Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Infection and RNAseq
2.2. Differential HERVs Expression Estimation
2.3. Correlation between DEHERVs and Nearby DEGs
2.4. The Construction of Protein-Protein Network of the HERVs Nearby Genes
3. Results
3.1. Modulation of HERVs Transcription during Arboviral Infections in Astrocytes
3.2. HERV Upregulated Nearby Genes: Distance, Gene Ontology, Reactome and Integration Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, T.J.; Rosenkrantz, J.L.; Carbone, L.; Chavez, S.L. Endogenous Retroviruses: With Us and against Us. Front. Chem. 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Cullen, H.; Schorn, A.J. Endogenous Retroviruses Walk a Fine Line between Priming and Silencing. Viruses 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, X.-E.; Jiang, Y.-Z.; Liu, Y.-R.; Sun, W.; Guo, Y.-J.; Ren, Y.-X.; Zuo, W.-J.; Hu, X.; Huang, S.-L.; et al. The Endogenous Retrovirus-Derived Long Noncoding RNA TROJAN Promotes Triple-Negative Breast Cancer Progression via ZMYND8 Degradation. Sci. Adv. 2019, 5, eaat9820. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Ma, H.; Liu, D. Endogenous Retroviruses Function as Gene Expression Regulatory Elements during Mammalian Pre-Implantation Embryo Development. IJMS 2019, 20, 790. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, F.; Wu, F.; Nie, H.; Song, Y.; Shao, L.; Han, J.; Wu, Z.; Saiyin, H.; Wei, G.; et al. Endogenous Retrovirus-Derived Long Noncoding RNA Enhances Innate Immune Responses via Derepressing RELA Expression. mBio 2019, 10, e00937-19. [Google Scholar] [CrossRef]
- Ha, H.; Song, J.; Wang, S.; Kapusta, A.; Feschotte, C.; Chen, K.C.; Xing, J. A Comprehensive Analysis of PiRNAs from Adult Human Testis and Their Relationship with Genes and Mobile Elements. BMC Genom. 2014, 15, 545. [Google Scholar] [CrossRef]
- Lu, X.; Sachs, F.; Ramsay, L.; Jacques, P.-É.; Göke, J.; Bourque, G.; Ng, H.-H. The Retrovirus HERVH Is a Long Noncoding RNA Required for Human Embryonic Stem Cell Identity. Nat. Struct. Mol. Biol. 2014, 21, 423–425. [Google Scholar] [CrossRef]
- Xing, J.; Witherspoon, D.J.; Jorde, L.B. Mobile Element Biology: New Possibilities with High-Throughput Sequencing. Trends Genet. 2013, 29, 280–289. [Google Scholar] [CrossRef]
- Jern, P.; Coffin, J.M. Effects of Retroviruses on Host Genome Function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef]
- Malassiné, A.; Frendo, J.-L.; Blaise, S.; Handschuh, K.; Gerbaud, P.; Tsatsaris, V.; Heidmann, T.; Evain-Brion, D. Human Endogenous Retrovirus-FRD Envelope Protein (Syncytin 2) Expression in Normal and Trisomy 21-Affected Placenta. Retrovirology 2008, 5, 6. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.-Y.; Edouard, P.; Howes, S.; et al. Syncytin Is a Captive Retroviral Envelope Protein Involved in Human Placental Morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Blond, J.-L.; Besème, F.; Duret, L.; Bouton, O.; Bedin, F.; Perron, H.; Mandrand, B.; Mallet, F. Molecular Characterization and Placental Expression of HERV-W, a New Human Endogenous Retrovirus Family. J. Virol. 1999, 73, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; De Toma, I.; Vilor-Tejedor, N.; Ghamsari, M.R.E.; Sadeghi, I. Transposable Elements in Brain Health and Disease. Ageing Res. Rev. 2020, 64, 101153. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.M.; van Marle, G.; Opii, W.; Butterfield, D.A.; Mallet, F.; Yong, V.W.; Wallace, J.L.; Deacon, R.M.; Warren, K.; Power, C. Human Endogenous Retrovirus Glycoprotein—Mediated Induction of Redox Reactants Causes Oligodendrocyte Death and Demyelination. Nat. Neurosci. 2004, 7, 1088–1095. [Google Scholar] [CrossRef]
- Salimi, H.; Cain, M.D.; Klein, R.S. Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis. Neurotherapeutics 2016, 13, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.T. Viral Encephalitis. Arq. Neuro-Psiquiatr. 2013, 71, 703–709. [Google Scholar] [CrossRef]
- Zacks, M.A.; Paessler, S. Encephalitic Alphaviruses. Vet. Microbiol. 2010, 140, 281–286. [Google Scholar] [CrossRef]
- Gubler, D.J. Human Arbovirus Infections Worldwide. Ann. N. Y. Acad. Sci. 2006, 951, 13–24. [Google Scholar] [CrossRef]
- Brito Ferreira, M.L.; Militão de Albuquerque, M.d.F.P.; de Brito, C.A.A.; de Oliveira França, R.F.; Porto Moreira, Á.J.; de Morais Machado, M.Í.; da Paz Melo, R.; Medialdea-Carrera, R.; Dornelas Mesquita, S.; Lopes Santos, M.; et al. Neurological Disease in Adults with Zika and Chikungunya Virus Infection in Northeast Brazil: A Prospective Observational Study. Lancet Neurol. 2020, 19, 826–839. [Google Scholar] [CrossRef]
- de Souza Costa, M.C.; Maia, L.M.S.; de Souza, V.C.; Gonzaga, A.M.; de Azevedo, V.C.; Martins, L.R.; Pavoni, J.H.C.; Naveca, F.G.; Slhessarenko, R.D. Arbovirus Investigation in Patients from Mato Grosso during Zika and Chikungunya Virus Introdution in Brazil, 2015–2016. Acta Trop. 2019, 190, 395–402. [Google Scholar] [CrossRef]
- Clé, M.; Eldin, P.; Briant, L.; Lannuzel, A.; Simonin, Y.; Van de Perre, P.; Cabié, A.; Salinas, S. Neurocognitive Impacts of Arbovirus Infections. J. Neuroinflamm. 2020, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern Recognition Receptors and Central Nervous System Repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Geddes, V.E.V.; Brustolini, O.J.B.; Cavalcante, L.T.d.F.; Moreira, F.R.R.; de Castro, F.L.; Guimarães, A.P.d.C.; Gerber, A.L.; Figueiredo, C.M.; Diniz, L.P.; Neto, E.d.A.; et al. Common Dysregulation of Innate Immunity Pathways in Human Primary Astrocytes Infected With Chikungunya, Mayaro, Oropouche, and Zika Viruses. Front. Cell. Infect. Microbiol. 2021, 11, 641261. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bendall, M.L.; de Mulder, M.; Iñiguez, L.P.; Lecanda-Sánchez, A.; Pérez-Losada, M.; Ostrowski, M.A.; Jones, R.B.; Mulder, L.C.F.; Reyes-Terán, G.; Crandall, K.A.; et al. Telescope: Characterization of the Retrotranscriptome by Accurate Estimation of Transposable Element Expression. PLoS Comput. Biol. 2019, 15, e1006453. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Almeida, G.M.; Souza, J.P.; Mendes, N.D.; Pontelli, M.C.; Pinheiro, N.R.; Nogueira, G.O.; Cardoso, R.S.; Paiva, I.M.; Ferrari, G.D.; Veras, F.P.; et al. Neural Infection by Oropouche Virus in Adult Human Brain Slices Induces an Inflammatory and Toxic Response. Front. Neurosci. 2021, 15, 674576. [Google Scholar] [CrossRef]
- Suntsova, M.; Garazha, A.; Ivanova, A.; Kaminsky, D.; Zhavoronkov, A.; Buzdin, A. Molecular Functions of Human Endogenous Retroviruses in Health and Disease. Cell. Mol. Life Sci. 2015, 72, 3653–3675. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.O.; Azevedo, R.S.; Justino, M.C.A.; Matos, H.J.; Cabeça, H.L.S.; Silva, S.P.; Henriques, D.F.; Silva, E.V.P.; Andrade, G.S.S.; Vasconcelos, P.F.C.; et al. Neurological Disease Caused by Oropouche Virus in Northern Brazil: Should It Be Included in the Scope of Clinical Neurological Diseases? J. Neurovirol. 2021, 27, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Vernal, S.; Martini, C.C.R.; da Fonseca, B.A.L. Oropouche Virus–Associated Aseptic Meningoencephalitis, Southeastern Brazil. Emerg. Infect. Dis. 2019, 25, 380–382. [Google Scholar] [CrossRef]
- Pavon, J.A.R.; da Silva Neves, N.A.; Silva, L.C.F.; de Azevedo, F.K.; de Figueiredo, J.A.B., Jr.; Nunes, M.R.T.; Slhessarenko, R.D. Neurological Infection by Chikungunya and a Triple Arbovirus Co-Infection in Mato Grosso, Central Western Brazil during 2019. J. Clin. Virol. 2022, 146, 105056. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; He, S.; Contreras-Galindo, A.C.; Gonzalez-Hernandez, M.J.; Kappes, F.; Dube, D.; Chan, S.M.; Robinson, D.; Meng, F.; et al. HIV Infection Reveals Widespread Expansion of Novel Centromeric Human Endogenous Retroviruses. Genome Res. 2013, 23, 1505–1513. [Google Scholar] [CrossRef]
- Hurst, T.; Magiorkinis, G. Epigenetic Control of Human Endogenous Retrovirus Expression: Focus on Regulation of Long-Terminal Repeats (LTRs). Viruses 2017, 9, 130. [Google Scholar] [CrossRef]
- Liu, H.; Bergant, V.; Frishman, G.; Ruepp, A.; Pichlmair, A.; Vincendeau, M.; Frishman, D. Influenza A Virus Infection Reactivates Human Endogenous Retroviruses Associated with Modulation of Antiviral Immunity. Viruses 2022, 14, 1591. [Google Scholar] [CrossRef]
- Toufaily, C.; Landry, S.; Leib-Mosch, C.; Rassart, E.; Barbeau, B. Activation of LTRs from Different Human Endogenous Retrovirus (HERV) Families by the HTLV-1 Tax Protein and T-Cell Activators. Viruses 2011, 3, 2146–2159. [Google Scholar] [CrossRef]
- Lee, W.J.; Kwun, H.J.; Kim, H.S.; Jang, K.L. Activation of the Human Endogenous Retrovirus W Long Terminal Repeat by Herpes Simplex Virus Type 1 Immediate Early Protein 1. Mol. Cells 2003, 15, 75–80. [Google Scholar]
- Nellåker, C.; Yao, Y.; Jones-Brando, L.; Mallet, F.; Yolken, R.H.; Karlsson, H. Transactivation of Elements in the Human Endogenous Retrovirus W Family by Viral Infection. Retrovirology 2006, 3, 44. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; López, P.; Vélez, R.; Yamamura, Y. HIV-1 Infection Increases the Expression of Human Endogenous Retroviruses Type K (HERV-K) In Vitro. AIDS Res. Hum. Retrovir. 2007, 23, 116–122. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Wang, X.; Liu, Y.; Wang, M.; Zhu, F. HBV X Protein Induces Overexpression of HERV-W Env through NF-ΚB in HepG2 Cells. Virus Genes 2017, 53, 797–806. [Google Scholar] [CrossRef]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the Pathogenic HERV-W Envelope Expression in T Lymphocytes in Association with the Respiratory Outcome of COVID-19 Patients. EBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Del Valle, L.; Miley, W.; Whitby, D.; Ochoa, A.C.; Flemington, E.K.; Qin, Z. Transactivation of Human Endogenous Retrovirus K (HERV-K) by KSHV Promotes Kaposi’s Sarcoma Development. Oncogene 2018, 37, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Galliano, I.; Montanari, P.; Gambarino, S.; Mareschi, K.; Ferro, F.; Fagioli, F.; Tovo, P.-A.; Ravanini, P. CMV Induces HERV-K and HERV-W Expression in Kidney Transplant Recipients. J. Clin. Virol. 2015, 68, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Sutkowski, N.; Chen, G.; Calderon, G.; Huber, B.T. Epstein-Barr Virus Latent Membrane Protein LMP-2A Is Sufficient for Transactivation of the Human Endogenous Retrovirus HERV-K18 Superantigen. J. Virol. 2004, 78, 7852–7860. [Google Scholar] [CrossRef]
- Turcanova, V.L.; Bundgaard, B.; Höllsberg, P. Human Herpesvirus-6B Induces Expression of the Human Endogenous Retrovirus K18-Encoded Superantigen. J. Clin. Virol. 2009, 46, 15–19. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, Y.; Liu, H.; Liang, B.; Fan, B.; Zhou, X.; Liu, D. Transcription Profile of Human Endogenous Retroviruses in Response to Dengue Virus Serotype 2 Infection. Virology 2020, 544, 21–30. [Google Scholar] [CrossRef]
- Taruscio, D.; Floridia, G.; Zoraqi, G.K.; Mantovani, A.; Falbo, V. Organization and Integration Sites in the Human Genome of Endogenous Retroviral Sequences Belonging to HERV-E Family. Mamm. Genome 2002, 13, 216–222. [Google Scholar] [CrossRef]
- Sokol, M.; Jessen, K.M.; Pedersen, F.S. Human Endogenous Retroviruses Sustain Complex and Cooperative Regulation of Gene-Containing Loci and Unannotated Megabase-Sized Regions. Retrovirology 2015, 12, 32. [Google Scholar] [CrossRef][Green Version]
- McGrath, E.L.; Rossi, S.L.; Gao, J.; Widen, S.G.; Grant, A.C.; Dunn, T.J.; Azar, S.R.; Roundy, C.M.; Xiong, Y.; Prusak, D.J.; et al. Differential Responses of Human Fetal Brain Neural Stem Cells to Zika Virus Infection. Stem Cell Rep. 2017, 8, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Herdy, B.; Karonitsch, T.; Vladimer, G.I.; Tan, C.S.H.; Stukalov, A.; Trefzer, C.; Bigenzahn, J.W.; Theil, T.; Holinka, J.; Kiener, H.P.; et al. The RNA-Binding Protein HuR/ELAVL1 Regulates IFN-β MRNA Abundance and the Type I IFN Response: Innate Immunity. Eur. J. Immunol. 2015, 45, 1500–1511. [Google Scholar] [CrossRef]
- Alfahad, T.; Nath, A. Retroviruses and Amyotrophic Lateral Sclerosis. Antivir. Res. 2013, 99, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Canet, G.; Dias, C.; Gabelle, A.; Simonin, Y.; Gosselet, F.; Marchi, N.; Makinson, A.; Tuaillon, E.; Van de Perre, P.; Givalois, L.; et al. HIV Neuroinfection and Alzheimer’s Disease: Similarities and Potential Links? Front. Cell. Neurosci. 2018, 12, 307. [Google Scholar] [CrossRef]
- De Kumar, B.; Parker, H.J.; Paulson, A.; Parrish, M.E.; Pushel, I.; Singh, N.P.; Zhang, Y.; Slaughter, B.D.; Unruh, J.R.; Florens, L.; et al. HOXA1 and TALE Proteins Display Cross-Regulatory Interactions and Form a Combinatorial Binding Code on HOXA1 Targets. Genome Res. 2017, 27, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.R.; Heikel, G.; Michlewski, G. TRIM25 and Its Emerging RNA-binding Roles in Antiviral Defense. WIREs RNA 2020, 11, e1588. [Google Scholar] [CrossRef]
- van Gent, M.; Sparrer, K.M.J.; Gack, M.U. TRIM Proteins and Their Roles in Antiviral Host Defenses. Annu. Rev. Virol. 2018, 5, 385–405. [Google Scholar] [CrossRef]
- Dolei, A.; Ibba, G.; Piu, C.; Serra, C. Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases. IJMS 2019, 20, 3706. [Google Scholar] [CrossRef]
- Ryman, K.D.; Klimstra, W.B. Host Responses to Alphavirus Infection. Immunol. Rev. 2008, 225, 27–45. [Google Scholar] [CrossRef]
- Ward, A.M.; Bidet, K.; Yinglin, A.; Ler, S.G.; Hogue, K.; Blackstock, W.; Gunaratne, J.; Garcia-Blanco, M.A. Quantitative Mass Spectrometry of DENV-2 RNA-Interacting Proteins Reveals That the DEAD-Box RNA Helicase DDX6 Binds the DB1 and DB2 3’ UTR Structures. RNA Biol. 2011, 8, 1173–1186. [Google Scholar] [CrossRef]
- Ostareck, D.H.; Naarmann-de Vries, I.S.; Ostareck-Lederer, A. DDX6 and Its Orthologs as Modulators of Cellular and Viral RNA Expression: Functions of DDX6 and Its Orthologs. WIREs RNA 2014, 5, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.E.; Locke, M.C.; Young, A.R.; Monte, K.; Hedberg, M.L.; Shimak, R.M.; Sheehan, K.C.F.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Distinct Roles of Interferon Alpha and Beta in Controlling Chikungunya Virus Replication and Modulating Neutrophil-Mediated Inflammation. J. Virol. 2019, 94, e00841-19. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Budt, M.; Saenger, S.; Paki, K.; Arnold, U.; Sadewasser, A.; Wolff, T. The RNA Helicase DDX6 Associates with RIG-I to Augment Induction of Antiviral Signaling. IJMS 2018, 19, 1877. [Google Scholar] [CrossRef]
- Aguiar, R.S.; Pohl, F.; Morais, G.L.; Nogueira, F.C.S.; Carvalho, J.B.; Guida, L.; Arge, L.W.P.; Melo, A.; Moreira, M.E.L.; Cunha, D.P.; et al. Molecular Alterations in the Extracellular Matrix in the Brains of Newborns with Congenital Zika Syndrome. Sci. Signal. 2020, 13, eaay6736. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Yonezawa, T.; Hasegawa, H.; Hattori, S.; Greenhill, N.S.; Davis, P.F.; Sage, E.H.; Ninomiya, Y. Astrocytes Express Type VIII Collagen during the Repair Process of Brain Cold Injury. Biochem. Biophys. Res. Commun. 2004, 317, 437–443. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Birke, M.; Welge-Lussen, U.; Kook, D.; Lütjen-Drecoll, E. Transforming Growth Factor-Β2 Modulated Extracellular Matrix Component Expression in Cultured Human Optic Nerve Head Astrocytes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 568. [Google Scholar] [CrossRef]
- Kottler, U.B.; Jünemann, A.G.M.; Aigner, T.; Zenkel, M.; Rummelt, C.; Schlötzer-Schrehardt, U. Comparative Effects of TGF-Β1 and TGF-Β2 on Extracellular Matrix Production, Proliferation, Migration, and Collagen Contraction of Human Tenon’s Capsule Fibroblasts in Pseudoexfoliation and Primary Open-Angle Glaucoma. Exp. Eye Res. 2005, 80, 121–134. [Google Scholar] [CrossRef]
- Marceau, C.D.; Puschnik, A.S.; Majzoub, K.; Ooi, Y.S.; Brewer, S.M.; Fuchs, G.; Swaminathan, K.; Mata, M.A.; Elias, J.E.; Sarnow, P.; et al. Genetic Dissection of Flaviviridae Host Factors through Genome-Scale CRISPR Screens. Nature 2016, 535, 159–163. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Blanco, M.R.; Bruce, E.A.; Honson, D.D.; Chen, L.M.; Chow, A.; Bhat, P.; Ollikainen, N.; Quinodoz, S.A.; Loney, C.; et al. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell 2020, 183, 1325–1339. [Google Scholar] [CrossRef]
- Nordholm, J.; Petitou, J.; Östbye, H.; da Silva, D.V.; Dou, D.; Wang, H.; Daniels, R. Translational Regulation of Viral Secretory Proteins by the 5′ Coding Regions and a Viral RNA-Binding Protein. J. Cell Biol. 2017, 216, 2283–2293. [Google Scholar] [CrossRef]
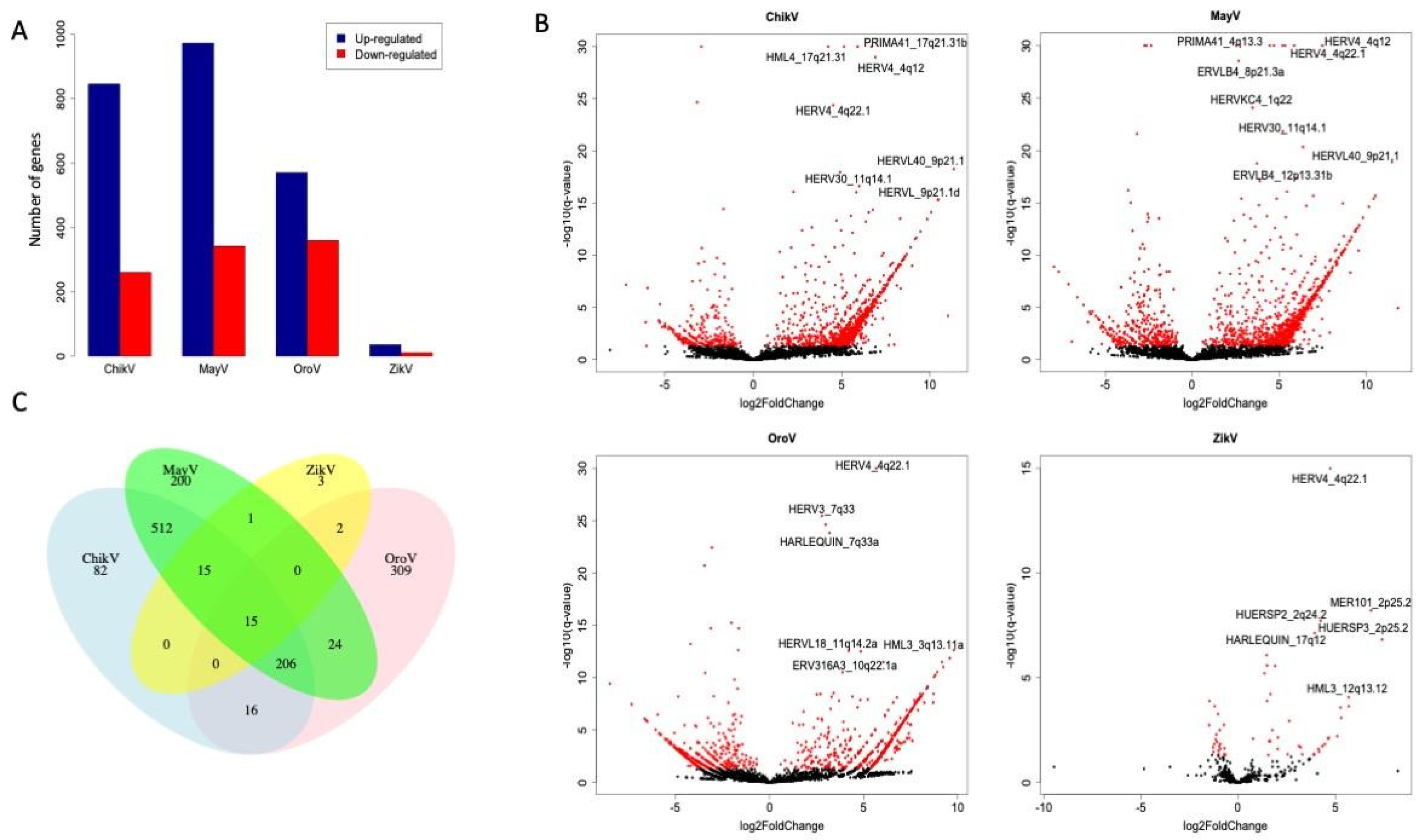
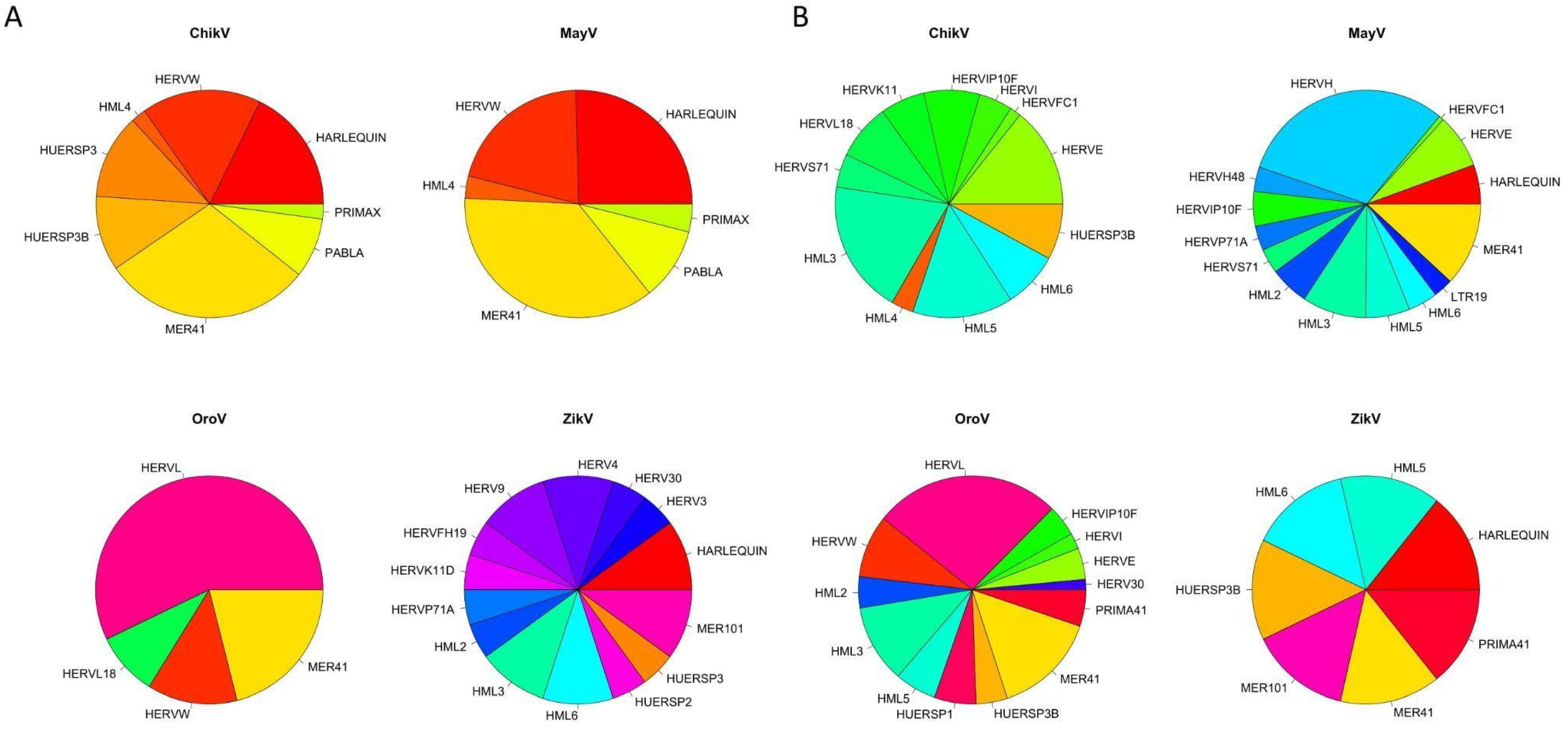
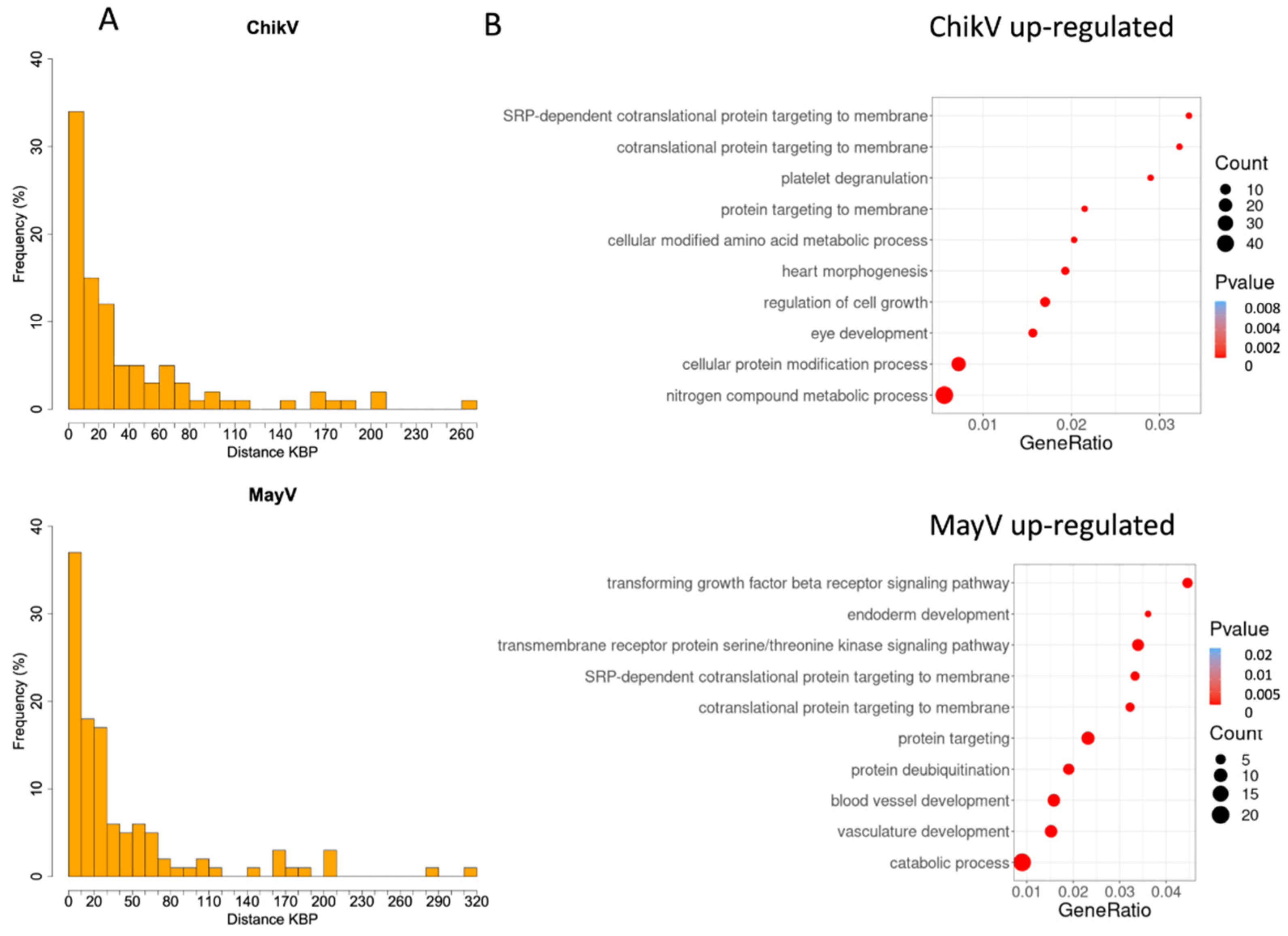
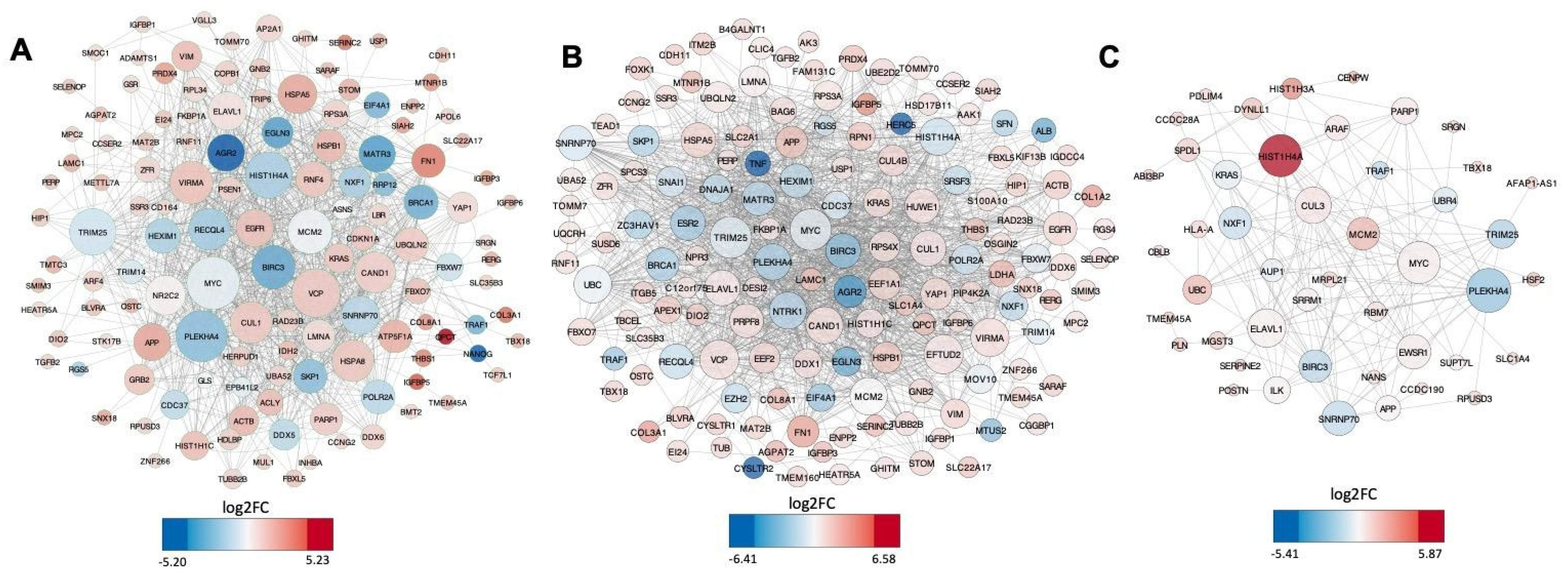
| HERV | ChikV | MayV | OroV | ZikV | ||||
|---|---|---|---|---|---|---|---|---|
| log2FC | q-Value | log2FC | q-Value | log2FC | q-Value | log2FC | q-Value | |
| ERV316A3_12q24.13 | 6.366 | 1.90 × 10−6 | 7.004 | 4.73 × 10−8 | 8.006 | 3.94 × 10−9 | 5.681 | 9.04 × 10−5 |
| ERV316A3_3q27.3e | 3.946 | 0.00099 | 5.164 | 1.22 × 10−6 | 3.913 | 0.001330 | 3.228 | 0.021470 |
| ERV316A3_7q34a | 6.362 | 1.29 × 10−5 | 7.813 | 8.46 × 10−9 | 6.249 | 0.000368 | 3.913 | 0.048601 |
| ERVLB4_12p13.2a | 7.738 | 9.68 × 10−10 | 8.414 | 9.95 × 10−12 | 5.440 | 2.98 × 10−5 | 5.266 | 0.000273 |
| HARLEQUIN_17q12 | 3.698 | 9.65 × 10−7 | 3.860 | 4.38 × 10−8 | 6.680 | 3.17 × 10−6 | 3.946 | 7.72 × 10−8 |
| HERV4_4q22.1 | 4.520 | 4.21 × 10−25 | 5.181 | 4.01 × 10−36 | 5.665 | 6.00 × 10−53 | 4.734 | 7.63 × 10−30 |
| HERV9_11q21 | 3.363 | 1.01 × 10−11 | 3.886 | 8.34 × 10−18 | 2.613 | 7.21 × 10−5 | 1.773 | 0.003343 |
| HERVK11D_2q11.2 | 4.270 | 4.14 × 10−10 | 4.494 | 3.63 × 10−12 | 2.058 | 0.035437 | 2.051 | 0.025747 |
| HML3_12q13.12 | 7.832 | 9.26 × 10−9 | 8.662 | 5.28 × 10−11 | 6.631 | 3.77 × 10−5 | 5.694 | 0.000241 |
| HML3_16p13.3 | 7.785 | 1.21 × 10−8 | 8.547 | 1.01 × 10−10 | 6.079 | 0.001019 | 3.909 | 0.046599 |
| HML6_14q24.2 | 9.465 | 4.98 × 10−13 | 9.569 | 1.42 × 10−13 | 9.166 | 3.57 × 10−12 | 5.300 | 0.000859 |
| HML6_19p13.2c | 5.274 | 3.84 × 10−7 | 5.421 | 6.02 × 10−8 | 2.418 | 0.000147 | 3.131 | 0.018769 |
| HUERSP3_2p25.2 | 7.397 | 6.92 × 10−8 | 8.553 | 7.31 × 10−11 | 9.796 | 2.60 × 10−13 | 7.404 | 1.56 × 10−7 |
| MER101_1p22.2a | 3.510 | 2.21 × 10−8 | 3.662 | 3.83 × 10−10 | 3.876 | 3.34 × 10−11 | 2.064 | 0.007506 |
| MER101_2p25.2 | 6.205 | 6.93 × 10−8 | 7.081 | 1.47 × 10−10 | 4.254 | 0.013413 | 6.832 | 6.34 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, F.L.d.; Brustolini, O.J.B.; Geddes, V.E.V.; Souza, J.P.B.M.d.; Alves-Leon, S.V.; Aguiar, R.S.; Vasconcelos, A.T.R. Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes. Viruses 2022, 14, 2505. https://doi.org/10.3390/v14112505
Castro FLd, Brustolini OJB, Geddes VEV, Souza JPBMd, Alves-Leon SV, Aguiar RS, Vasconcelos ATR. Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes. Viruses. 2022; 14(11):2505. https://doi.org/10.3390/v14112505
Chicago/Turabian StyleCastro, Fernando Luz de, Otávio José Bernandes Brustolini, Victor Emmanuel Viana Geddes, Jorge Paes Barreto Marcondes de Souza, Soniza Vieira Alves-Leon, Renato Santana Aguiar, and Ana Tereza Ribeiro Vasconcelos. 2022. "Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes" Viruses 14, no. 11: 2505. https://doi.org/10.3390/v14112505
APA StyleCastro, F. L. d., Brustolini, O. J. B., Geddes, V. E. V., Souza, J. P. B. M. d., Alves-Leon, S. V., Aguiar, R. S., & Vasconcelos, A. T. R. (2022). Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes. Viruses, 14(11), 2505. https://doi.org/10.3390/v14112505





