Trajectories of CD4+/CD8+ T-Cells Ratio 96 Weeks after Switching to Dolutegravir-Based Two-Drug Regimens: Results from a Multicenter Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
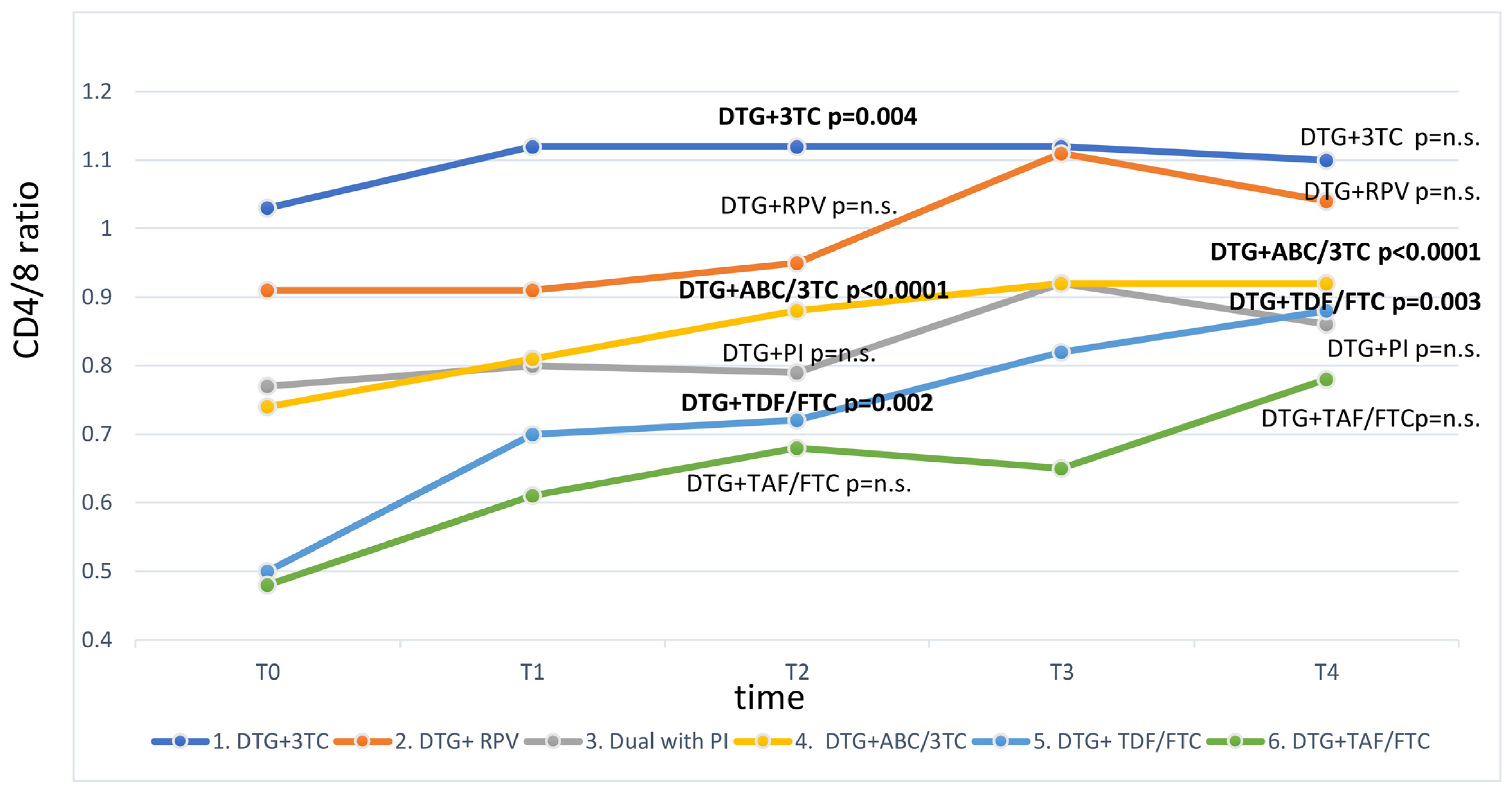
3. Results
Predictors of CD4/CD8 Restoration to ≥1.0
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antela, A.; Rivero, A.; Llibre, J.M.; Moreno, S.; RET Group. Redefining therapeutic success in HIV patients: An expert view. J. Antimicrob. Chemother. 2021, 76, 2501–2518. [Google Scholar] [CrossRef] [PubMed]
- Buell, K.G.; Chung, C.; Chaudhry, Z.; Puri, A.; Nawab, K.; Ravindran, R.P. Lifelong antiretroviral therapy or HIV cure: The benefits for the individual patient. AIDS Care 2016, 28, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Milic, J.; Marcotullio, S.; Mussini, C. A patient-centred approach to deprescribing antiretroviral therapy in people living with HIV. J. Antimicrob. Chemother. 2020, 75, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Llibre, J.M.; Hung, C.-C.; Brinson, C.; Castelli, F.; Girard, P.-M.; Kahl, L.P.; Blair, E.A.; Angelis, K.; Wynne, B.; Vandermeulen, K.; et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: Phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018, 391, 839–849. [Google Scholar] [CrossRef]
- Saracino, A.; Bruno, G.; Scudeller, L.; Volpe, A.; Caricato, P.; Ladisa, N.; Monno, L.; Angarano, G. Chronic Inflammation in a Long-Term Cohort of HIV-Infected Patients According to the Normalization of the CD4:CD8 Ratio. AIDS Res. Hum. Retroviruses 2014, 30, 1178–1184. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Pérez-Elias, M.J.; Dronda, F.; Casado, J.L.; Moreno, A.; Royuela, A.; Pérez-Molina, J.A.; Sainz, T.; Navas, E.; Hermida, J.M.; et al. Increased Risk of Serious Non-AIDS-Related Events in HIV-Infected Subjects on Antiretroviral Therapy Associated with a Low CD4/CD8 Ratio. PLoS ONE 2014, 9, e85798. [Google Scholar] [CrossRef]
- Trickey, A.; May, M.T.; Schommers, P.; Tate, J.; Ingle, S.M.; Guest, J.; Gill, M.J.; Zangerle, R.; Saag, M.; Reiss, P.; et al. CD4:CD8 Ratio and CD8 Count as Prognostic Markers for Mortality in Human Immunodeficiency Virus–Infected Patients on Antiretroviral Therapy: The Antiretroviral Therapy Cohort Collaboration (ART-CC). Clin. Infect. Dis. 2017, 65, 959–966. [Google Scholar] [CrossRef]
- Mussini, C.; Lorenzini, P.; Cozzi-Lepri, A.; Marchetti, G.; Rusconi, S.; Gori, A.; Nozza, S.; Lichtner, M.; Antinori, A.; Cossarizza, A.; et al. Switching to dual/monotherapy determines an increase in CD8+ in HIV-infected individuals: An observational cohort study. BMC Med. 2018, 16, 79. [Google Scholar] [CrossRef]
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.-C.; Rockstroh, J.K.; Girard, P.-M.; Sievers, J.; et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment–Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 83, 310–318. [Google Scholar] [CrossRef]
- Van Wyk, J.; Ait-Khaled, M.; Santos, J.; Scholten, S.; Wohlfeiler, M.; Ajana, F.; Jones, B.; Nascimento, M.-C.; Tenorio, A.R.; Smith, D.E.; et al. Brief Report: Improvement in Metabolic Health Parameters at Week 48 after Switching From a Tenofovir Alafenamide–Based 3- or 4-Drug Regimen to the 2-Drug Regimen of Dolutegravir/Lamivudine: The TANGO Study. J. Acquir. Immune Defic. Syndr. 2021, 87, 794–800. [Google Scholar] [CrossRef]
- Capetti, A.F.; Cossu, M.V.; Sterrantino, G.; Barbarini, G.; Di Giambenedetto, S.; De Socio, G.V.; Orofino, G.; Di Biagio, A.; Celesia, B.M.; Rusconi, S.; et al. Dolutegravir Plus Rilpivirine as a Switch Option in cART-Experienced Patients: 96-Week Data. Ann. Pharmacother. 2018, 52, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Ciccullo, A.; Baldin, G.; Capetti, A.; Borghi, V.; Sterrantino, G.; Latini, A.; Madeddu, G.; Celani, L.; Vignale, F.; Rossetti, B.; et al. Cohort profile: The Observational cohort for the study of DOlutegravir in Antiretroviral Combination REgimens (ODOACRE). BMJ Open 2019, 9, e029960. [Google Scholar] [CrossRef] [PubMed]
- Baldin, G.; Ciccullo, A.; Rusconi, S.; Capetti, A.; Sterrantino, G.; Colafigli, M.; D’Ettorre, G.; Giacometti, A.; Cossu, M.V.; Borghetti, A.; et al. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int. J. Antimicrob. Agents 2019, 54, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, P.; Martinelli, C.; Ricci, E.; Carradori, S.; Parruti, G.; Armignacco, O.; Magnani, C.; Quirino, T.; CISAI Group (Italian Coordinators for the Study of Allergies HIV Infection). An Italian Approach to Postmarketing Monitoring: Preliminary Results From the SCOLTA (Surveillance Cohort Long-Term Toxicity Antiretrovirals) Project on the Safety of Lopinavir/Ritonavir. J. Acquir. Immune Defic. Syndr. 2005, 39, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; De Vito, A.; Ricci, E.D.; Orofino, G.; Squillace, N.; Menzaghi, B.; Molteni, C.; Gulminetti, R.; De Socio, G.V.; Pellicanò, G.F.; et al. Durability of Dolutegravir-Based Regimens: A 5-Year Prospective Observational Study. AIDS Patient Care STDs 2021, 35, 342–353. [Google Scholar] [CrossRef]
- Taramasso, L.; Orofino, G.; Ricci, E.; Menzaghi, B.; De Socio, G.V.; Squillace, N.; Madeddu, G.; Vichi, F.; Celesia, B.M.; Molteni, C.; et al. Reversibility of Central Nervous System Adverse Events in Course of Art. Viruses 2022, 14, 1028. [Google Scholar] [CrossRef]
- Davy-Mendez, T.; Napravnik, S.; Zakharova, O.; Kuruc, J.; Gay, C.; Hicks, C.B.; Mcgee, K.S.; Eron, J.J. Acute HIV Infection and CD4/CD8 Ratio Normalization After Antiretroviral Therapy Initiation. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 79, 510–518. [Google Scholar] [CrossRef]
- Sainz, T.; Serrano-Villar, S.; Díaz, L.; Tomé, M.I.G.; Gurbindo, M.D.; de José, M.I.; Mellado, M.J.; Ramos, J.T.; Zamora, J.; Moreno, S.; et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013, 27, 1513–1516. [Google Scholar] [CrossRef]
- Mondi, A.; Cozzi-Lepri, A.; Tavelli, A.; Rusconi, S.; Vichi, F.; Ceccherini-Silberstein, F.; Calcagno, A.; De Luca, A.; Maggiolo, F.; Marchetti, G.; et al. Effectiveness of dolutegravir-based regimens as either first-line or switch antiretroviral therapy: Data from the Icona cohort. J. Int. AIDS Soc. 2019, 22, e25227. [Google Scholar] [CrossRef]
- Masiá, M.; Padilla, S.; Barber, X.; Sanchis, M.; Terol, G.; Lidón, F.; Gutiérrez, F. Comparative Impact of Suppressive Antiretroviral Regimens on the CD4/CD8 T-Cell Ratio: A Cohort Study. Medicine 2016, 95, e3108. [Google Scholar] [CrossRef]
- Hocqueloux, L.; Avettand-Fenoel, V.; Jacquot, S.; Prazuck, T.; Legac, E.; Mélard, A.; Niang, M.; Mille, C.; Le Moal, G.; Viard, J.-P.; et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J. Antimicrob. Chemother. 2013, 68, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef] [PubMed]
- Mussini, C.; Lorenzini, P.; Cozzi-Lepri, A.; Lapadula, G.; Marchetti, G.; Nicastri, E.; Cingolani, A.; Lichtner, M.; Antinori, A.; Gori, A.; et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: An observational cohort study. Lancet HIV 2015, 2, e98–e106. [Google Scholar] [CrossRef]
- Monsalvo, M.; Vallejo, A.; Fontecha, M.; Vivancos, M.J.; Vizcarra, P.; Casado, J.L. CD4/CD8 ratio improvement in HIV-1-infected patients receiving dual antiretroviral treatment. Int. J. STD AIDS 2019, 30, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.; Durant, J.; Fabre, R.; Ticchioni, M.; Lotte, L.; Sindt, A.; Puchois, A.; De Monte, A.; Cezar, R.; Corbeau, P.; et al. Switching to a Dual-Drug Regimen in HIV-Infected Patients Could Be Associated With Macrophage Activation? Front. Med. 2021, 8, 712880. [Google Scholar] [CrossRef] [PubMed]
- Dyavar, S.R.; Gautam, N.; Podany, A.T.; Winchester, L.C.; Weinhold, J.A.; Mykris, T.M.; Campbell, K.M.; Alnouti, Y.; Fletcher, C.V. Assessing the lymphoid tissue bioavailability of antiretrovirals in human primary lymphoid endothelial cells and in mice. J. Antimicrob. Chemother. 2019, 74, 2974–2978. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef]
- Caby, F.; Guihot, A.; Lambert-Niclot, S.; Guiguet, M.; Boutolleau, D.; Agher, R.; Valantin, M.-A.; Tubiana, R.; Calvez, V.; Marcelin, A.-G.; et al. Determinants of a Low CD4/CD8 Ratio in HIV-1–Infected Individuals Despite Long-term Viral Suppression. Clin. Infect. Dis. 2016, 62, 1297–1303. [Google Scholar] [CrossRef]
- Smith, D.; Nakazawa, M.; Freeman, M.L.; Anderson, C.M.; de Oliveira, M.F.; Little, S.J.; Gianella, S. Asymptomatic CMV Replication During Early Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower CD4/CD8 Ratio During HIV Treatment. Clin. Infect. Dis. 2016, 63, 1517–1524. [Google Scholar] [CrossRef]
| Total | DTG + 3TC | DTG + PI | DTG + RPV | DTG + TDF/FTC | DTG + TAF/FTC | DTG + ABC/3TC | p | |
|---|---|---|---|---|---|---|---|---|
| N = 533 | N = 120 (22.5%) | N = 38 (7.1%) | N = 67 (12.6%) | N = 49 (9.2%) | N = 27 (5.1%) | N = 232 (43.5%) | ||
| Age (years), mean ± SD | 51.0 ± 10.9 | 50.7 ± 11.1 | 53.4 ± 8.6 | 53.4 ± 11.1 | 48.9 ± 10.4 | 50.6 ± 12.3 | 50.4 ± 10.8 | 0.13 |
| Sex F, n (%) | 145 (27.2) | 28 (23.3) | 6 (15.8) | 27 (40.3) | 14 (28.5) | 7 (25.9) | 63 (27.1) | 0.10 |
| Caucasian ethnicity, n (%) | 492 (92.3) | 111 (92.5) | 34 (89.5) | 63 (94.0) | 48 (97.9) | 23 (85.1) | 213 (91.8) | 0.42 |
| Risk Factor for HIV acquisition, n (%) | ||||||||
| Heterosexual | 210 (39.4) | 49 (40.8) | 13 (34.2) | 29 (43.2) | 16 (32.6) | 9 (33.3) | 94 (40.5) | <0.0001 |
| MSM | 165 (31.0) | 49 (40.8) | 11 (29.0) | 16 (23.8) | 9 (18.3) | 12 (44.4) | 68 (29.3) | |
| IDU | 94 (17.6) | 14 (11.6) | 6 (15.8) | 20 (29.8) | 10 (20.4) | 6 (22.2) | 38 (16.3) | |
| Other | 64 (12.0) | 8 (6.6) | 8 (21.0) | 2 (2.9) | 14 (28.5) | 0 (0.0) | 32 (13.7) | |
| BMI (Kg/m2), n (%) | ||||||||
| ≤25.0 | 269 (56.3) | 52 (51.5) | 20 (58.8) | 34 (56.7) | 26 (57.8) | 9 (37.5) | 128 (59.8) | 0.31 |
| >25.0–30.0 | 168 (35.2) | 39 (38.6) | 13 (38.2) | 21 (35.0) | 12 (26.7) | 11 (45.8) | 72 (33.6) | |
| >30.0 | 41 (8.6) | 10 (9.9) | 1 (2.9) | 5 (8.3) | 7 (15.6) | 4 (16.7) | 14 (6.5) | |
| HBsAg positive, n (%) | 14 (2.8) | 3 (2.5) | 0 (0.0) | 0 (0.0) | 3 (6.8) | 4 (15.3) | 4 (1.8) | 0.0007 |
| HCV coinfection, n (%) | 127 (24.5) | 22 (18.8) | 8 (21.1) | 22 (33.8) | 14 (30.4) | 6 (23.0) | 55 (24.1) | 0.29 |
| Cirrhosis | 6 (1.1) | 0 | 0 | 1 (1.5) | 3 (1.3) | 0 | 2 (7.4) | 0.03 |
| CDC stage, n (%) | ||||||||
| A | 262 (49.2) | 71 (59.2) | 20 (52.6) | 28 (41.8) | 24 (49.0) | 13 (48.2) | 106 (45.7) | 0.004 |
| B | 166 (31.1) | 37 (30.8) | 8 (21.0) | 29 (43.3) | 13 (26.5) | 9 (33.3) | 70 (30.2) | |
| C | 105 (19.7) | 12 (10.0) | 10 (26.3) | 10 (14.9) | 12 (24.5) | 5 (18.5) | 56 (24.1) | |
| CD4 (cells/mL), median (IQR) | 688 (466–908) | 735 (594–1001) | 644 (407–904) | 684 (490–841) | 555 (300–867) | 652 (429–869) | 656 (455–900) | 0.01 |
| CD8 (cells/mL), median (IQR) | 816 (620–1120) | 786 (614–1018) | 778 (618–1193) | 816 (614–1120) | 922 (615–1177) | 838 (744–1033) | 834 (620–1175) | 0.40 |
| CD4/CD8, mean ± SD | 0.92 ± 0.58 | 1.13 ± 0.63 | 0.90 ± 0.67 | 0.95 ± 0.62 | 0.74 ± 0.47 | 0.88 ± 0.64 | 0.86 ± 0.50 | 0.0003 |
| Nadir CD4 (cells/mL), median (IQR) (N = 86) | 263 (171–368) | 328 (211–447) | - | 201 (58–263) | - | 250 (99–294) | 214 (161–311) | 0.06 |
| Diabetes, n (%) | 29 (5.4) | 5 (4.17) | 1 (2.6) | 2 (2.9) | 5 (10.2) | 3 (11.1) | 13 (5.6) | 0.35 |
| Hypertension, n (%) | 126 (23.6) | 31 (25.8) | 8 (21.0) | 13 (19.4) | 7 (14.2) | 5 (18.5) | 62 (26.7) | 0.40 |
| Other cardiovascular diseases, n (%) | 30 (5.6) | 9 (7.5) | 6 (15.8) | 4 (6.0) | 8 (3.4) | 2 (4.1) | 1 (3.7) | 0.06 |
| Malignancies, n (%) | 15 (2.8) | 1 (0.8) | 1 (2.6) | 5 (7.5) | 6 (2.6) | 2 (4.1) | 0 | 0.15 |
| Auto-immune diseases, n (%) | 9 (1.7) | 1 (0.8) | 2 (5.3) | 0 | 5 (2.2) | 1 (2.0) | 0 | 0.37 |
| eGFR < 60 (mL/min), n (%) | 44 (8.2) | 9 (7.5) | 3 (8.1) | 8 (11.9) | 3 (6.1) | 3 (11.1) | 18 (7.7) | 0.86 |
| DTG + 3TC | DTG + PI | DTG + RPV | DTG + TDF/FTC | DTG + TAF/FTC | DTG + ABC/3TC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| CD4 | ||||||||||||||||||
| T1-T0 | 120 | 45 | 183 | 38 | −1 | 156 | 67 | −1 | 192 | 49 | 82 | 313 | 27 | 12 | 170 | 232 | 38 | 198 |
| T2-T0 | 93 | 32 | 237 | 36 | 14 | 179 | 51 | 19 | 174 | 43 | 89 | 202 | 16 | −39 | 174 | 199 | 76 | 218 |
| T3-T0 | 68 | 55 | 231 | 32 | 69 | 215 | 35 | 25 | 224 | 44 | 146 | 333 | 7 | 54 | 314 | 160 | 94 | 259 |
| T4-T0 | 40 | 127 | 268 | 30 | 39 | 179 | 32 | 8 | 219 | 36 | 98 | 261 | 5 | 44 | 330 | 142 | 145 | 273 |
| CD8 | ||||||||||||||||||
| T1-T0 | 120 | 0 | 200 | 38 | −50 | 216 | 67 | 8 | 258 | 49 | 27 | 328 | 27 | −8 | 272 | 232 | 19 | 266 |
| T2-T0 | 91 | −11 | 316 | 33 | −19 | 262 | 48 | 11 | 221 | 41 | 34 | 276 | 16 | 62 | 348 | 193 | −3 | 265 |
| T3-T0 | 67 | 53 | 466 | 31 | 150 | 369 | 33 | 17 | 493 | 40 | 375 | 623 | 6 | 200 | 371 | 156 | 284 | 462 |
| T4-T0 | 40 | 201 | 449 | 29 | 221 | 342 | 28 | 12 | 393 | 34 | 268 | 448 | 5 | 123 | 385 | 135 | 332 | 509 |
| CD4/CD8 | ||||||||||||||||||
| T1-T0 | 120 | 0.05 | 0.22 | 38 | −0.01 | 0.2 | 67 | −0.03 | 0.37 | 49 | 0.11 | 0.36 | 27 | −0.05 | 0.37 | 232 | 0.02 | 0.23 |
| T2-T0 | 91 | 0.08 | 0.26 | 33 | −0.01 | 0.21 | 48 | −0.04 | 0.5 | 41 | 0.10 | 0.19 | 16 | −0.04 | 0.27 | 193 | 0.08 | 0.25 |
| T3-T0 | 67 | 0.11 | 0.32 | 31 | 0.02 | 0.4 | 33 | 0.13 | 0.25 | 40 | 0.16 | 0.39 | 6 | 0.23 | 0.51 | 155 | 0.08 | 0.30 |
| T4-T0 | 40 | 0.08 | 0.45 | 29 | 0.07 | 0.41 | 28 | 0.08 | 0.25 | 33 | 0.16 | 0.28 | 5 | 0.34 | 0.59 | 135 | 0.10 | 0.30 |
| Variable | CD4/CD8 < 1.0 (n = 241, 70.5%) | CD4/CD8 ≥ 1.0 (n = 101, 29.5%) | p |
|---|---|---|---|
| Baseline CD4/CD8 ratio, mean ± SD | 0.55 ± 0.22 | 0.75 ± 0.23 | <0.0001 |
| Age, years, mean ± SD | 51.7 ± 10.8 | 48.8 ± 12.4 | 0.03 |
| Sex, n (%) | |||
| F | 55 (62.5) | 33 (37.5) | |
| M | 186 (73.2) | 68 (67.3) | 0.058 |
| Caucasian ethnicity, n (%) | 220 (71.0) | 90 (29.0) | 0.53 |
| Risk factor for HIV acquisition, n (%) | |||
| Heterosexual | 94 (70.2) | 40 (29.8) | |
| MSM | 70 (68.6) | 32 (31.4) | |
| IDU | 52 (80.0) | 13 (20.0) | |
| Other | 25 (61.0) | 16 (39.0) | 0.19 |
| HCV coinfection, n (%) | |||
| HCV RNA − | 48 (76.2) | 15 (23.8) | |
| HCV RNA + | 20 (87.0) | 3 (13.0) | 0.30 |
| HBsAg positive, n (%) | 7 (63.6) | 4 (36.4) | 0.54 |
| Time on ART (years), median (IQR) | 10.7 (4.5–18.8) | 10.2 (4.4–16.5) | 0.55 |
| CDC stage, n (%) | |||
| A | 93 (63.3) | 54 (36.7) | |
| B | 78 (69.6) | 34 (30.4) | |
| C | 70 (84.3) | 13 (15.7) | 0.001 |
| CD4 (cells/mm3), n (%) | |||
| <250 | 19 (73.1) | 7 (26.9) | |
| 250–499 | 89 (78.1) | 25 (21.9) | |
| 500–749 | 73 (65.8) | 38 (34.2) | |
| ≥750 | 60 (65.9) | 31 (34.1) | 0.07 |
| eGFR (mL/min), n (%) | |||
| ≥90 | 98 (64.5) | 54 (35.5) | |
| 80–89 | 37 (68.5) | 17 (31.5) | |
| 70–79 | 40 (72.7) | 15 (27.3) | |
| 60–69 | 37 (77.1) | 11 (22.9) | |
| ≤60 | 29 (87.9) | 4 (12.1) | 0.003 |
| Regimen, n (%) | |||
| DTG + 3TC | 35 (60.3) | 23 (39.7) | |
| DTG + RPV | 19 (70.4) | 8 (29.6) | |
| DTG + PI | 35 (83.3) | 7 (16.7) | |
| DTG + TDF/FTC | 24 (66.7) | 12 (33.3) | |
| DTG + TAF/FTC | 16 (80.0) | 4 (20.0) | |
| DTG + ABC/3TC | 112 (70.4) | 47 (29.6) | 0.20 |
| Variable | Crude HR | 95% CI | p | Adjusted HR * | 95% CI | p |
|---|---|---|---|---|---|---|
| Sex F (ref. M) | 1.55 | 1.02–2.35 | 0.04 | 1.05 | 0.67–1.65 | 0.82 |
| Age (by 1 year) | 0.98 | 0.96–1.00 | 0.04 | |||
| Age (ref. < 50 years) | 0.66 | 0.45–0.97 | 0.04 | 0.84 | 0.55–1.26 | 0.39 |
| Risk factor for HIV (ref. hetero) | ||||||
| MSM | 1.12 | 0.71–1.79 | 0.62 | |||
| IDU | 0.72 | 0.38–1.35 | 0.31 | |||
| Other | 1.12 | 0.63–2.01 | 0.69 | |||
| BMI (Kg/m2) (by 1) | 1.00 | 0.95–1.06 | 0.92 | |||
| BMI (Kg/m2) (ref. ≤ 25.0) | ||||||
| >25.0–30.0 | 0.90 | 0.56–1.44 | 0.65 | |||
| >30.0 | 1.12 | 0.58–2.15 | 0.74 | |||
| Ethnicity (ref. Caucasian) | 1.35 | 0.72–2.52 | 0.35 | |||
| HCV coinfection (ref. N) | ||||||
| HCV-Ab+/HCV RNA - | 0.91 | 0.53–1.58 | 0.75 | |||
| HCV-Ab+/HCV RNA + | 0.36 | 0.11–1.13 | 0.08 | |||
| HBsAg positive (ref. HBsAg negative) | 1.26 | 0.46–3.43 | 0.65 | |||
| Years of ART (by 1 year) | 1.01 | 0.99–1.04 | 0.32 | |||
| CDC stage (ref. A) | ||||||
| B | 0.90 | 0.58–1.38 | 0.63 | 0.95 | 0.61–1.48 | 0.82 |
| C | 0.34 | 0.18–0.62 | 0.0005 | 0.44 | 0.24–0.82 | 0.009 |
| CD4 (cells/mm3) (ref. < 250) | ||||||
| 250–499 | 1.03 | 0.45–2.39 | 0.94 | |||
| 500–749 | 1.98 | 0.88–4.44 | 0.10 | |||
| ≥750 | 2.07 | 0.91–4.71 | 0.08 | |||
| INSTI in previous regimen | 0.57 | 0.32–1.00 | 0.051 | 0.54 | 0.30–0.97 | 0.04 |
| Baseline CD4/CD8 ratio (by 0.01) | 1.04 | 1.03–1.05 | <0.0001 | 1.04 | 1.03–1.06 | <0.0001 |
| eGFR (mL/min) (ref. ≥ 90) | ||||||
| 80–89 | 1.01 | 0.58–1.74 | 0.98 | 0.69 | 0.40–1.21 | 0.20 |
| 70–79 | 0.79 | 0.44–1.40 | 0.42 | 0.70 | 0.39–1.26 | 0.23 |
| 60–69 | 0.59 | 0.31–1.13 | 0.11 | 0.48 | 0.24–0.93 | 0.03 |
| ≤60 | 0.35 | 0.13–0.97 | 0.04 | 0.34 | 0.12–0.96 | 0.04 |
| Regimen (ref. DTG + ABC/3TC) | ||||||
| DTG + PI | 0.82 | 0.39–1.74 | 0.60 | 0.85 | 0.39–1.84 | 0.68 |
| DTG + 3TC | 1.91 | 1.15–3.17 | 0.01 | 1.34 | 0.80–2.23 | 0.27 |
| DTG + RPV | 0.74 | 0.34–1.64 | 0.46 | 0.55 | 0.24–1.54 | 0.15 |
| DTG + TDF/FTC | 0.94 | 0.50–1.77 | 0.84 | 0.92 | 0.48–1.78 | 0.79 |
| DTG + TAF/FTC | 1.57 | 0.56–4.40 | 0.40 | 0.97 | 0.34–2.73 | 0.95 |
| Variable | Crude HR | 95% CI | p | Adjusted HR * | 95% CI | p |
|---|---|---|---|---|---|---|
| Sex F (ref. M) | 1.12 | 0.56–2.26 | 0.75 | |||
| Age (by 1 year) | 0.98 | 0.95–1.01 | 0.20 | |||
| Age (ref. < 50 years) | 0.68 | 0.35–1.29 | 0.23 | |||
| Risk factor for HIV (ref. hetero) | ||||||
| MSM | 1.62 | 0.79–3.32 | 0.19 | |||
| IDU | 0.51 | 0.17–1.54 | 0.23 | |||
| Other | 0.95 | 0.32–2.87 | 0.93 | |||
| BMI (Kg/m2) (by 1) | 1.01 | 0.92–1.11 | 0.86 | |||
| BMI (Kg/m2) (ref. ≤ 25.0) | ||||||
| >25.0–30.0 | 0.95 | 0.44–2.04 | 0.89 | |||
| >30.0 | 1.18 | 0.40–3.56 | 0.76 | |||
| Ethnicity (ref. Caucasian) | 1.39 | 0.54–3.57 | 0.49 | |||
| HCV-Ab- (ref.) | ||||||
| HCV-Ab+/HCV RNA − | 0.77 | 0.34–1.74 | 0.53 | |||
| HCV-Ab+/HCV RNA + | n.e. | n.e. | n.e. | |||
| HBsAg (ref. HBsAg negative) | 1.10 | 0.15–8.09 | 0.92 | |||
| Years of ART (by 1 year) | 0.98 | 0.94–1.02 | 0.27 | |||
| INSTI in previous regimen | 0.85 | 0.40–1.80 | 0.67 | |||
| Baseline CD4/CD8 ratio (by 0.01) | 1.05 | 1.03–1.08 | <0.0001 | 1.05 | 1.03–1.08 | <0.0001 |
| CDC stage (ref. A) | ||||||
| B | 0.73 | 0.36–1.46 | 0.37 | 1.03 | 0.50–2.11 | 0.94 |
| C | 0.28 | 0.10–0.82 | 0.02 | 0.52 | 0.18–1.57 | 0.25 |
| CD4 (cells/mm3) (ref. < 250) | ||||||
| 250–499 | 0.28 | 0.06–1.37 | 0.12 | |||
| 500–749 | 1.03 | 0.24–4.41 | 0.97 | |||
| ≥750 | 0.88 | 0.19–4.05 | 0.87 | |||
| eGFR (mL/min) (ref. ≥ 90) | ||||||
| 80–89 | 1.59 | 0.72–3.52 | 0.25 | |||
| 70–79 | 0.92 | 0.36–2.35 | 0.86 | |||
| 60–69 | 0.62 | 0.20–1.86 | 0.39 | |||
| ≤60 | 0.36 | 0.08–1.55 | 0.17 | |||
| Regimen (ref. DTG + 3TC) | ||||||
| DTG + PI | 0.43 | 0.19–0.99 | 0.04 | 0.59 | 0.25–1.40 | 0.23 |
| DTG + RPV | 0.38 | 0.16–0.91 | 0.02 | 0.38 | 0.16–0.95 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taramasso, L.; Falletta, A.; Ricci, E.; Orofino, G.; Squillace, N.; Menzaghi, B.; De Socio, G.V.; Molteni, C.; Pellicanò, G.F.; Gulminetti, R.; et al. Trajectories of CD4+/CD8+ T-Cells Ratio 96 Weeks after Switching to Dolutegravir-Based Two-Drug Regimens: Results from a Multicenter Prospective Cohort Study. Viruses 2022, 14, 2315. https://doi.org/10.3390/v14112315
Taramasso L, Falletta A, Ricci E, Orofino G, Squillace N, Menzaghi B, De Socio GV, Molteni C, Pellicanò GF, Gulminetti R, et al. Trajectories of CD4+/CD8+ T-Cells Ratio 96 Weeks after Switching to Dolutegravir-Based Two-Drug Regimens: Results from a Multicenter Prospective Cohort Study. Viruses. 2022; 14(11):2315. https://doi.org/10.3390/v14112315
Chicago/Turabian StyleTaramasso, Lucia, Antonio Falletta, Elena Ricci, Giancarlo Orofino, Nicola Squillace, Barbara Menzaghi, Giuseppe Vittorio De Socio, Chiara Molteni, Giovanni Francesco Pellicanò, Roberto Gulminetti, and et al. 2022. "Trajectories of CD4+/CD8+ T-Cells Ratio 96 Weeks after Switching to Dolutegravir-Based Two-Drug Regimens: Results from a Multicenter Prospective Cohort Study" Viruses 14, no. 11: 2315. https://doi.org/10.3390/v14112315
APA StyleTaramasso, L., Falletta, A., Ricci, E., Orofino, G., Squillace, N., Menzaghi, B., De Socio, G. V., Molteni, C., Pellicanò, G. F., Gulminetti, R., Madeddu, G., Sarchi, E., Vichi, F., Celesia, B. M., Bonfanti, P., & Di Biagio, A., on behalf of the CISAI Study Group. (2022). Trajectories of CD4+/CD8+ T-Cells Ratio 96 Weeks after Switching to Dolutegravir-Based Two-Drug Regimens: Results from a Multicenter Prospective Cohort Study. Viruses, 14(11), 2315. https://doi.org/10.3390/v14112315








