Brazilian Populations of Aedes aegypti Resistant to Pyriproxyfen Exhibit Lower Susceptibility to Infection with Zika Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
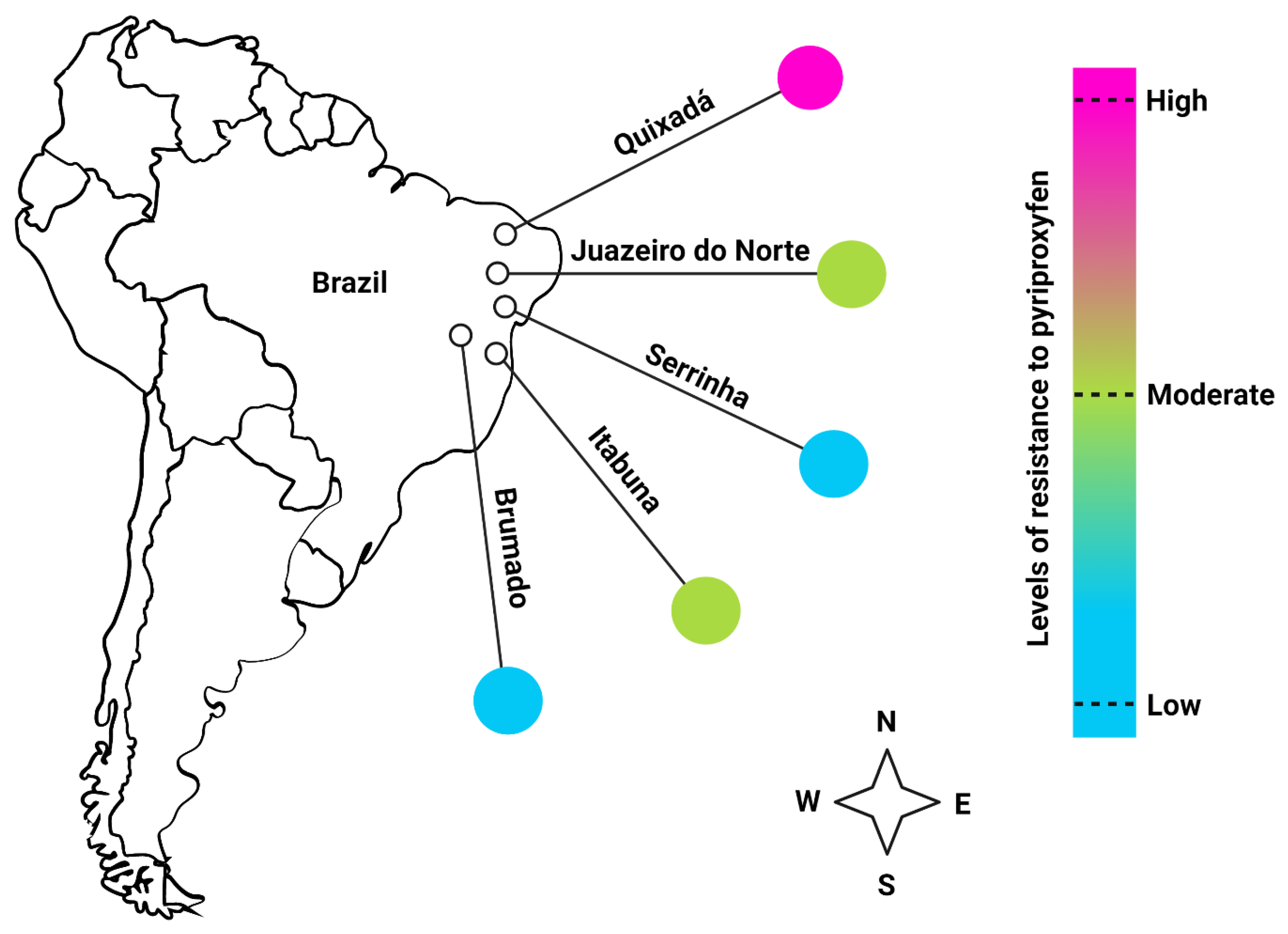
2.2. Mosquito Populations
2.3. Zika Virus and Cells
2.4. Zika Virus-Infected Blood and per os Infection
2.5. Cationic-(Q)-Paper and Saliva Collection
2.6. Ribonucleic Acid Extraction and Real-Time PCR for Zika Virus Quantification
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Slavov, S.N.; Otaguiri, K.K.; Kashima, S.; Covas, D.T. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz. J. Med. Biol. Res. 2016, 49, e5420. [Google Scholar] [CrossRef] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef]
- Besnard, M.; Lastere, S.; Teissier, A.; Cao-Lormeau, V.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014, 19, 20751. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Peixoto, T.M.; Siqueira, A.M.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Pupella, S.; Vaglio, S.; Liumbruno, G.M.; Grazzini, G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus. 2016, 14, 95–100. [Google Scholar] [CrossRef]
- Lessler, J.; Chaisson, L.H.; Kucirka, L.M.; Bi, Q.; Grantz, K.; Salje, H.; Carcelen, A.C.; Ott, C.T.; Sheffield, J.S.; Ferguson, N.M.; et al. Assessing the global threat from Zika virus. Science 2016, 353, aaf8160. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Roth, A.; Mercier, A.; Lepers, C.; Hoy, D.; Duituturaga, S.; Benyon, E.; Guillaumot, L.; Souares, Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—An unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eur. Surveill. 2014, 19, 20929. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Faria, N.R.; Azevedo, R.D.S.D.S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; de Oliveira, W.K.; de Góes Cavalcanti, L.P. Zika virus outbreak in Brazil. J. Infect Dev. Ctries. 2016, 10, 116–120. [Google Scholar] [CrossRef]
- Brito, C. Zika virus: A new chapter in the history of medicine. Acta Med. Port. 2015, 28, 679–680. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; de Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; da Costa, A.C.; et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. Zika virus in the Americas—yet another arbovirus threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Alomar, A.A.; Eastmond, B.H.; Alto, B.W. The effects of exposure to pyriproxyfen and predation on Zika virus infection and transmission in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008846. [Google Scholar] [CrossRef]
- Alomar, A.A.; Alto, B.W. Mosquito responses to lethal and nonlethal effects of predation and an insect growth regulator. Ecosphere 2021, 12, e03452. [Google Scholar] [CrossRef]
- Alomar, A.A.; Alto, B.W. Evaluation of pyriproxyfen effects on Aedes aegypti and predatory mosquito Toxorhynchites rutilus (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 585–590. [Google Scholar] [CrossRef]
- Lima, E.P.; Paiva, M.H.S.; de Araújo, A.P.; da Silva, E.V.G.; da Silva, U.M.; de Oliveira, L.N.; Santana, A.E.G.; Barbosa, C.N.; de Paiva Neto, C.C.; Goulart, M.O.F.; et al. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasites Vectors 2011, 4, 5. [Google Scholar] [CrossRef]
- Campos, K.B.; Martins, A.J.; Rodovalho, C.d.M.; Bellinato, D.F.; Dias, L.d.S.; Macoris, M.d.L.d.G.; Andrighetti, M.T.M.; Lima, J.B.P.; Obara, M.T. Assessment of the susceptibility status of Aedes aegypti (Diptera: Culicidae) populations to pyriproxyfen and malathion in a nation-wide monitoring of insecticide resistance performed in Brazil from 2017 to 2018. Parasites Vectors 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Paris, M.; David, J.-P.; Despres, L. Fitness costs of resistance to Bti toxins in the dengue vector Aedes aegypti. Ecotoxicology 2011, 20, 1184–1194. [Google Scholar] [CrossRef]
- Martins, A.J.; Ribeiro, C.D.; Bellinato, D.F.; Peixoto, A.A.; Valle, D.; Lima, J.B. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE 2012, 7, e31889. [Google Scholar] [CrossRef]
- Belinato, T.A.; Martins, A.J.; Valle, D. Fitness evaluation of two Brazilian Aedes aegypti field populations with distinct levels of resistance to the organophosphate temephos. Mem. Inst. Oswaldo Cruz 2012, 107, 916–922. [Google Scholar] [CrossRef]
- Diniz, D.F.A.; de Melo-Santos, M.A.; Santos, E.M.M.; Beserra, E.B.; Helvecio, E.; de Carvalho-Leandro, D.; de Santos, B.S.; Lima, V.L.d.M.; Ayres, C.F.J. Fitness cost in field and laboratory Aedes aegypti populations associated with resistance to the insecticide temephos. Parasites Vectors 2015, 8, 662. [Google Scholar] [CrossRef]
- Ndiath, M.O. Insecticides and insecticide resistance. Methods Mol. Biol. 2019, 2013, 287–304. [Google Scholar] [CrossRef]
- LIma, J.B.P.; Da-Cunha, M.P.; JÚNIOR, R.C.D.S.; Galardo, A.K.R.; Soares, S.S.; Braga, I.A.; Ramos, R.P.; Valle, D. Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espírito Santo, Brazil. Am. J. Trop. Med. Hyg. 2003, 68, 329–333. [Google Scholar] [CrossRef]
- Braga, I.A.; Valle, D. Aedes aegypti: Surveillance, resistance monitoring, and control alternatives in Brazil. Epidemiol. Serv. Saude 2007, 16, 295–302. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Entomological Surveillance for Aedes spp. in the Context of Zika Virus: Interim Guidance for Entomologists. Available online: https://www.who.int/publications/i/item/WHO-ZIKV-VC-16.2 (accessed on 8 August 2022).
- Alomar, A.A.; Eastmond, B.H.; Alto, B.W. Juvenile hormone analog enhances Zika virus infection in Aedes aegypti. Sci. Rep. 2021, 11, 21062. [Google Scholar] [CrossRef]
- Alomar, A.A.; Alto, B.W. Temperature-mediated effects on Mayaro virus vector competency of Florida Aedes aegypti mosquito vectors. Viruses 2022, 14, 880. [Google Scholar] [CrossRef]
- Alomar, A.A.; Eastmond, B.H.; Rapti, Z.; Walker, E.D.; Alto, B.W. Ingestion of spinosad-containing toxic sugar bait alters Aedes alobpictus vector competence and vectorial capacity for dengue virus. Front. Microbiol. 2022, 13, 933482. [Google Scholar] [CrossRef]
- Lanteri, M.C.; Kleinman, S.H.; Glynn, S.A.; Musso, D.; Keith Hoots, W.; Custer, B.S.; Sabino, E.C.; Busch, M.P. Zika virus: A new threat to the safety of the blood supply with worldwide impact and implications. Transfusion 2016, 56, 1907–1914. [Google Scholar] [CrossRef]
- Rivero, A.; Vézilier, J.; Weill, M.; Read, A.F.; Gandon, S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 2010, 6, e1001000. [Google Scholar] [CrossRef]
- Friedlander, E.; Steinrücken, M. A numerical framework for genetic hitchhiking in populations of variable size. Genetics 2022, 220, iyac012. [Google Scholar] [CrossRef]
- Yan, G.; Chadee, D.D.; Severson, D.W. Evidence of genetic hitchhiking effect associated with insecticide resistance in Aedes aegypti. Genetics 1998, 148, 793–800. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Haigh, J. The hitch-hiking effect of a favorable gene. Genet. Res. 1974, 23, 23–35. [Google Scholar] [CrossRef]
- Vézilier, J.; Nicot, A.; De Lorgeril, J.; Gandon, S.; Rivero, A. The impact of insecticide resistance on Culex pipiens immunity. Evol. Appl. 2012, 6, 497–509. [Google Scholar] [CrossRef]
- Yunta, C.; Grisales, N.; Nász, S.; Hemmings, K.; Pignatelli, P.; Voice, M.; Ranson, H.; Paine, M.J.I. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect Biochem. Mol. Biol. 2016, 78, 50–57. [Google Scholar] [CrossRef]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 9, e101992. [Google Scholar] [CrossRef]
- Stephenson, C.J.; Coatsworth, H.; Waits, C.M.; Nazario-Maldonado, N.M.; Mathias, D.K.; Dinglasan, R.R.; Lednicky, J.A. Geographic partitioning of dengue virus transmission risk in Florida. Viruses 2021, 13, 2232. [Google Scholar] [CrossRef]
- Deng, J.; Guo, Y.; Su, X.; Liu, S.; Yang, W.; Wu, Y.; Kun, W.; Guiyun, Y.; Xiao-Guang, C. Impact of deltamethrin-resistance in Aedes albopictus on its fitness cost and vector competence. PLoS Negl. Trop. Dis. 2021, 15, e0009391. [Google Scholar] [CrossRef]
- Wang, L.; Fontaine, A.; Gaborit, P.; Guidez, A.; Issaly, J.; Girod, R.; Kazanji, M.; Rousset, D.; Vignuzzi, M.; Epelboin, Y.; et al. Interactions between vector competence to chikungunya virus and resistance to deltamethrin in Aedes aegypti laboratory lines? Med. Vet. Entomol 2022, 1–10, (Online ahead of print). [Google Scholar] [CrossRef]
- Atyame, C.M.; Alout, H.; Mousson, L.; Vazeille, M.; Diallo, M.; Weill, M.; Failloux, A.-B. Insecticide resistance genes affect Culex quinquefasciatus vector competence for West Nile virus. Proc. R. Soc. B 2019, 286, 20182273. [Google Scholar] [CrossRef]
- Parker-Crockett, C.; Connelly, C.R.; Siegfried, B.; Alto, B. Influence of pyrethroid resistance on vector competency for Zika virus by Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 1908–1916. [Google Scholar] [CrossRef]
- Chen, T.Y.; Smartt, C.T.; Shin, D. Permethrin resistance in Aedes aegypti affects aspects of vectorial capacity. Insects 2021, 12, 71. [Google Scholar] [CrossRef]

| Population (Resistance Level) | Mean (No. Samples) | Std. Error of Mean | Lower Mean | Upper Mean |
|---|---|---|---|---|
| Rockefeller (reference strain) | 0.8491 (51) a | 0.04917 | 0.7262 | 0.9227 |
| Serrinha (Low) | 0.9512 (39) a | 0.03364 | 0.8248 | 0.9878 |
| Brumado (Low) | 0.8600 (48) a | 0.04907 | 0.7343 | 0.9318 |
| Juazeiro do Norte (Moderate) | 0.8200 (48) a | 0.05433 | 0.6889 | 0.9036 |
| Itabuna (Moderate) | 0.4694 (47) b | 0.07129 | 0.3354 | 0.6079 |
| Quixadá (High) | 0.5111 (43) b | 0.07452 | 0.3682 | 0.6523 |
| Population (Resistance Level) | Mean (No. Samples) | Std. Error of Mean | Lower Mean | Upper Mean |
|---|---|---|---|---|
| Rockefeller (reference strain) | 0.5869 (44) a | 0.07260 | 0.4414 | 0.7187 |
| Serrinha (Low) | 0.6750 (38) a | 0.07406 | 0.5173 | 0.8010 |
| Brumado (Low) | 0.7273 (42) a | 0.06714 | 0.5787 | 0.8381 |
| Juazeiro do Norte (Moderate) | 0.7561 (40) a | 0.06707 | 0.6031 | 0.8634 |
| Itabuna (Moderate) | 0.6667 (22) a | 0.09623 | 0.4612 | 0.8237 |
| Quixadá (High) | 0.6667 (22) a | 0.09623 | 0.4612 | 0.8237 |
| Population (Resistance Level) | Mean (No. Samples) | Std. Error of Mean | Lower Mean | Upper Mean |
|---|---|---|---|---|
| Rockefeller (reference strain) | 0.4286 (26) a | 0.09352 | 0.2619 | 0.6132 |
| Serrinha (Low) | 0.2143 (26) a | 0.07754 | 0.09957 | 0.4021 |
| Brumado (Low) | 0.1212 (31) a | 0.05682 | 0.04625 | 0.2818 |
| Juazeiro do Norte (Moderate) | 0.2188 (31) a | 0.07308 | 0.1080 | 0.3930 |
| Itabuna (Moderate) | 0.1765 (15) a | 0.09246 | 0.05801 | 0.4271 |
| Quixadá (High) | 0.2941 (15) a | 0.1105 | 0.1280 | 0.5419 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, K.B.; Alomar, A.A.; Eastmond, B.H.; Obara, M.T.; Alto, B.W. Brazilian Populations of Aedes aegypti Resistant to Pyriproxyfen Exhibit Lower Susceptibility to Infection with Zika Virus. Viruses 2022, 14, 2198. https://doi.org/10.3390/v14102198
Campos KB, Alomar AA, Eastmond BH, Obara MT, Alto BW. Brazilian Populations of Aedes aegypti Resistant to Pyriproxyfen Exhibit Lower Susceptibility to Infection with Zika Virus. Viruses. 2022; 14(10):2198. https://doi.org/10.3390/v14102198
Chicago/Turabian StyleCampos, Kauara Brito, Abdullah A. Alomar, Bradley H. Eastmond, Marcos Takashi Obara, and Barry W. Alto. 2022. "Brazilian Populations of Aedes aegypti Resistant to Pyriproxyfen Exhibit Lower Susceptibility to Infection with Zika Virus" Viruses 14, no. 10: 2198. https://doi.org/10.3390/v14102198
APA StyleCampos, K. B., Alomar, A. A., Eastmond, B. H., Obara, M. T., & Alto, B. W. (2022). Brazilian Populations of Aedes aegypti Resistant to Pyriproxyfen Exhibit Lower Susceptibility to Infection with Zika Virus. Viruses, 14(10), 2198. https://doi.org/10.3390/v14102198






