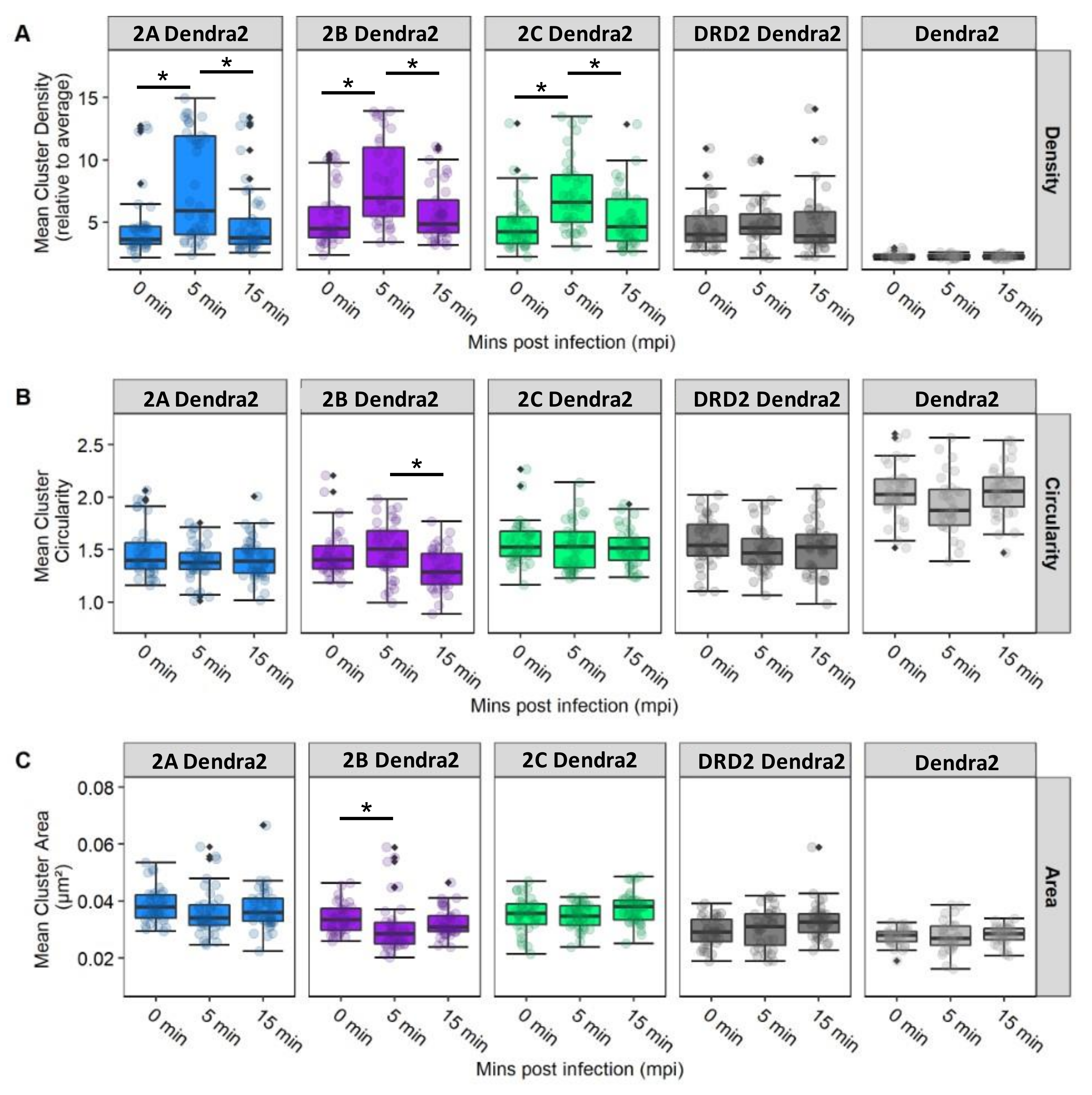
3.6. JCPyV Changes Cluster Properties for 5-HT2 Receptor Subtypes in Infected Cells
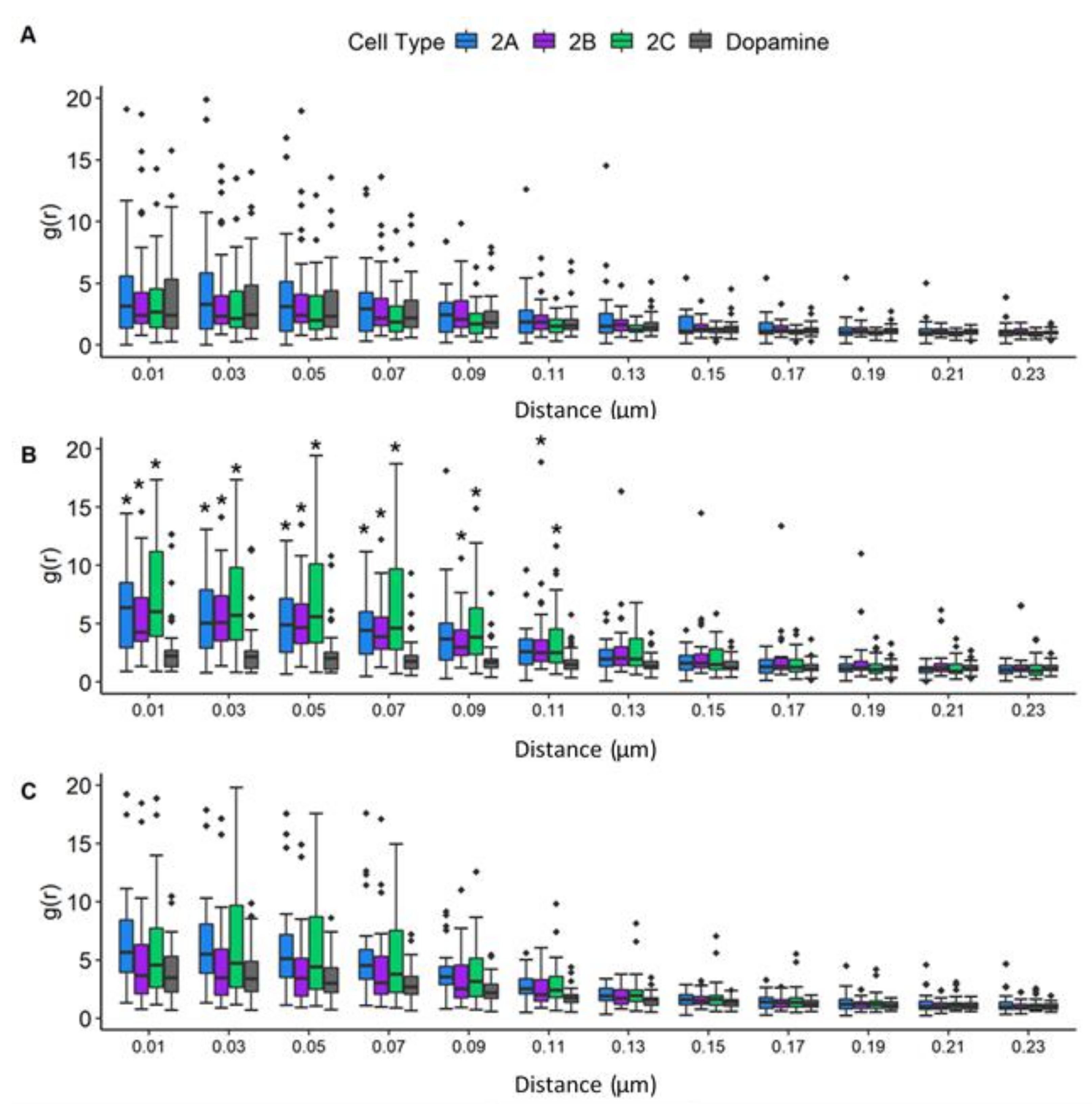
3.7. Radial Distribution Function Analysis: JCPyV Aggregation within and Adjacent to 5-HT2 Receptor Clusters
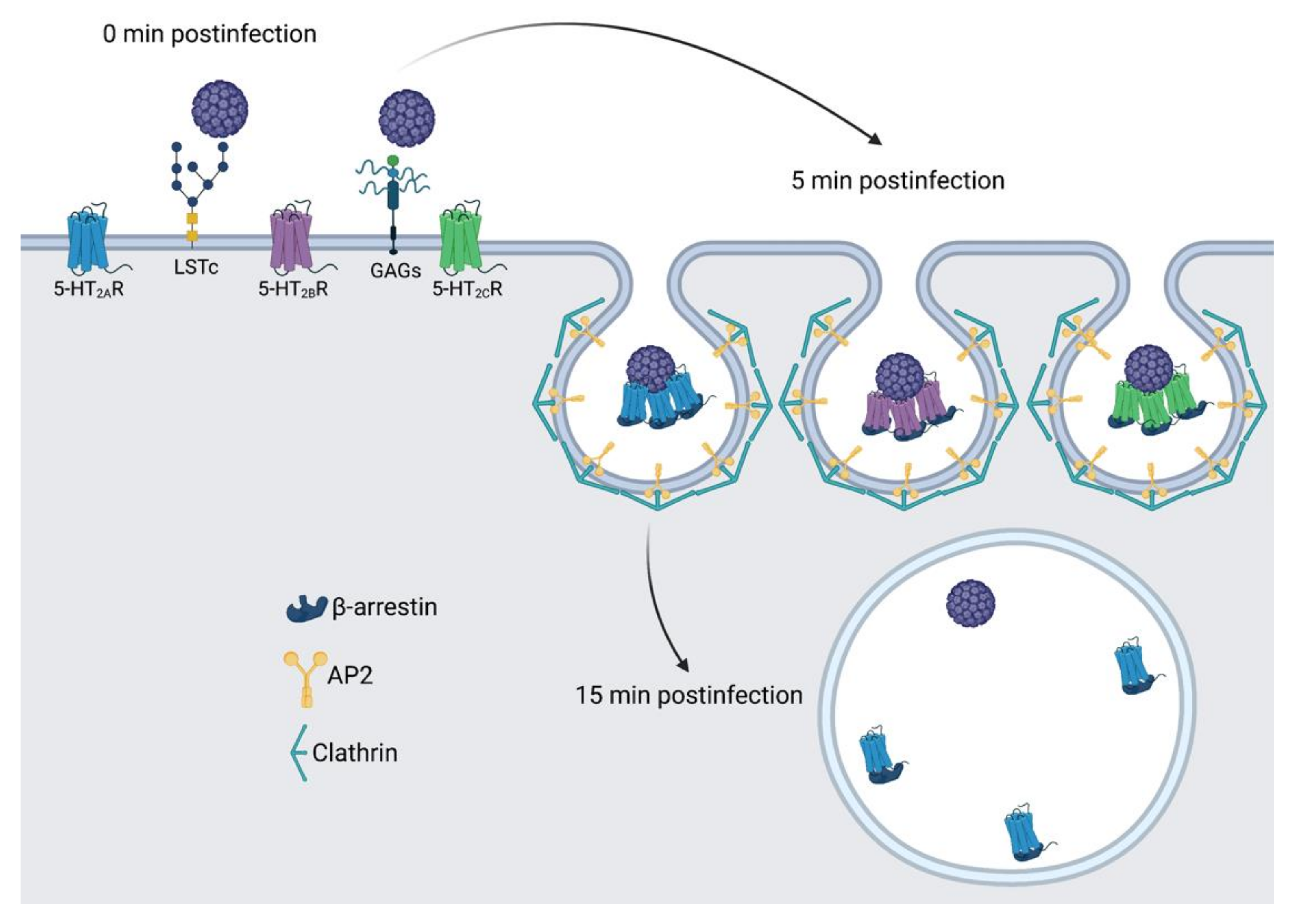
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartman, N.C.; Groves, J.T. Signaling clusters in the cell membrane. Curr. Opin. Cell Biol. 2011, 23, 370–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulant, S.; Stanifer, M.; Lozach, P.Y. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses 2015, 7, 2794–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, C.D.; Andrew, D.J. Outside-in signaling—A brief review of GPCR signaling with a focus on the Drosophila GPCR family. J. Cell Sci. 2015, 128, 3533–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrami, L.; Liu, S.; Cosson, P.; Leppla, S.H.; van der Goot, F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 2003, 160, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eierhoff, T.; Hrincius, E.R.; Rescher, U.; Ludwig, S.; Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010, 6, e1001099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchanda, Y.; Bitsi, S.; Kang, Y.; Jones, B.; Tomas, A. Spatiotemporal control of GLP-1 receptor activity. Curr. Opin. Endocr. Metab. Res. 2021, 16, 19–27. [Google Scholar] [CrossRef]
- Hanyaloglu, A.C.; Reiter, E. Editorial: G protein–coupled receptors: From molecules to medicine. Curr. Opin. Endocr. Metab. Res. 2021, 16, iv–vi. [Google Scholar] [CrossRef]
- Casaletto, J.B.; McClatchey, A.I. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat. Rev. Cancer 2012, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sams, M.; Silye, R.; Gohring, J.; Muresan, L.; Schilcher, K.; Jacak, J. Spatial cluster analysis of nanoscopically mapped serotonin receptors for classification of fixed brain tissue. J. Biomed. Opt. 2014, 19, 011021. [Google Scholar] [CrossRef] [Green Version]
- Caetano Crowley, F.A.; Heit, B.; Ferguson, S.S.G. Super-Resolution Imaging of G Protein-Coupled Receptors Using Ground State Depletion Microscopy. In G Protein-Coupled Receptor Signaling: Methods and Protocols; Tiberi, M., Ed.; Springer: New York, NY, USA, 2019; pp. 323–336. [Google Scholar]
- Hess, S.T.; Girirajan, T.P.; Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grove, J. Super-resolution microscopy: A virus’ eye view of the cell. Viruses 2014, 6, 1365–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacki, J.; Staudt, T.; Glass, B.; Bingen, P.; Engelhardt, J.; Anders, M.; Schneider, J.; Muller, B.; Hell, S.W.; Krausslich, H.G. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science 2012, 338, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muranyi, W.; Malkusch, S.; Muller, B.; Heilemann, M.; Krausslich, H.G. Super-resolution microscopy reveals specific recruitment of HIV-1 envelope proteins to viral assembly sites dependent on the envelope C-terminal tail. PLoS Pathog. 2013, 9, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Yuan, Z.; Rice, C.M.; MacDonald, M.R. Flavivirus replication complex assembly revealed by DNAJC14 functional mapping. J. Virol. 2012, 86, 11815–11832. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Sun, E.; Bujny, M.V.; Kim, D.; Davidson, M.W.; Zhuang, X. Dual function of CD81 in influenza virus uncoating and budding. PLoS Pathog. 2013, 9, e1003701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggert, D.; Rosch, K.; Reimer, R.; Herker, E. Visualization and analysis of hepatitis C virus structural proteins at lipid droplets by super-resolution microscopy. PLoS ONE 2014, 9, e102511. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.H.; Suomalainen, M.; Andriasyan, V.; Kilcher, S.; Mercer, J.; Neef, A.; Luedtke, N.W.; Greber, U.F. Tracking viral genomes in host cells at single-molecule resolution. Cell Host Microbe 2013, 14, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Gray, R.D.; Beerli, C.; Pereira, P.M.; Scherer, K.M.; Samolej, J.; Bleck, C.K.; Mercer, J.; Henriques, R. VirusMapper: Open-source nanoscale mapping of viral architecture through super-resolution microscopy. Sci. Rep. 2016, 6, 29132. [Google Scholar] [CrossRef]
- Long, R.K.M.; Moriarty, K.P.; Cardoen, B.; Gao, G.; Vogl, A.W.; Jean, F.; Hamarneh, G.; Nabi, I.R. Super resolution microscopy and deep learning identify Zika virus reorganization of the endoplasmic reticulum. Sci. Rep. 2020, 10, 20937. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.J.; Verkhusha, V.V.; Hess, S.T. Imaging biological structures with fluorescence photoactivation localization microscopy. Nat. Protoc. 2009, 4, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, N.M.; Parent, M.; Mlodzianoski, M.; Nelson, A.J.; Lilieholm, J.; Butler, M.B.; Valles, M.; Hess, S.T. Dances with Membranes: Breakthroughs from Super-resolution Imaging. Curr. Top Membr. 2015, 75, 59–123. [Google Scholar] [PubMed] [Green Version]
- Curthoys, N.M.; Mlodzianoski, M.J.; Parent, M.; Butler, M.B.; Raut, P.; Wallace, J.; Lilieholm, J.; Mehmood, K.; Maginnis, M.S.; Waters, H.; et al. Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering. Biophys. J. 2019, 116, 893–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahl, S.J.; Schönle, A.; Hell, S.W. Fluorescence Microscopy with Nanometer Resolution. In Springer Handbook of Microscopy; Hawkes, P.W., Spence, J.C.H., Eds.; Spinger: Cham, Switzerland, 2019; pp. 1089–1143. [Google Scholar]
- Astrom, K.E.; Mancall, E.L.; Richardson, E.P.J. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain 1958, 81, 93–111. [Google Scholar] [PubMed]
- Berger, J.R.; Major, E.O. Progressive multifocal leukoencephalopathy. Semin. Neurol. 1999, 19, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Weissert, R. Progressive multifocal leukoencephalopathy. J. Neuroimmunol. 2011, 231, 73–77. [Google Scholar] [CrossRef]
- Knowles, W.A.; Luxton, R.W.; Hand, J.F.; Gardner, S.D.; Brown, D.W. The JC virus antibody response in serum and cerebrospinal fluid in progressive multifocal leucoencephalopathy. Clin. Diagn. Virol. 1995, 4, 183–194. [Google Scholar] [CrossRef]
- Stolt, A.; Sasnauskas, K.; Koskela, P.; Lehtinen, M.; Dillner, J. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 2003, 84, 1499–1504. [Google Scholar] [CrossRef]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 2009, 199, 837–846. [Google Scholar] [CrossRef]
- Kean, J.M.; Rao, S.; Wang, M.; Garcea, R.L. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009, 5, e1000363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, B.V.; Palacio, V.C. JC virus: A brief review. World J. Neurosci. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Dorries, K.; Arendt, G.; Eggers, C.; Roggendorf, W.; Dorries, R. Nucleic acid detection as a diagnostic tool in polyomavirus JC induced progressive multifocal leukoencephalopathy. J. Med. Virol. 1998, 54, 196–203. [Google Scholar] [CrossRef]
- Yogo, Y.; Kitamura, T.; Sugimoto, C.; Ueki, T.; Aso, Y.; Hara, K.; Taguchi, F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J. Virol. 1990, 64, 3139–3143. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, A.L.; Atwood, W.J. Fifty Years of JC Polyomavirus: A Brief Overview and Remaining Questions. Viruses 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Zurhein, G.; Chou, S.M. Particles Resembling Papova Viruses in Human Cerebral Demyelinating Disease. Science 1965, 148, 1477–1479. [Google Scholar] [CrossRef]
- Silverman, L.; Rubinstein, L.J. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathol. 1965, 5, 215–224. [Google Scholar] [CrossRef]
- Ferenczy, M.W.; Marshall, L.J.; Nelson, C.D.; Atwood, W.J.; Nath, A.; Khalili, K.; Major, E.O. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 2012, 25, 471–506. [Google Scholar] [CrossRef] [Green Version]
- Maas, R.P.; Muller-Hansma, A.H.; Esselink, R.A.; Murk, J.L.; Warnke, C.; Killestein, J.; Wattjes, M.P. Drug-associated progressive multifocal leukoencephalopathy: A clinical, radiological, and cerebrospinal fluid analysis of 326 cases. J. Neurol. 2016, 263, 2004–2021. [Google Scholar] [CrossRef] [Green Version]
- Kleinschmidt-DeMasters, B.K.; Tyler, K.L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005, 353, 369–374. [Google Scholar] [CrossRef]
- Carson, K.R.; Focosi, D.; Major, E.O.; Petrini, M.; Richey, E.A.; West, D.P.; Bennett, C.L. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: A Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009, 10, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Major, E.O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 2010, 61, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Bloomgren, G.; Richman, S.; Hotermans, C.; Subramanyam, M.; Goelz, S.; Natarajan, A.; Lee, S.; Plavina, T.; Scanlon, J.V.; Sandrock, A.; et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012, 366, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Wolbers, M.; Mueller, N.J.; Garzoni, C.; Du Pasquier, R.A.; Fux, C.A.; Vernazza, P.; Bernasconi, E.; Viscidi, R.; Battegay, M. JC virus-specific immune responses in human immunodeficiency virus type 1 patients with progressive multifocal leukoencephalopathy. J. Virol. 2009, 83, 4404–4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Dong-Si, T.; Gheuens, S.; Gangadharan, A.; Wenten, M.; Philip, J.; McIninch, J.; Datta, S.; Richert, N.; Bozic, C.; Bloomgren, G.; et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J. NeuroVirology 2015, 21, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, D.; Patera, A.C.; Nyberg, F.; Gerber, M.; Liu, M. Progressive multifocal leukoencephalopathy: Current treatment options and future perspectives. Ther. Adv. Neurol. Disord. 2015, 8, 255–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartau, M.; Sipila, J.O.; Auvinen, E.; Palomaki, M.; Verkkoniemi-Ahola, A. Progressive Multifocal Leukoencephalopathy: Current Insights. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, H.H.; Kardas, P.; Kranz, D.; Leboeuf, C. The human JC polyomavirus (JCPyV): Virological background and clinical implications. Apmis 2013, 121, 685–727. [Google Scholar] [CrossRef]
- Brew, B.J.; Davies, N.W.; Cinque, P.; Clifford, D.B.; Nath, A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat. Rev. Neurol. 2010, 6, 667–679. [Google Scholar] [CrossRef]
- Clifford, D.B.; De Luca, A.; Simpson, D.M.; Arendt, G.; Giovannoni, G.; Nath, A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: Lessons from 28 cases. Lancet Neurol. 2010, 9, 438–446. [Google Scholar] [CrossRef]
- Steiner, I.; Berger, J.R. Update on progressive multifocal leukoencephalopathy. Curr. Neurol. Neurosci. Rep. 2012, 12, 680–686. [Google Scholar] [CrossRef]
- Tan, K.; Roda, R.; Ostrow, L.; McArthur, J.; Nath, A. PML-IRIS in patients with HIV infection: Clinical manifestations and treatment with steroids. Neurology 2009, 72, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- DuShane, J.K.; Maginnis, M.S. Human DNA Virus Exploitation of the MAPK-ERK Cascade. Int. J. Mol. Sci. 2019, 20, 3427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maginnis, M.S.; Nelson, C.D.; Atwood, W.J. JC polyomavirus attachment, entry, and trafficking: Unlocking the keys to a fatal infection. J. Neurovirol. 2015, 21, 601–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assetta, B.; Atwood, W.J. The biology of JC polyomavirus. J. Biol. Chem. 2017, 398, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Stehle, T.; Harrison, S.C. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 1998, 17, 3233–3240. [Google Scholar] [CrossRef]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles To Infect Target Cells. mBio 2019, 10, e00379-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neu, U.; Maginnis, M.S.; Palma, A.S.; Ströh, L.J.; Nelson, C.D.; Feizi, T.; Atwood, W.J.; Stehle, T. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe 2010, 8, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Maginnis, M.S.; Stroh, L.J.; Gee, G.V.; O’Hara, B.A.; Derdowski, A.; Stehle, T.; Atwood, W.J. Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding. mBio 2013, 4, e00247-13. [Google Scholar] [CrossRef]
- Stroh, L.J.; Maginnis, M.S.; Blaum, B.S.; Nelson, C.D.; Neu, U.; Gee, G.V.; O’Hara, B.A.; Motamedi, N.; DiMaio, D.; Atwood, W.J.; et al. The Greater Affinity of JC Polyomavirus Capsid for alpha2,6-Linked Lactoseries Tetrasaccharide c than for Other Sialylated Glycans Is a Major Determinant of Infectivity. J. Virol. 2015, 89, 6364–6375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geoghegan, E.M.; Pastrana, D.V.; Schowalter, R.M.; Ray, U.; Gao, W.; Ho, M.; Pauly, G.T.; Sigano, D.M.; Kaynor, C.; Cahir-McFarland, E.; et al. Infectious Entry and Neutralization of Pathogenic JC Polyomaviruses. Cell Rep. 2017, 21, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elphick, G.F.; Querbes, W.; Jordan, J.A.; Gee, G.V.; Eash, S.; Manley, K.; Dugan, A.; Stanifer, M.; Bhatnagar, A.; Kroeze, W.K.; et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 2004, 306, 1380–1383. [Google Scholar] [CrossRef] [Green Version]
- Assetta, B.; Maginnis, M.S.; Ahufinger, I.G.; Haley, S.A.; Gee, G.V.; Nelson, C.D.; O’Hara, B.A.; Stacy-ann, A.; Atwood, W.J. 5-HT2 receptors facilitate JC polyomavirus entry. J. Virol. 2013, 87, 13490–13498. [Google Scholar] [CrossRef] [Green Version]
- Mayberry, C.L.; Soucy, A.N.; Lajoie, C.R.; DuShane, J.K.; Maginnis, M.S. JC polyomavirus entry by clathrin-mediated endocytosis is driven by β-arrestin. J. Virol. 2019, 93, e01948-18. [Google Scholar] [CrossRef] [Green Version]
- Assetta, B.; Morris-Love, J.; Gee, G.V.; Atkinson, A.L.; O’Hara, B.A.; Maginnis, M.S.; Haley, S.A.; Atwood, W.J. Genetic and Functional Dissection of the Role of Individual 5-HT2 Receptors as Entry Receptors for JC Polyomavirus. Cell Rep. 2019, 27, 1960–1966.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnovskaya, M.N.; Nebigil, C.G.; Arthur, J.M.; Spurney, R.F.; Raymond, J.R. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol. Pharmacol. 1995, 48, 230–237. [Google Scholar]
- Xu, J.; Yao, B.; Fan, X.; Langworthy, M.M.; Zhang, M.Z.; Harris, R.C. Characterization of a putative intrarenal serotonergic system. Am. J. Physiol. Renal. Physiol. 2007, 293, F1468–F1475. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Alenina, N.; Klempin, F. The role of serotonin in adult hippocampal neurogenesis. Behav. Brain Res. 2015, 277, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Nikoui, V.; Javadi-Paydar, M.; Salehi, M.; Behestani, S.; Dehpour, A.R. Protective Effects of Lithium on Sumatriptan-Induced Memory Impairment in Mice. Acta Med. Iran. 2016, 54, 226–232. [Google Scholar] [PubMed]
- Blackburn, T.P. Serotonin (5-Hydroxytryptamine; 5-HT): Receptors. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 701–714. [Google Scholar]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Gavarini, S.; Becamel, C.; Altier, C.; Lory, P.; Poncet, J.; Wijnholds, J.; Bockaert, J.; Marin, P. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol. Biol. Cell 2006, 17, 4619–4631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, S.A.; Shah, M.C.; Khan, N.; Roth, B.L. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol. Pharmacol. 1996, 50, 306–313. [Google Scholar]
- Halls, M.L.; Yeatman, H.R.; Nowell, C.J.; Thompson, G.L.; Gondin, A.B.; Civciristov, S.; Bunnett, N.W.; Lambert, N.A.; Poole, D.P.; Canals, M. Plasma membrane localization of the mu-opioid receptor controls spatiotemporal signaling. Sci. Signal 2016, 9, ra16. [Google Scholar] [CrossRef]
- Yanagawa, M.; Hiroshima, M.; Togashi, Y.; Abe, M.; Yamashita, T.; Shichida, Y.; Murata, M.; Ueda, M.; Sako, Y. Single-molecule diffusion-based estimation of ligand effects on G protein-coupled receptors. Sci. Signal 2018, 11, eaao1917. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, Z.Y.; Puthenveedu, M.A. Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic 2019, 20, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Baltanas, T.; Olivares, J.M.; Martinez-Villamarin, J.R.; Fenton, E.Y.; Kalynchuk, L.E.; Caruncho, H.J. Serotonin 2A receptor clustering in peripheral lymphocytes is altered in major depression and may be a biomarker of therapeutic efficacy. J. Affect. Disord. 2014, 163, 47–55. [Google Scholar] [CrossRef]
- Benredjem, B.; Gallion, J.; Pelletier, D.; Dallaire, P.; Charbonneau, J.; Cawkill, D.; Nagi, K.; Gosink, M.; Lukasheva, V.; Jenkinson, S.; et al. Exploring use of unsupervised clustering to associate signaling profiles of GPCR ligands to clinical response. Nat. Commun. 2019, 10, 4075. [Google Scholar] [CrossRef] [Green Version]
- Major, E.O.; Vacante, D.A. Human fetal astrocytes in culture support the growth of the neurotropic human polyomavirus, JCV. J. Neuropathol. Exp. Neurol. 1989, 48, 425–436. [Google Scholar] [CrossRef]
- Vacante, D.A.; Traub, R.; Major, E.O. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 1989, 170, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.D.S.; Carney, D.W.; Derdowski, A.; Lipovsky, A.; Gee, G.V.; O’Hara, B.; Williard, P.; DiMaio, D.; Sello, J.K.; Atwood, W.J. A Retrograde Trafficking Inhibitor of Ricin and Shiga-Like Toxins Inhibits Infection of Cells by Human and Monkey Polyomaviruses. mBio 2013, 4, e00729-13. [Google Scholar] [CrossRef] [PubMed]
- Gudheti, M.V.; Curthoys, N.M.; Gould, T.J.; Kim, D.; Gunewardene, M.S.; Gabor, K.A.; Gosse, J.A.; Kim, C.H.; Zimmerberg, J.; Hess, S.T. Actin mediates the nanoscale membrane organization of the clustered membrane protein influenza hemagglutinin. Biophys. J. 2013, 104, 2182–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuShane, J.K.; Wilczek, M.P.; Mayberry, C.L.; Maginnis, M.S. ERK Is a Critical Regulator of JC Polyomavirus Infection. J. Virol. 2018, 92, e01529-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczek, M.P.; DuShane, J.K.; Armstrong, F.J.; Maginnis, M.S. Correction for Wilczek et al. “JC Polyomavirus Infection Reveals Delayed Progression of the Infectious Cycle in Normal Human Astrocytes”. J. Virol. 2020, 94, e00174-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maginnis, M.S.; Haley, S.A.; Gee, G.V.; Atwood, W.J. Role of N-linked glycosylation of the 5-HT2A receptor in JC virus infection. J. Virol. 2010, 84, 9677–9684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. In Use R! 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Curthoys, N.M.; Mlodzianoski, M.J.; Kim, D.; Hess, S.T. Simultaneous multicolor imaging of biological structures with fluorescence photoactivation localization microscopy. J. Vis. Exp. 2013, 82, e50680. [Google Scholar] [CrossRef] [Green Version]
- Gunewardene, M.S.; Subach, F.V.; Gould, T.J.; Penoncello, G.P.; Gudheti, M.V.; Verkhusha, V.V.; Hess, S.T. Superresolution imaging of multiple fluorescent proteins with highly overlapping emission spectra in living cells. Biophys. J. 2011, 101, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- van de Linde, S.; Loschberger, A.; Klein, T.; Heidbreder, M.; Wolter, S.; Heilemann, M.; Sauer, M. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 2011, 6, 991–1009. [Google Scholar] [CrossRef]
- Hoogendoorn, E.; Crosby, K.C.; Leyton-Puig, D.; Breedijk, R.M.P.; Jalink, K.; Gadella, T.W.J.; Postma, M. The fidelity of stochastic single-molecule super-resolution reconstructions critically depends upon robust background estimation. Sci. Rep. 2014, 4, 3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurskaya, N.G.; Verkhusha, V.V.; Shcheglov, A.S.; Staroverov, D.B.; Chepurnykh, T.V.; Fradkov, A.F.; Lukyanov, S.; Lukyanov, K.A. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 2006, 24, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Almo, S.C.; Wu, Y. General principles of binding between cell surface receptors and multi-specific ligands: A computational study. PLoS Comput. Biol. 2017, 13, e1005805. [Google Scholar] [CrossRef]
- Koutsoudakis, G.; Herrmann, E.; Kallis, S.; Bartenschlager, R.; Pietschmann, T. The Level of CD81 Cell Surface Expression Is a Key Determinant for Productive Entry of Hepatitis C Virus into Host Cells. J. Virol. 2007, 81, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Franke, C.; Chum, T.; Kvíčalová, Z.; Glatzová, D.; Rodriguez, A.; Helmerich, D.A.; Frank, O.; Brdička, T.; van de Linde, S.; Cebecauer, M. Unraveling nanotopography of cell surface receptors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Neu, U.; Allen, S.A.; Blaum, B.S.; Liu, Y.; Frank, M.; Palma, A.S.; Stroh, L.J.; Feizi, T.; Peters, T.; Atwood, W.J.; et al. A structure-guided mutation in the major capsid protein retargets BK polyomavirus. PLoS Pathog. 2013, 9, e1003688. [Google Scholar] [CrossRef] [Green Version]
- Handala, L.; Fiore, T.; Rouille, Y.; Helle, F. QuantIF: An ImageJ Macro to Automatically Determine the Percentage of Infected Cells after Immunofluorescence. Viruses 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Becker, B.; Shaebani, M.R.; Rammo, D.; Bubel, T.; Santen, L.; Schmitt, M.J. Cargo binding promotes KDEL receptor clustering at the mammalian cell surface. Sci. Rep. 2016, 6, 28940. [Google Scholar] [CrossRef] [Green Version]
- Parent, M. Quantification of Interactions Between Influenza Hemagglutinin and Host Cell Phosphoinositides by Super-Resolution Microscopy; University of Maine: Orono, ME, USA, 2020. [Google Scholar]
- Querol-Audí, J.; Fita, I.; Verdaguer, N. X-ray crystallography of virus-receptor complexes: Structure of a minor group rhinovirus bound to its cellular receptor protein. Crystallogr. Rev. 2005, 11, 73–81. [Google Scholar] [CrossRef]
- Sewald, X. Visualizing Viral Infection In Vivo by Multi-Photon Intravital Microscopy. Viruses 2018, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Mittler, E.; Alkutkar, T.; Jangra, R.K.; Chandran, K. Direct Intracellular Visualization of Ebola Virus-Receptor Interaction by In Situ Proximity Ligation. mBio 2021, 12, e03100-20. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.; Barr, V.; Samelson, L.E. Super-resolution characterization of TCR-dependent signaling clusters. Immunol. Rev. 2013, 251, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Reinhard, B.M. Characterizing Large-Scale Receptor Clustering on the Single Cell Level: A Comparative Plasmon Coupling and Fluorescence Superresolution Microscopy Study. J. Phys. Chem. B 2019, 123, 5494–5505. [Google Scholar] [CrossRef]
- Yi, J.; Balagopalan, L.; Nguyen, T.; McIntire, K.M.; Samelson, L.E. TCR microclusters form spatially segregated domains and sequentially assemble in calcium-dependent kinetic steps. Nat. Commun. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pho, M.; Ashok, A.; Atwood, W.J. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 2000, 74, 2288–2292. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Puri, S.; Miledi, R.; Panicker, M.M. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc. Natl. Acad. Sci. USA 2002, 99, 14470–14475. [Google Scholar] [CrossRef] [Green Version]
- Bjork, K.; Sjogren, B.; Svenningsson, P. Regulation of serotonin receptor function in the nervous system by lipid rafts and adaptor proteins. Exp. Cell Res. 2010, 316, 1351–1356. [Google Scholar] [CrossRef]
- Allen, J.A.; Yadav, P.N.; Roth, B.L. Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology 2008, 55, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S.; Clayton, A.H.; Chattopadhyay, A. Organization of higher-order oligomers of the serotonin1A receptor explored utilizing homo-FRET in live cells. Biophys. J. 2011, 100, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Herrick-Davis, K. Functional significance of serotonin receptor dimerization. Exp. Brain Res. 2013, 230, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Brea, J.; Castro, M.; Giraldo, J.; Lopez-Gimenez, J.F.; Padin, J.F.; Quintian, F.; Cadavid, M.I.; Vilaro, M.T.; Mengod, G.; Berg, K.A.; et al. Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine2A receptor homodimers. Mol. Pharmacol. 2009, 75, 1380–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukasiewicz, S.; Polit, A.; Kedracka-Krok, S.; Wedzony, K.; Mackowiak, M.; Dziedzicka-Wasylewska, M. Hetero-dimerization of serotonin 5-HT2A and dopamine D2 receptors. Biochim. Biophys. Acta 2010, 1803, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Albizu, L.; Holloway, T.; Gonzalez-Maeso, J.; Sealfon, S.C. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology 2011, 61, 770–777. [Google Scholar] [CrossRef] [PubMed]

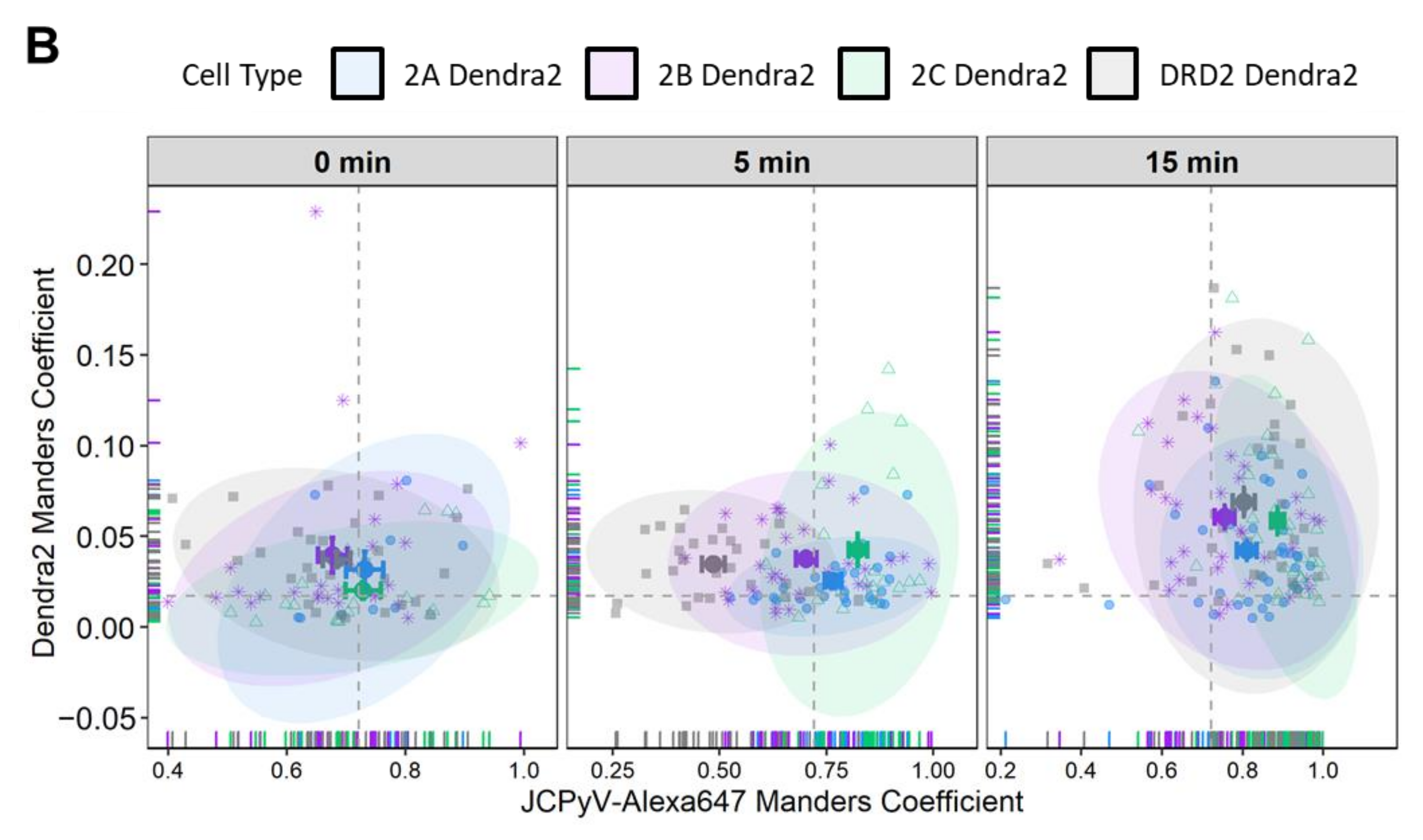

| Time Point | Cell Line | Manders’ Coefficient | Standard Error of Mean | % Difference with DRD2 Dendra2 (± Error) | p Value |
|---|---|---|---|---|---|
| 5 mpi | 2A Dendra2 | 0.766 | 0.020 | 58 (±6) | <0.001 |
| 2B Dendra2 | 0.703 | 0.024 | 45 (±6) | <0.001 | |
| 2C Dendra2 | 0.823 | 0.022 | 69 (±6) | <0.001 | |
| DRD2 Dendra2 | 0.486 | 0.026 | |||
| 15 mpi | 2A Dendra2 | 0.810 | 0.025 | 0.9 (±5) | >0.05 |
| 2B Dendra2 | 0.756 | 0.024 | −6 (±5) | >0.05 | |
| 2C Dendra2 | 0.886 | 0.016 | 10 (±4) | >0.05 | |
| DRD2 Dendra2 | 0.803 | 0.027 |
| Percent Difference | 2A Dendra2 | 2B Dendra2 | 2C Dendra2 | DRD2 Dendra2 | |
|---|---|---|---|---|---|
| 5 mpi | 2A Dendra2 | 9% (±4%) | −7% (±4%) | 58% (±6%) | |
| 2B Dendra2 | −8% (±4%) | −14% (±4%) | 45% (±6%) | ||
| 2C Dendra2 | 7% (±4%) | 17% (±4%) | 69% (±6%) | ||
| DRD2 Dendra2 | −36% (±6%) | −31% (±6%) | −41% (±6%) | ||
| 15 mpi | 2A Dendra2 | 7% (±4%) | −9% (±4%) | 0.9% (±5%) | |
| 2B Dendra2 | −7% (±4%) | −14% (±4%) | −6% (±5%) | ||
| 2C Dendra2 | 9% (±5%) | 17% (±4%) | 10% (±4%) | ||
| DRD2 Dendra2 | −1% (± 5%) | 6% (±5%) | −9% (±4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, K.; Wilczek, M.P.; DuShane, J.K.; Parent, M.T.; Mayberry, C.L.; Wallace, J.N.; Levasseur, F.L.; Fong, T.M.; Hess, S.T.; Maginnis, M.S. Dynamics and Patterning of 5-Hydroxytryptamine 2 Subtype Receptors in JC Polyomavirus Entry. Viruses 2022, 14, 2597. https://doi.org/10.3390/v14122597
Mehmood K, Wilczek MP, DuShane JK, Parent MT, Mayberry CL, Wallace JN, Levasseur FL, Fong TM, Hess ST, Maginnis MS. Dynamics and Patterning of 5-Hydroxytryptamine 2 Subtype Receptors in JC Polyomavirus Entry. Viruses. 2022; 14(12):2597. https://doi.org/10.3390/v14122597
Chicago/Turabian StyleMehmood, Kashif, Michael P. Wilczek, Jeanne K. DuShane, Matthew T. Parent, Colleen L. Mayberry, Jaqulin N. Wallace, Francois L. Levasseur, Tristan M. Fong, Samuel T. Hess, and Melissa S. Maginnis. 2022. "Dynamics and Patterning of 5-Hydroxytryptamine 2 Subtype Receptors in JC Polyomavirus Entry" Viruses 14, no. 12: 2597. https://doi.org/10.3390/v14122597
APA StyleMehmood, K., Wilczek, M. P., DuShane, J. K., Parent, M. T., Mayberry, C. L., Wallace, J. N., Levasseur, F. L., Fong, T. M., Hess, S. T., & Maginnis, M. S. (2022). Dynamics and Patterning of 5-Hydroxytryptamine 2 Subtype Receptors in JC Polyomavirus Entry. Viruses, 14(12), 2597. https://doi.org/10.3390/v14122597






