Effects of Klebsiella pneumoniae Bacteriophages on IRAK3 Knockdown/Knockout THP-1 Monocyte Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. THP-1 Monocyte Wild Type, IRAK3 Knockdown and IRAK3 Knockout Cell Culture
2.2. Bacteriophage Isolation and Purification
2.3. Transmission Electron Microscopy
2.4. Bacteriophage Whole Genome Sequencing and In Silico Analysis
2.5. Assessment of THP-1 Monocyte In-Vitro Cytokine Production
2.6. Statistical Analysis
3. Results
3.1. Characterisation of Klebsiella Bacteriophages KPN7 and KPN8
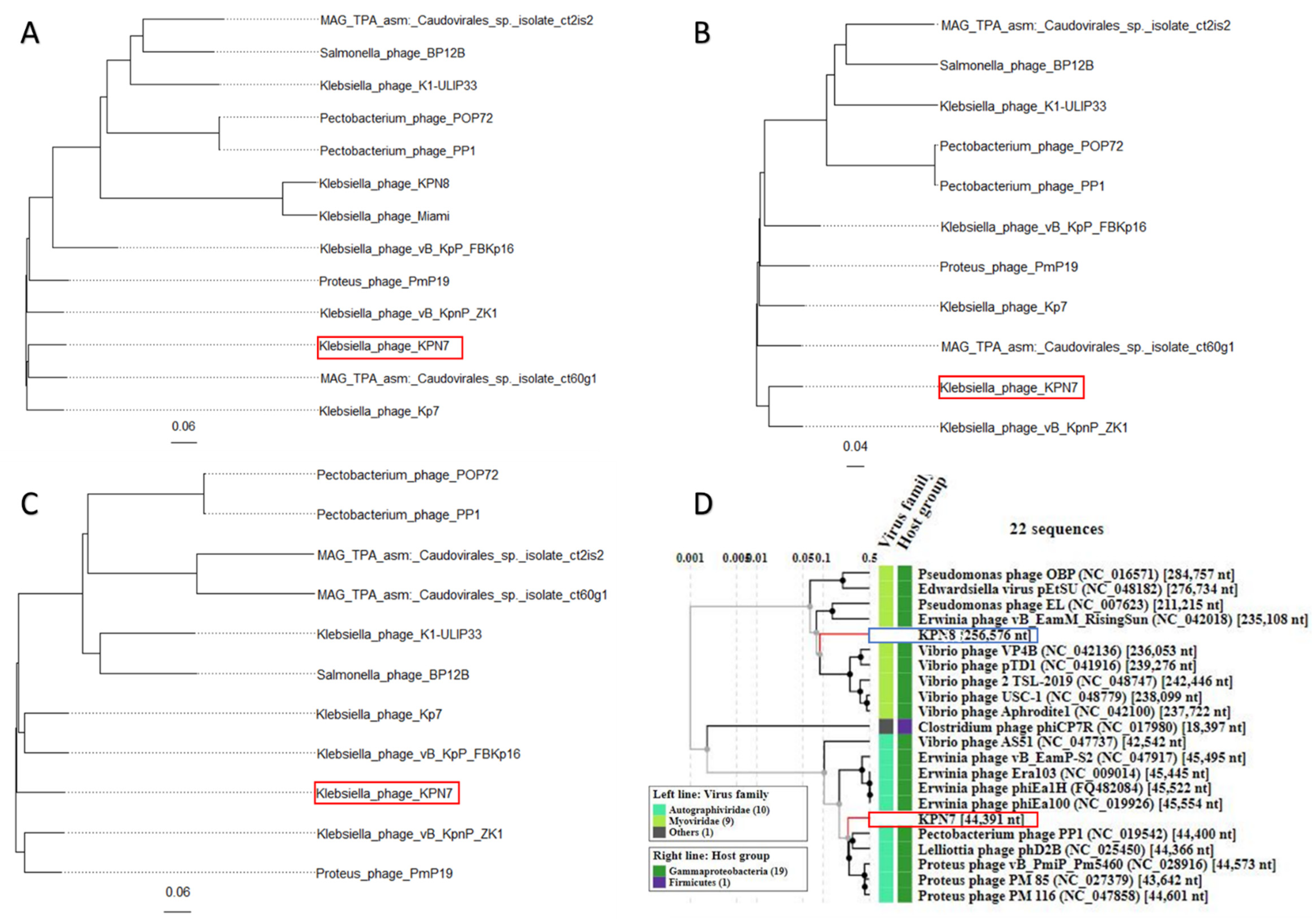
3.2. Phylogeny of Klebsiella Bacteriophages KPN7 and KPN8
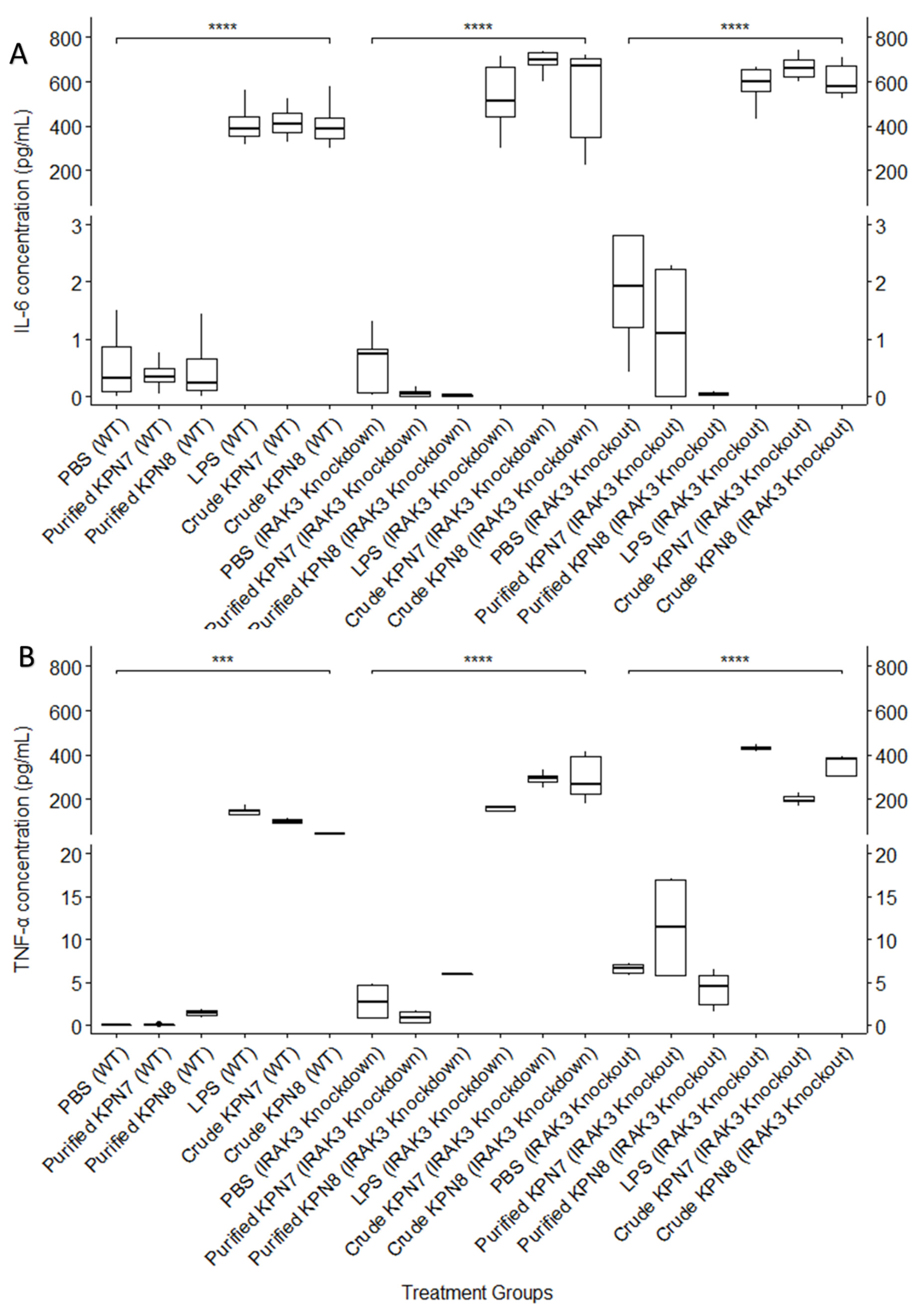
3.3. Effect of KPN7 and KPN8 Bacteriophages on IL-6 and TNF-α Cytokine Production in Wild Type, IRAK3 Knockdown and IRAK3 Knockout THP-1 Monocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Kabwe, M.; Tembo, J.; Chilukutu, L.; Chilufya, M.; Ngulube, F.; Lukwesa, C.; Kapasa, M.; Enne, V.; Wexner, H.; Mwananyanda, L.; et al. Etiology, Antibiotic Resistance and Risk Factors for Neonatal Sepsis in a Large Referral Center in Zambia. Pediatr. Infect. Dis. J. 2016, 35, e191–e198. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Shimaoka, M.; Park, E.J. Advances in understanding sepsis. Eur. J. Anaesthesiol. 2008, 42, 146–153. [Google Scholar] [CrossRef]
- Lu, X.; Xue, L.; Sun, W.; Ye, J.; Zhu, Z.; Mei, H. Identification of key pathogenic genes of sepsis based on the Gene Expression Omnibus database. Mol. Med. Rep. 2018, 17, 3042–3054. [Google Scholar] [CrossRef]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef]
- Jain, A.; Kaczanowska, S.; Davila, E. IL-1 Receptor-Associated Kinase Signaling and Its Role in Inflammation, Cancer Progression, and Therapy Resistance. Front. Immunol. 2014, 5, 553. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A., Jr.; Medzhitov, R.; Flavell, R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef]
- Wesche, H.; Gao, X.; Li, X.; Kirschning, C.J.; Stark, G.R.; Cao, Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 1999, 274, 19403–19410. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Turek, I.; Meehan-Andrews, T.; Zacharias, A.; Irving, H.R. A systematic review and meta-analyses of interleukin-1 receptor associated kinase 3 (IRAK3) action on inflammation in in vivo models for the study of sepsis. PLoS ONE 2022, 17, e0263968. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Li, W.; Yang, Y.; Liu, Q.; Li, S.; Zheng, P.; Zheng, X.; Zhang, Y.; He, J.; Chen, Y.; et al. Inappropriate use of antibiotics exacerbates inflammation through OMV-induced pyroptosis in MDR Klebsiella pneumoniae infection. Cell Rep. 2021, 36, 109750. [Google Scholar] [CrossRef]
- van Langevelde, P.; Kwappenberg, K.M.; Groeneveld, P.H.; Mattie, H.; van Dissel, J.T. Antibiotic-induced lipopolysaccharide (LPS) release from Salmonella typhi: Delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob. Agents Chemother. 1998, 42, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Tharakan, A.; Lane, A.P.; Ramanathan, M., Jr. Bactericidal antibiotics promote reactive oxygen species formation and inflammation in human sinonasal epithelial cells. Int. Forum Allergy Rhinol. 2016, 6, 191–200. [Google Scholar] [CrossRef]
- Hopkin, D.A. Frapper fort ou frapper doucement: A Gram-negative dilemma. Lancet 1978, 2, 1193–1194. [Google Scholar] [CrossRef]
- Piel, D.; Bruto, M.; Labreuche, Y.; Blanquart, F.; Goudenège, D.; Barcia-Cruz, R.; Chenivesse, S.; Le Panse, S.; James, A.; Dubert, J.; et al. Phage–host coevolution in natural populations. Nat. Microbiol. 2022, 7, 1075–1086. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dabrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.D.; Buckley, R.H.; Kobayashi, R.H.; Kobayashi, A.L.; Sorensen, R.U.; Douglas, S.D.; Hamilton, B.L.; Hershfield, M.S. Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood 1992, 80, 1163–1171. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Khalid, A.; Maddocks, S.; Ho, J.; Gilbey, T.; Sandaradura, I.; Lin, R.C.; Ben Zakour, N.; Venturini, C.; Bowring, B.; et al. Phage therapy for severe bacterial infections: A narrative review. Med. J. Aust. 2020, 212, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Petrovic Fabijan, A.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.A.; Samara, O.; Monzon, A.; Montalbano, G.; Scherger, S.; DeSanto, K.; Chastain, D.B.; Sillau, S.; Montoya, J.G.; Franco-Paredes, C.; et al. Proinflammatory Cytokine Levels in Sepsis and in Health and TNFα Association with Sepsis Mortality and patient characteristics: A Systematic Review and Meta-analysis. medRxiv 2021, 12, 267720. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.-J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef]
- Weber-Dabrowska, B.; Zimecki, M.; Mulczyk, M. Effective phage therapy is associated with normalization of cytokine production by blood cell cultures. Arch. Immunol. Ther. Exp. Warsz. 2000, 48, 31–37. [Google Scholar]
- Wiersinga, W.J.; van Veer, C.; van den Pangaart, P.S.; Dondorp, A.M.; Day, N.P.; Peacock, S.J.; van der Poll, T. Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in Gram-negative sepsis (melioidosis). Crit. Care Med. 2009, 37, 569–576. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Axell, A.; Turek, I.; Wright, B.; Meehan-Andrews, T.; Irving, H.R. Modulation of Inflammatory Cytokine Production in Human Monocytes by cGMP and IRAK3. Int. J. Mol. Sci. 2022, 23, 2552. [Google Scholar] [CrossRef]
- Brown, T.L.; Petrovski, S.; Hoyle, D.; Chan, H.T.; Lock, P.; Tucci, J. Characterization and formulation into solid dosage forms of a novel bacteriophage lytic against Klebsiella oxytoca. PLoS ONE 2017, 12, e0183510. [Google Scholar] [CrossRef]
- Kabwe, M.; Brown, T.; Ku, H.; Dashper, S.; Tucci, J. Isolation and Functional Characterization of Fusobacterium nucleatum Bacteriophage. Methods Mol. Biol. 2021, 2327, 51–68. [Google Scholar] [CrossRef]
- Branston, S.D.; Wright, J.; Keshavarz-Moore, E. A non-chromatographic method for the removal of endotoxins from bacteriophages. Biotechnol. Bioeng. 2015, 112, 1714–1719. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Mount, D.W. Using the Basic Local Alignment Search Tool (BLAST). CSH Protoc. 2007, 2007, pdb-top17. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.Y. ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Mora, D.; Lessor, L.; Le, T.; Clark, J.; Gill, J.J.; Liu, M. Complete Genome Sequence of Klebsiella pneumoniae Jumbo Phage Miami. Microbiol. Resour. Announc. 2021, 10, e01404–e01420. [Google Scholar] [CrossRef]
- Kabwe, M.; Brown, T.; Speirs, L.; Ku, H.; Leach, M.; Chan, H.T.; Petrovski, S.; Lock, P.; Tucci, J. Novel Bacteriophages Capable of Disrupting Biofilms From Clinical Strains of Aeromonas hydrophila. Front. Microbiol. 2020, 11, 194. [Google Scholar] [CrossRef]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the intriguing presence of tRNAs in phages. Genome Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef]
- Perea, L.; Rodriguez-Rubio, L.; Nieto, J.C.; Zamora, C.; Canto, E.; Soriano, G.; Poca, M.; Blanco-Picazo, P.; Navarro, F.; Muniesa, M.; et al. Bacteriophages immunomodulate the response of monocytes. Exp. Biol. Med. 2021, 246, 1263–1268. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Wahida, A.; Tang, F.; Barr, J.J. Rethinking phage-bacteria-eukaryotic relationships and their influence on human health. Cell Host Microbe 2021, 29, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.D.; Davis, S.D.; Wedgwood, R.J. Immunologic responses to bacteriophage ϕX 174 in immunodeficiency diseases. J. Clin. Investig. 1971, 50, 2559–2568. [Google Scholar] [CrossRef]
- Jariah, R.O.A.; Hakim, M.S. Interaction of phages, bacteria, and the human immune system: Evolutionary changes in phage therapy. Rev. Med. Virol. 2019, 29, e2055. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Maurice, C.F. Bacteriophages: Uncharacterized and Dynamic Regulators of the Immune System. Mediat. Inflamm. 2019, 2019, 3730519. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro- and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci. Rep. 2017, 7, 8004. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, X.; Sun, L.; He, T.; Wei, R.; Pang, M.; Wang, R. Staphylococcus aureus Bacteriophage Suppresses LPS-Induced Inflammation in MAC-T Bovine Mammary Epithelial Cells. Front. Microbiol. 2018, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e288. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.Y.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Bacteriophage Therapy of Ventilator-associated Pneumonia and Empyema Caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
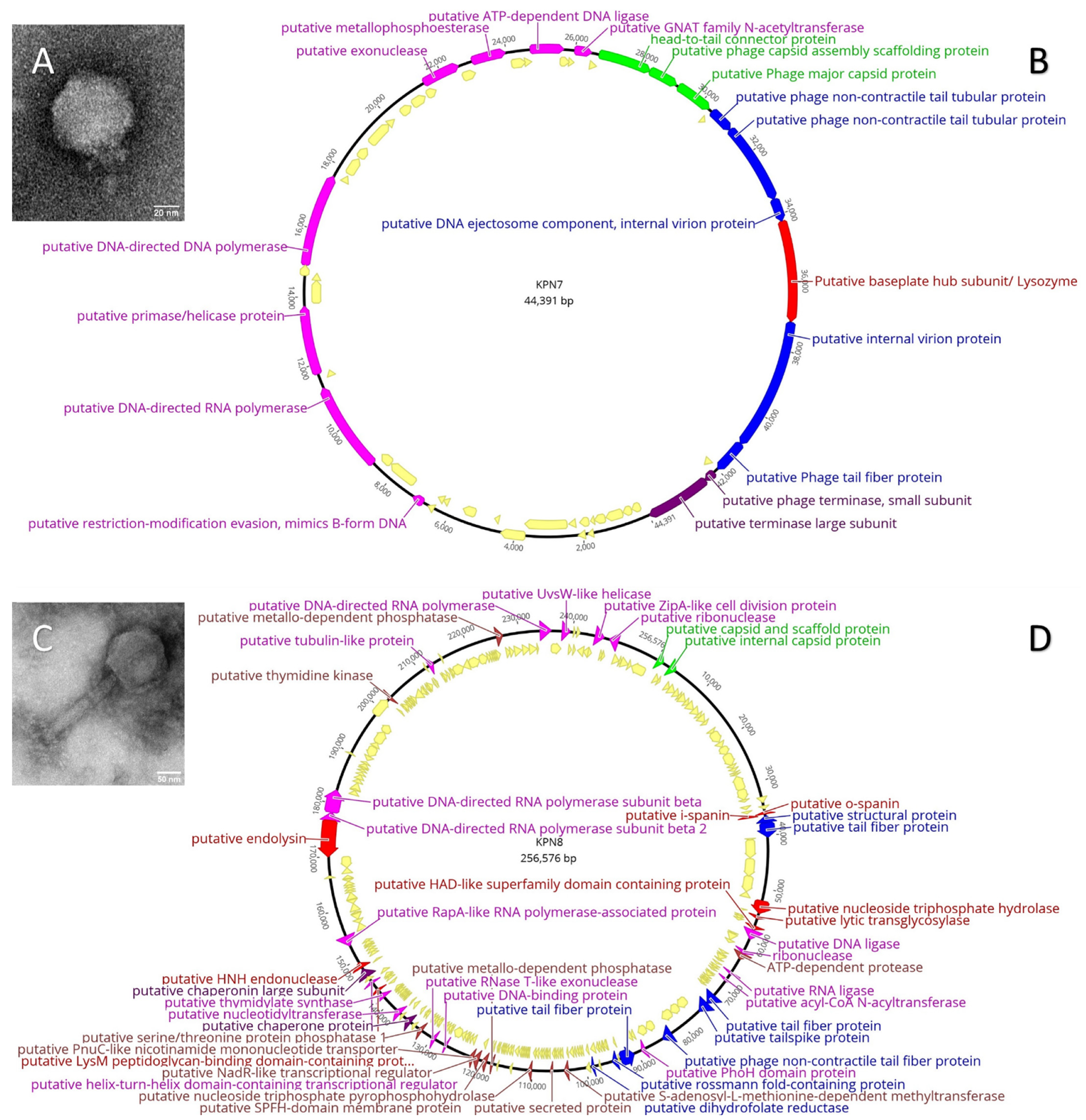
| Characteristics | KPN7 | KPN8 | |
|---|---|---|---|
| TEM | Morphology | Podovirus | Myovirus |
| Capsid diameter, mean (SE) | 62.3 (1.1) nm | 130.2 (3.2) nm | |
| Tail length, mean (SE) | 15.3 (2.7) nm | 196.1 (3.5) nm | |
| Tail width, mean (SE) | 15.6 (1.4) nm | 53.7 (11.4) nm | |
| Genomics | Genome size | 44 391 bases | 256 576 bases |
| GC% content | 51.80% | 44.10% | |
| Hypothetical genes, n (%) | 38/57 (66.7%) | 249/298 (83.6%) | |
| Putative viral family | Autographiviridae | Myoviridae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, B.D.; Ku, H.; Kabwe, M.; Nguyen, T.H.; Irving, H.; Tucci, J. Effects of Klebsiella pneumoniae Bacteriophages on IRAK3 Knockdown/Knockout THP-1 Monocyte Cell Lines. Viruses 2022, 14, 2582. https://doi.org/10.3390/v14112582
Schubert BD, Ku H, Kabwe M, Nguyen TH, Irving H, Tucci J. Effects of Klebsiella pneumoniae Bacteriophages on IRAK3 Knockdown/Knockout THP-1 Monocyte Cell Lines. Viruses. 2022; 14(11):2582. https://doi.org/10.3390/v14112582
Chicago/Turabian StyleSchubert, Bryce Dylan, Heng Ku, Mwila Kabwe, Trang Hong Nguyen, Helen Irving, and Joseph Tucci. 2022. "Effects of Klebsiella pneumoniae Bacteriophages on IRAK3 Knockdown/Knockout THP-1 Monocyte Cell Lines" Viruses 14, no. 11: 2582. https://doi.org/10.3390/v14112582
APA StyleSchubert, B. D., Ku, H., Kabwe, M., Nguyen, T. H., Irving, H., & Tucci, J. (2022). Effects of Klebsiella pneumoniae Bacteriophages on IRAK3 Knockdown/Knockout THP-1 Monocyte Cell Lines. Viruses, 14(11), 2582. https://doi.org/10.3390/v14112582







