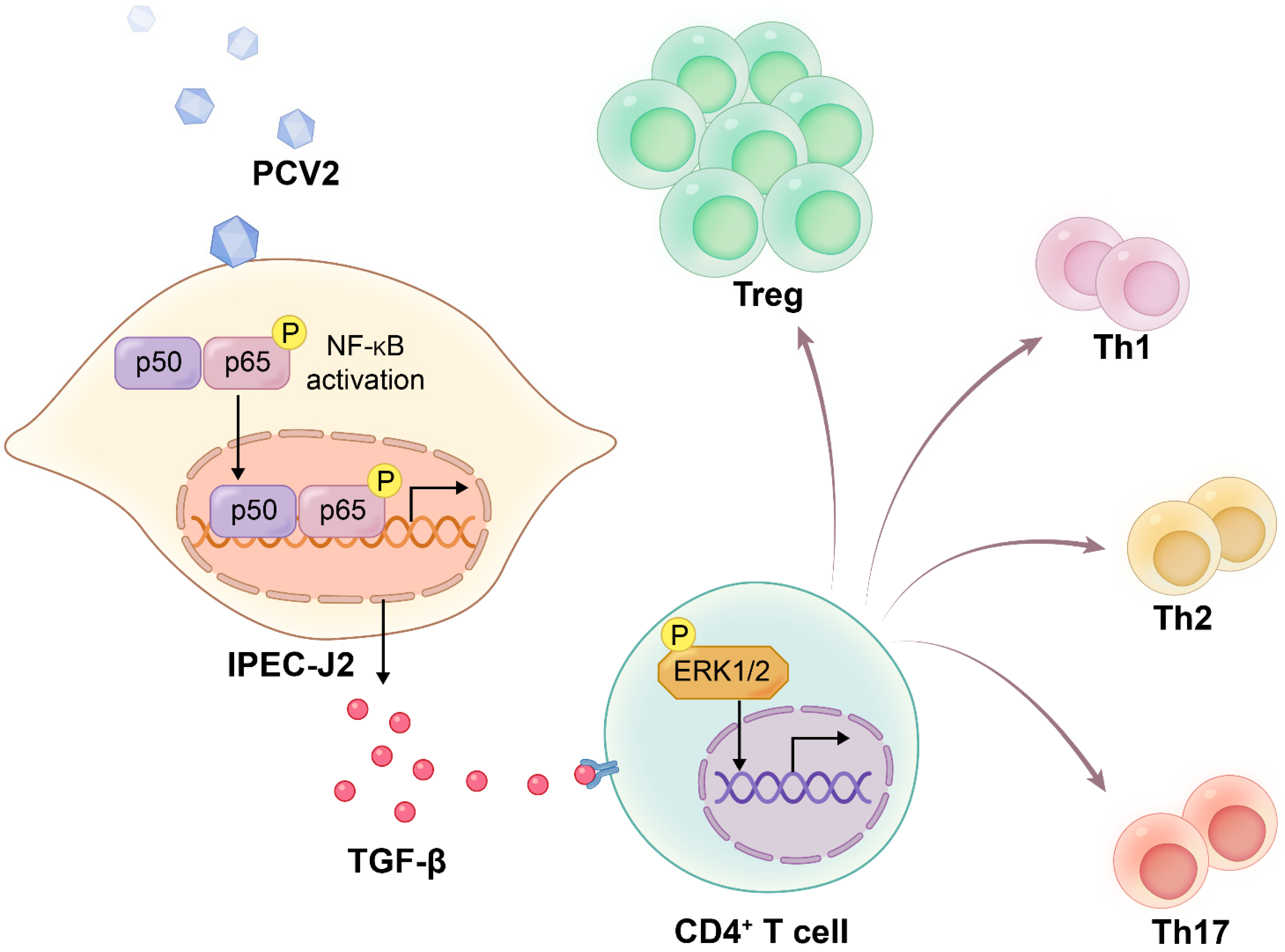
TGF-β from the Porcine Intestinal Cell Line IPEC-J2 Induced by Porcine Circovirus 2 Increases the Frequency of Treg Cells via the Activation of ERK (in CD4+ T Cells) and NF-κB (in IPEC-J2)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal, Cell and Virus
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMCs), Total T Cells and CD4+ T Cells
2.3. Co-Culture System
2.4. Inhibitor Assay
2.5. Extraction of Total RNA and Quantitative PCR
2.6. Confocal Microscopy
2.7. Flow Cytometry
2.8. Western Blot Assay
2.9. ELISA of TGF-β
2.10. Ethics Statement
2.11. Statistical Analysis
3. Results
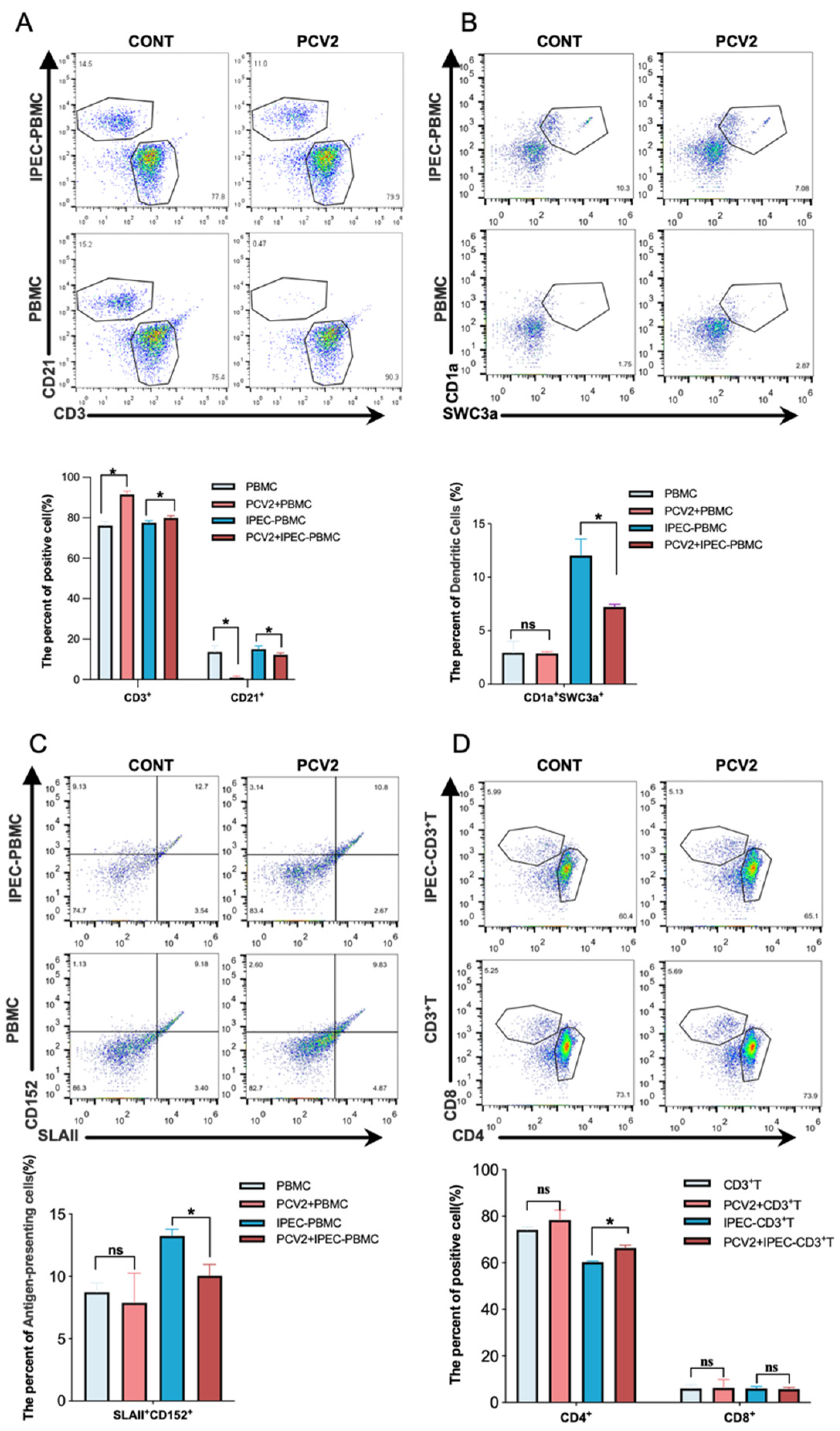
3.1. PCV2-Infected IPEC-J2 Leads to the Expansion of the Treg Cells Population
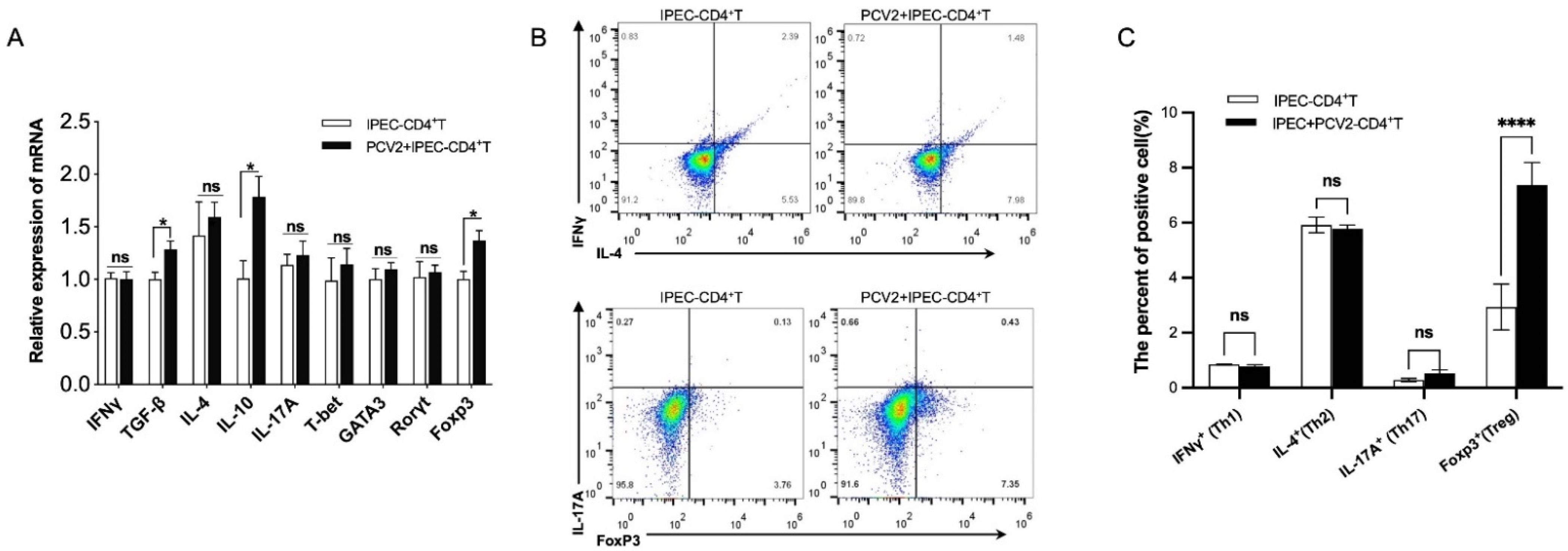
3.2. PCV2-Infected IPEC-J2 Enhanced the Frequency of Treg Cells by ERK Activation in CD4+ T
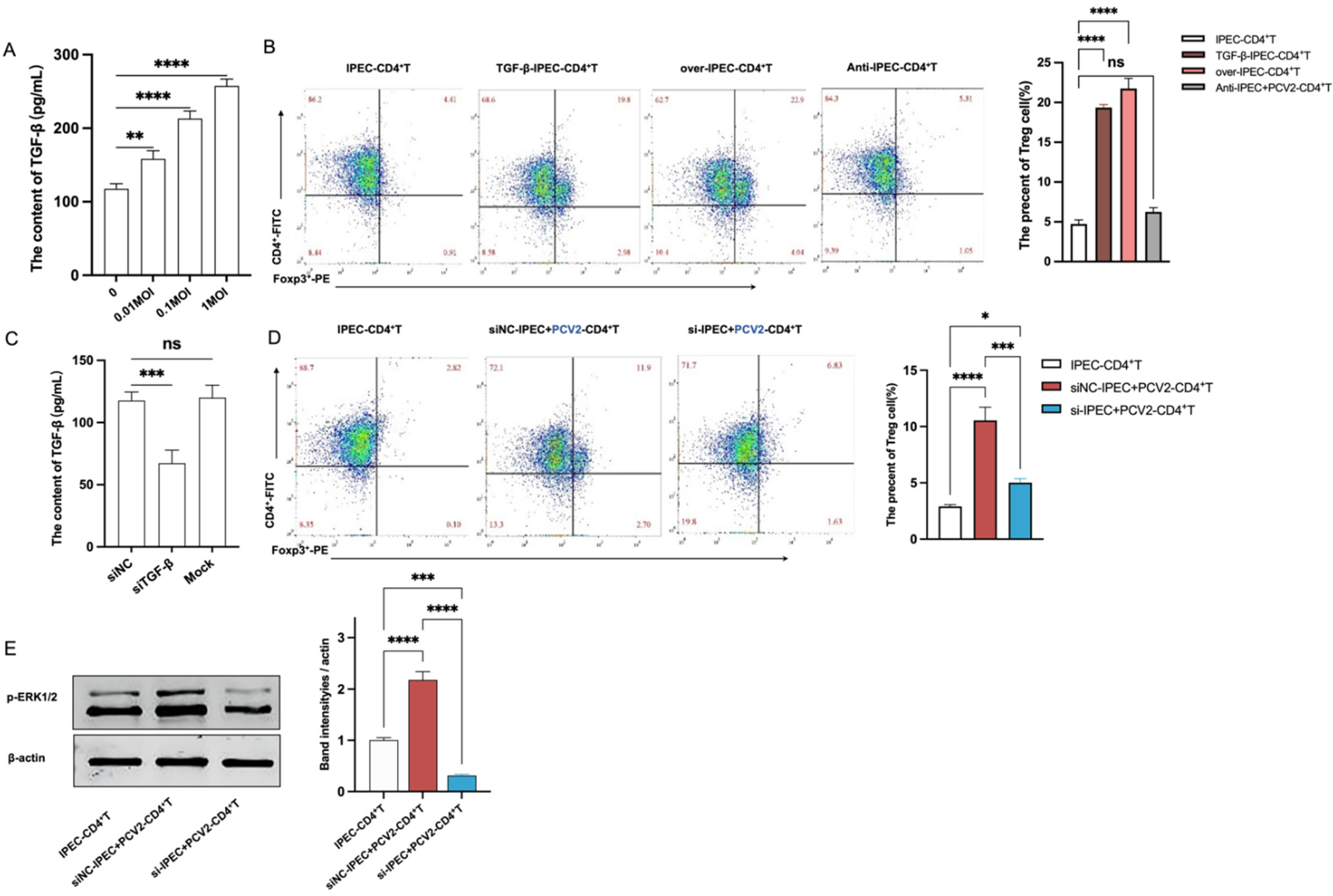
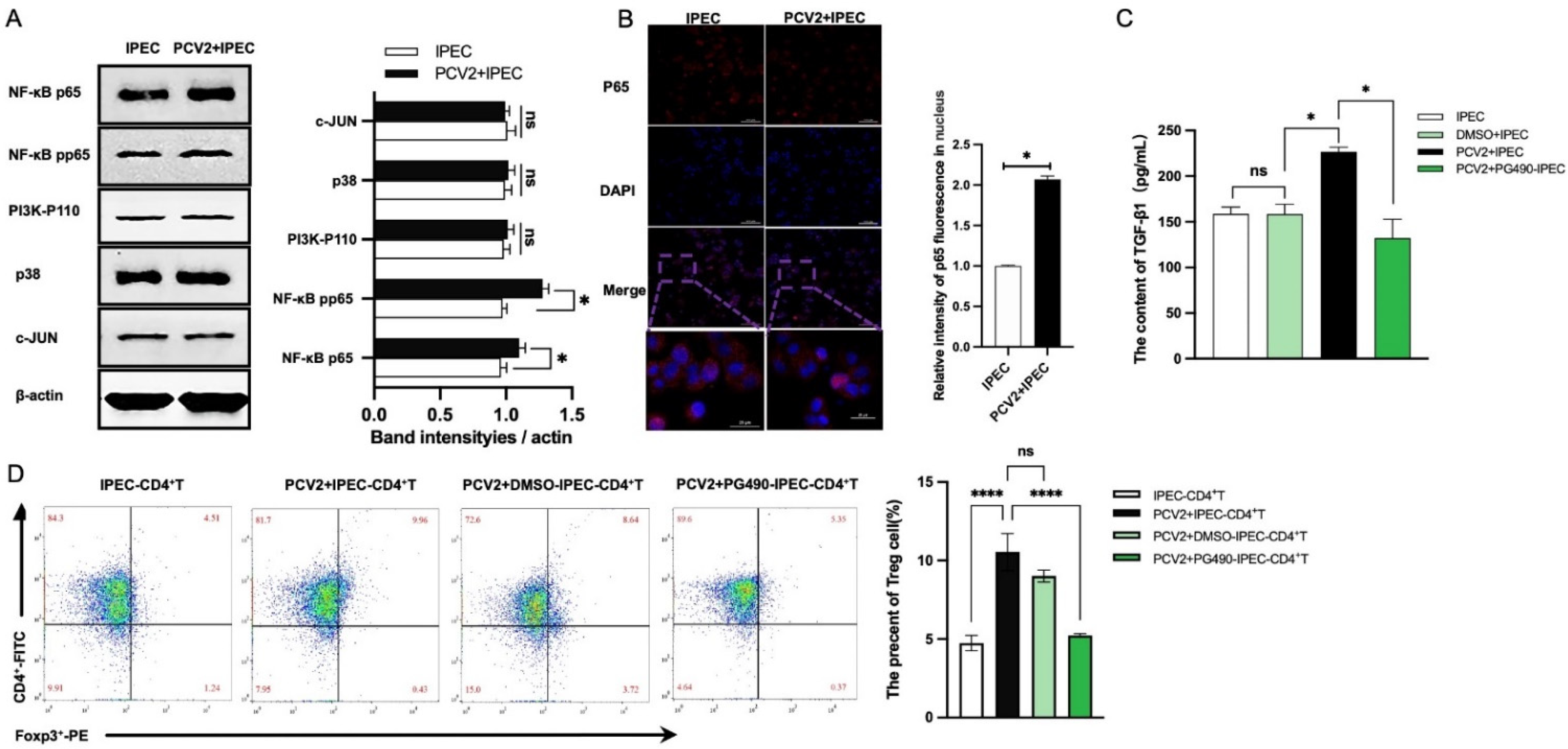
3.3. The Increase of Treg Cells Was Dependent on TGF-β from the PCV2-Infected IPEC-J2 and the NF-κB Activation in IPEC-J2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagan Tregaskis, P.; Staines, A.; Gordon, A.; Sheridan, P.; McMenamy, M.; Duffy, C.; Collins, P.J.; Mooney, M.H.; Lemon, K. Co-infection status of novel parvovirus’s (PPV2 to 4) with porcine circovirus 2 in porcine respiratory disease complex and porcine circovirus-associated disease from 1997 to 2012. Transbound. Emerg. Dis. 2021, 68, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Mateu, E. Immunology of porcine circovirus type 2 (PCV2). Virus Res. 2012, 164, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Segalés, J.; Domingo, M.; Mateu, E. Changes in CD4(+), CD8(+), CD4(+) CD8(+), and immunoglobulin M-positive peripheral blood mononuclear cells of postweaning multisystemic wasting syndrome-affected pigs and age-matched uninfected wasted and healthy pigs correlate with lesions and porcine circovirus type 2 load in lymphoid tissues. Clin. Diagn Lab. Immunol. 2002, 9, 236–242. [Google Scholar] [CrossRef]
- Grierson, S.S.; King, D.P.; Tucker, A.W.; Donadeu, M.; Mellencamp, M.A.; Haverson, K.; Banks, M.; Bailey, M. Ontogeny of systemic cellular immunity in the neonatal pig: Correlation with the development of post-weaning multisystemic wasting syndrome. Vet. Immunol. Immunopathol. 2007, 119, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef]
- Jacobson, M.; Andersson, M.; Lindberg, R.; Fossum, C.; Jensen-Waern, M. Microarray and cytokine analyses of field cases of pigs with diarrhoea. Vet. Microbiol. 2011, 153, 307–314. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Yang, Q. Infection of porcine circovirus 2 (PCV2) in intestinal porcine epithelial cell line (IPEC-J2) and interaction between PCV2 and IPEC-J2 microfilaments. Virol. J. 2014, 11, 193. [Google Scholar] [CrossRef]
- Liu, F.; Li, G.; Wen, K.; Bui, T.; Cao, D.; Zhang, Y.; Yuan, L. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010, 23, 135–149. [Google Scholar] [CrossRef]
- Opriessnig, T.; Madson, D.M.; Roof, M.; Layton, S.M.; Ramamoorthy, S.; Meng, X.J.; Halbur, P.G. Experimental Reproduction of Porcine Circovirus Type 2 (PCV2)-Associated Enteritis in Pigs Infected with PCV2 Alone or Concurrently with Lawsonia intracellularis or Salmonella typhimurium. J. Comp. Pathol. 2011, 145, 261–270. [Google Scholar] [CrossRef]
- Meng, X.J. Spread like a wildfire--the omnipresence of porcine circovirus type 2 (PCV2) and its ever-expanding association with diseases in pigs. Virus Res. Int. J. Mol. Cell. Virol. 2012, 164, 1–3. [Google Scholar] [CrossRef]
- Kim, J.; Ha, Y.; Jung, K.; Choi, C.; Chae, C. Enteritis associated with porcine circovirus 2 in pigs. Can. J. Vet. Res. Rev. Can. Rech. Vétérinaire 2004, 68, 218. [Google Scholar]
- Al-Shalan, H.; Hu, D.; Nicholls, P.K.; Greene, W.K.; Ma, B. Innervation and nerve-immune cell contacts in mouse Peyer’s patches. Histol. Histopathol. 2020, 35, 371–383. [Google Scholar] [PubMed]
- Stamataki, Z.; Swadling, L. The liver as an immunological barrier redefined by single-cell analysis. Immunology 2020, 160, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Li, Q.; Zou, Z.; Wang, Y.; Hou, X.; Zhang, Y.; Song, Q.; Zhou, S.; Li, H. The changes of immune-related molecules within the ileal mucosa of piglets infected with porcine circovirus type 2. J. Vet. Sci. 2020, 21, e78. [Google Scholar] [CrossRef]
- Andersson, M.; Ahlberg, V.; Jensen-Waern, M.; Fossum, C. Intestinal gene expression in pigs experimentally co-infected with PCV2 and PPV. Vet. Immunol. Immunopathol. 2011, 142, 72–80. [Google Scholar] [CrossRef]
- Lund, J.M.; Hsing, L.; Pham, T.T.; Rudensky, A.Y. Coordination of Early Protective Immunity to Viral Infection by Regulatory T Cells. Science 2008, 320, 1220–1224. [Google Scholar] [CrossRef]
- Zhu, J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb. Perspect. Biol. 2017, 10, a030338. [Google Scholar] [CrossRef]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef]
- Zhang, Y.; Nallaparaju, K.C.; Liu, X.; Jiao, H.; Reynolds, J.M.; Wang, Z.X.; Dong, C. MAPK phosphatase 7 regulates T cell differentiation via inhibiting ERK-mediated IL-2 expression. J. Immunol. 2015, 194, 3088–3095. [Google Scholar] [CrossRef]
- Di Mitri, D.; Sambucci, M.; Loiarro, M.; De Bardi, M.; Volpe, E.; Cencioni, M.T.; Gasperini, C.; Centonze, D.; Sette, C.; Akbar, A.N. The p38 mitogen-activated protein kinase cascade modulates T helper type 17 differentiation and functionality in multiple sclerosis. Immunology 2015, 146, 251–263. [Google Scholar] [CrossRef]
- Poli, A.; Fiume, R.; Mongiorgi, S.; Zaurito, A.; Sheth, B.; Vidalle, M.C.; Hamid, S.A.; Kimber, S.; Campagnoli, F.; Ratti, S.; et al. Exploring the controversial role of PI3K signalling in CD4(+) regulatory T (T-Reg) cells. Adv. Biol. Regul. 2020, 76, 100722. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lin, Z.; Wen, J.; Wu, K.; Xu, Y.; Zhang, Y.; Lu, G.; Xiao, W.; Ding, Y.; Jia, X.; et al. Astilbin promotes the induction of regulatory NK1.1(-) CD4(+) NKG2D(+) T cells through the PI3K, STAT3, and MAPK signaling pathways. Int. Immunopharmacol. 2020, 81, 106143. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; Zhang, D.; Zanvit, P.; Chia, C.; Zangarle-Murray, T.; Jin, W.; Wang, S.; Chen, W. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017, 46, 660–674. [Google Scholar] [CrossRef]
- Shevach, E.M.; Tran, D.Q.; Davidson, T.S.; Andersson, J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol. 2010, 38, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Cecere, T.E.; Meng, X.J.; Pelzer, K.; Todd, S.M.; Beach, N.M.; Ni, Y.Y.; Leroith, T. Co-infection of porcine dendritic cells with porcine circovirus type 2a (PCV2a) and genotype II porcine reproductive and respiratory syndrome virus (PRRSV) induces CD4(+)CD25(+)FoxP3(+) T cells in vitro. Vet. Microbiol. 2012, 160, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Wang, P.; Wang, L.; Sun, Y.; Liu, G.; Zhang, P.; Kang, L.; Jiang, S.; Jiang, Y. RNA-Seq Analysis Reveals Genes Underlying Different Disease Responses to Porcine Circovirus Type 2 in Pigs. PLoS ONE 2016, 11, e0155502. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, S.J.; Kim, S.R.; Min, K.H.; Lee, K.Y.; Choe, Y.H.; Hong, S.H.; Lee, Y.R.; Kim, J.S.; Hong, S.J.; et al. Inhibition of VEGF blocks TGF-beta1 production through a PI3K/Akt signalling pathway. Eur. Respir. J. 2008, 31, 523–531. [Google Scholar] [CrossRef]
- Li, Z.D.; Bork, J.P.; Krueger, B.; Patsenker, E.; Schulze-Krebs, A.; Hahn, E.G.; Schuppan, D. VEGF induces proliferation, migration, and TGF-beta1 expression in mouse glomerular endothelial cells via mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 2005, 334, 1049–1060. [Google Scholar] [CrossRef]
- He, J.; Du, Y.; Li, G.; Xiao, P.; Sun, X.; Song, W.; Lai, L.; Xia, M.; Zhang, J.; Wang, Q. Myeloid Fbxw7 Prevents Pulmonary Fibrosis by Suppressing TGF-β Production. Front. Immunol. 2021, 12, 760138. [Google Scholar] [CrossRef]
- Tang, F.; Wang, H.; Chen, E.; Bian, E.; Xu, Y.; Ji, X.; Yang, Z.; Hua, X.; Zhang, Y.; Zhao, B. LncRNA-ATB promotes TGF-β-induced glioma cells invasion through NF-κB and P38/MAPK pathway. J. Cell. Physiol. 2019, 234, 23302–23314. [Google Scholar] [CrossRef]
- Wu, W.; Chen, F.; Cui, X.; Yang, L.; Chen, J.; Zhao, J.; Huang, D.; Liu, J.; Yang, L.; Zeng, J.; et al. LncRNA NKILA suppresses TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer 2018, 143, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wu, X.; Wang, T.; Yang, X.; Wang, Z.; Niu, Y.; Zhao, X.; Liu, S.L.; Tong, D.; Huang, Y. Porcine Circovirus Type 2 Suppresses IL-12p40 Induction via Capsid/gC1qR-Mediated MicroRNAs and Signalings. J. Immunol. 2018, 201, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, X.; Ouyang, H. Interactions of porcine circovirus 2 with its hosts. Virus Genes 2016, 52, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Stambas, J.; Lu, C.; Tripp, R.A. Innate and adaptive immune responses in respiratory virus infection: Implications for the clinic. Expert Rev. Respir. Med. 2020, 14, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhai, N.; Wang, Z.; Song, H.; Yang, Y.; Cui, A.; Li, T.; Wang, G.; Niu, J.; Crispe, I.N.; et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut 2018, 67, 2035–2044. [Google Scholar] [CrossRef]
- Loh, L.; Koutsakos, M.; Kedzierska, K.; Hinks, T.S.C. Influenza A Virus-Infected Lung Epithelial Cell Co-Culture with Human Peripheral Blood Mononuclear Cells. Methods Mol. Biol. 2020, 2098, 141–147. [Google Scholar] [CrossRef]
- Autissier, P.; Soulas, C.; Burdo, T.H.; Williams, K.C. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytom. A 2010, 77, 410–419. [Google Scholar] [CrossRef]
- Raulf, M. T Cell: Primary Culture from Peripheral Blood. Methods Mol. Biol. 2019, 2020, 17–31. [Google Scholar] [CrossRef]
- Cabrera, R.; Tu, Z.; Xu, Y.; Firpi, R.J.; Rosen, H.R.; Liu, C.; Nelson, D.R. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 2004, 40, 1062–1071. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Savage, P.A.; Klawon, D.E.J.; Miller, C.H. Regulatory T Cell Development. Annu. Rev. Immunol. 2020, 38, 421–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, X.; Yu, S.; Ren, L.; Sun, X.; Long, Y.; Wang, X.; Lu, S.; Song, Y.; Sun, X.H.; et al. Up-regulated DNA-binding inhibitor Id3 promotes differentiation of regulatory T cell to influence antiviral immunity in chronic hepatitis B virus infection. Life Sci. 2021, 285, 119991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Li, H.; Su, W.; Xie, Y.; Pan, Y.; Chen, X.; Liang, D. Apremilast Regulates the Teff/Treg Balance to Ameliorate Uveitis via PI3K/AKT/FoxO1 Signaling Pathway. Front. Immunol. 2020, 11, 581673. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, W.; Le, Q. All-trans Retinoic Acid Regulates the Balance of Treg-Th17 Cells through ERK and P38 Signaling Pathway. Iran. J. Immunol. 2019, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yao, S.; Dann, S.M.; Qin, H.; Elson, C.O.; Cong, Y. ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 2013, 43, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, J.; Chen, Q.; Xu, X.; Gao, J.; Li, X.; Shao, Q.; Zhou, B.; Zhou, H.; Wei, S.; et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022, 39, 110986. [Google Scholar] [CrossRef]
- Yang, S.; Zafar Khan, M.U.; Liu, B.; Humza, M.; Yin, S.; Cai, J. In Vitro Analysis of TGF-β Signaling Modulation of Porcine Alveolar Macrophages in Porcine Circovirus Type 2b Infection. Vet. Sci. 2022, 9, 101. [Google Scholar] [CrossRef]
- Rowan, A.G.; Fletcher, J.M.; Ryan, E.J.; Moran, B.; Hegarty, J.E.; O’Farrelly, C.; Mills, K.H. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J. Immunol. 2008, 181, 4485–4494. [Google Scholar] [CrossRef]
- Li, H.; Zheng, H.W.; Chen, H.; Xing, Z.Z.; You, H.; Cong, M.; Jia, J.D. Hepatitis B virus particles preferably induce Kupffer cells to produce TGF-β1 over pro-inflammatory cytokines. Dig. Liver Dis. 2012, 44, 328–333. [Google Scholar] [CrossRef]

| Genes | Primer Sequence (5′-3′) |
|---|---|
| IFNγ | Forward:GAGCCAAATTGTCTCCTTCTACT |
| IL-4 | Reverse: CTGACTTCTCTTCCGCTTTCT |
| Forward: GCCGGGCCTCGACTGT | |
| IL-6 | Reverse: TCCGCTCAGGAGGCTCTTC |
| Forward:CTGGCAGAAAACAACTGAACC | |
| IL-10 | Reverse: TGATTCTCATCAAGCAGGTCTCC |
| Forward: CGGCGCTGTCATCAATTTCTG | |
| IL-17A | Reverse: CCCCTCTCTTGGAGCTTGCTA |
| Forward: CTCTCGTGAAGGCGGGAATC | |
| T-bet | Reverse: GTAATCTGAGGGCCGTCTGG |
| Forward: ACAAACCCGATATGGCTGAGA | |
| GATA3 | Reverse: CCTGCTTGCTTCTCCTGTTC |
| Forward:GCTCTACACAAAATGAAGGAC | |
| Rorγt | Reverse: TCGTTGTGGTTTGACAGTTTGC |
| Forward: TTCAGTACGTGGTGGAGTTC | |
| Foxp3 | Reverse: TGTGGTTGTCAGCGTTGTAG |
| Forward: TGCCATTCGCCACAACTT | |
| β-actin | Reverse: CCTGTCCATCCTTCTTTCCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Y.; Han, C.; Li, Q.; Hou, X.; Song, Q.; Zhou, S.; Li, H. TGF-β from the Porcine Intestinal Cell Line IPEC-J2 Induced by Porcine Circovirus 2 Increases the Frequency of Treg Cells via the Activation of ERK (in CD4+ T Cells) and NF-κB (in IPEC-J2). Viruses 2022, 14, 2466. https://doi.org/10.3390/v14112466
Liu X, Wang Y, Han C, Li Q, Hou X, Song Q, Zhou S, Li H. TGF-β from the Porcine Intestinal Cell Line IPEC-J2 Induced by Porcine Circovirus 2 Increases the Frequency of Treg Cells via the Activation of ERK (in CD4+ T Cells) and NF-κB (in IPEC-J2). Viruses. 2022; 14(11):2466. https://doi.org/10.3390/v14112466
Chicago/Turabian StyleLiu, Xuewei, Yang Wang, Cheng Han, Qiuming Li, Xiaolin Hou, Qinye Song, Shuanghai Zhou, and Huanrong Li. 2022. "TGF-β from the Porcine Intestinal Cell Line IPEC-J2 Induced by Porcine Circovirus 2 Increases the Frequency of Treg Cells via the Activation of ERK (in CD4+ T Cells) and NF-κB (in IPEC-J2)" Viruses 14, no. 11: 2466. https://doi.org/10.3390/v14112466
APA StyleLiu, X., Wang, Y., Han, C., Li, Q., Hou, X., Song, Q., Zhou, S., & Li, H. (2022). TGF-β from the Porcine Intestinal Cell Line IPEC-J2 Induced by Porcine Circovirus 2 Increases the Frequency of Treg Cells via the Activation of ERK (in CD4+ T Cells) and NF-κB (in IPEC-J2). Viruses, 14(11), 2466. https://doi.org/10.3390/v14112466





